RUNX3: A Location-oriented Genome Coordinator
1Department of Stomatology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China
2Nanhai Translational Innovation Center of Precision Immunology, Sun Yat-Sen Memorial Hospital, Foshan, China
3Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Guangdong-Hong Kong Joint Laboratory for RNA Medicine, Medical Research, Guangzhou, China
4Department of Oral and Maxillofacial Surgery, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, 107th, Yanjiang Xi Road, Guangzhou, Guangdong, China
5Medical Research Center, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, China
aJoint first authors: these authors contributed to this work equally.
*Correspondence to: Zhiquan Huang, E-mail: hzhquan@mail.sysu.edu.cn; Zixian Huang, E-mail: huangzx66@mail.sysu.edu.cn
Received: February 17 2023; Revised: March 27 2023; Accepted: April 13 2023; Published Online: April 24 2023
Cite this paper:
Tianshu Xu, Yancan Liang, Zhiquan Huang and Zixian Huang. RUNX3: A Location-oriented Genome Coordinator. BIO Integration 2023; 4(1): 30–37.
DOI: 10.15212/bioi-2023-0003. Available at: https://bio-integration.org/
Download citation
© 2023 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
Transcription factors are key components in gene expression and are associated with various diseases. Transcription factors maintain the stability of gene transcription and cell function. Among the transcription factors, the Runt-related transcription factor (RUNX) family regulates growth and development in a tissue-specific manner and is involved in tumorigenesis. The function of an important member of the RUNX family, RUNX3, was shown to be closely related to its subcellular localization. Normally, RUNX3 promotes or represses gene transcription in the nucleus; however, when RUNX3 is restricted in the cytoplasm, RUNX3 fails to function and only has a minor effect o gene expression. Hence, the risk of tumorigenesis cannot simply be equated with the level of RUNX3 expression, which makes the diagnosis and treatment of cancer more complicated. The cytoplasmic localization of RUNX3 has been shown to be associated with a variety of tumors. Herein we have summarized the current information on RUNX3 mis-localization and RUNX3 promotion of tumorigenesis, thus providing new insight for future investigations to elucidate the mechanisms by which RUNX3 regulates tumorigenesis.
Keywords
Cytoplasmic localization, mis-localization, RUNX3, tumorigenesis.
Introduction
Transcription factors are a group of proteins that bind to a specific sequence upstream of the 5’ end of a gene, thereby enabling the transcriptional regulation of downstream genes [1]. Transcription factors are key components in the nucleus that control gene expression, determine cell function, and the response to the environment.
As a main member of the Runt-related transcription factor (RUNX) family of transcription factors, RUNX3 is essential for regulating growth and development, and regulates the development, differentiation, and maintenance of the gastric epithelium [2, 3], nervous system [4], and immune system [5–7]. The RUNX3 N-terminus is mainly composed of the highly-conserved Runt homology domain (RHD) [8], which is the characteristic structure of the RUNX family and participates in the direct transcriptional regulation of DNA. The C-terminus is composed of similar transactivation domains (TADs), inhibitory domains (IDs), and proline-tyrosine (PY) and VWRPY motifs [9], but RUNX3 exhibits different protein interactions and post-translational modifications than RUNX1 and RUNX2. Moreover, the sequence structure of RUNX3 is more compact and conserved [10], thus RUNX3 is considered a unique gene in this family and has received much attention.
In recent years, an unusual relationship between RUNX3 cytoplasmic localization and a tumor-promoting phenotype have been reported [11]. Therefore, we focused on the impact of unusual localizations of the RUNX3 transcription factor in cancer by comparing localization in the nucleus and cytoplasm, and combining that information with literature reports to determine the possible causes of mis-localization and impact on cell fate.
RUNX3 in the nucleus, an important regulator of gene homeostasis
RUNX3 was first identified in the nucleus [10]. As a transcription factor, RUNX3 has long been considered a tumor suppressor gene that directly or indirectly suppresses the expression of cancer-related genes by binding to DNA promoters [12] or protein–protein interactions [13, 14]. Under different stimulation signals, RUNX3 can independently or cooperatively maintain homeostasis at the genomic and cellular levels.
RUNX3 balances TGF-β signaling
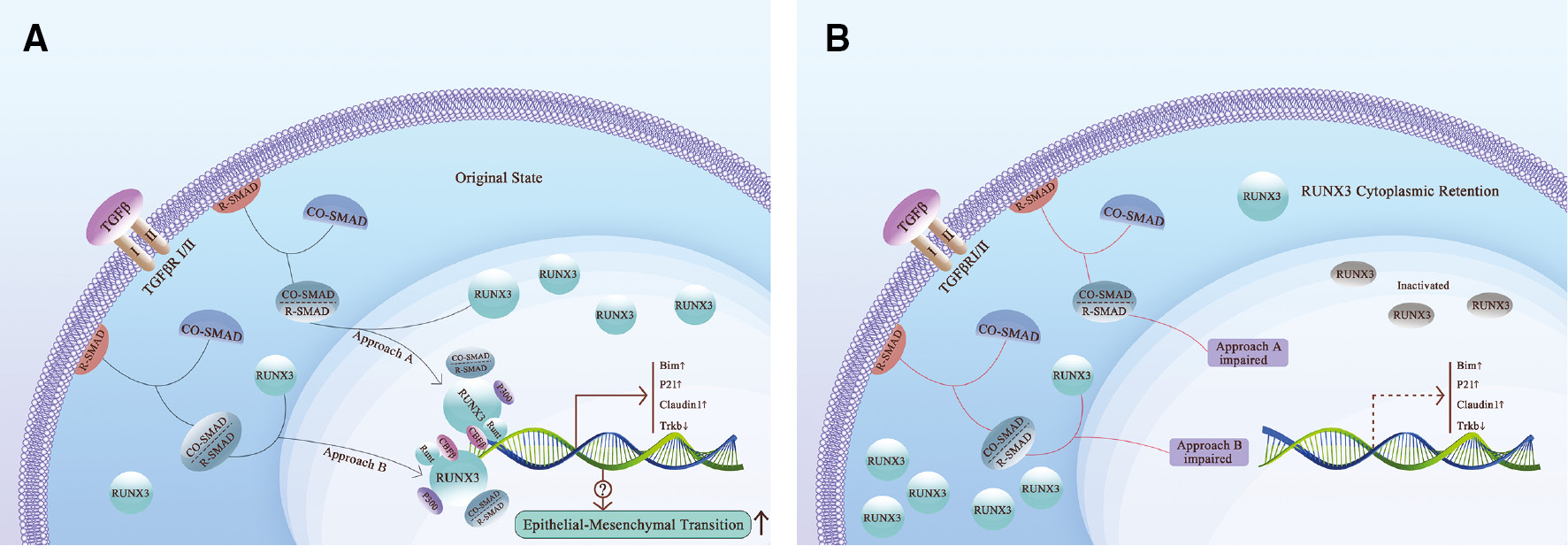
The TGF-β signaling pathway is a characteristic signaling pathway involved in cancer progression that promotes cell proliferation and malignant phenotype transformation in tumor cells, and also activates RUNX3 [2, 15]. Under the stimulation of TGF-β signaling, reactive-suppressor of mothers against decapentaplegic (R-SMAD) of the Smad protein family, which is anchored to the plasma membrane, forms heterodimeric SMAD complexes with cooperative SMAD (Co-Smad). The complexes bind to RUNX3 and transport to the nucleus together [16]. After reaching the nucleus, under promotion of CBF-β, the runt homology domain (RHD) of RUNX3 directly binds to the DNA promoter [17, 18], upregulates the expression of p21, Bim, and Claudin1 [12, 19, 20], silences Trkb, promotes apoptosis and inhibits tumor formation and progression [21–25] (Figure 1A). Moreover, RUNX3 also inhibits the transcriptional activity of TGF-β [26], thus forming a negative feedback pathway to balance the tumor-promoting effect brought about by the upregulation of TGF-β [27].
Figure 1 TGFβ pathway and RUNX3 cytoplasmic retention. A. RUNX3 enters the nucleus under TGFβ signaling. RUNX3 exerts DNA binding ability with the assistance of CBFβ. After stimulation by TGFβ, R-Smad combined with Co-Smad form the Smads complex, which binds to RUNX3 and promotes RUNX3 nuclear translocation and transactivation. In the original state, Smads complex and P300 activate RUNX3, the Runt domain binds to DNA, promotes the upregulation of Bim, P21, and Claudin 1, and down-regulation of TrkB. B. Impaired TGFβ pathway leads to cytoplasmic retention of RUNX3. When the TGFβ signaling pathway is injured, malfunctioning of TGFβ signaling or SMAD protein family occurs. RUNX3 fails to bind with SMADs and is retained in the cytoplasm in an inactive state. The aforementioned dysregulation result in downregulation of Bim, P21, and Claudin 1, and upregulation of TrkB.
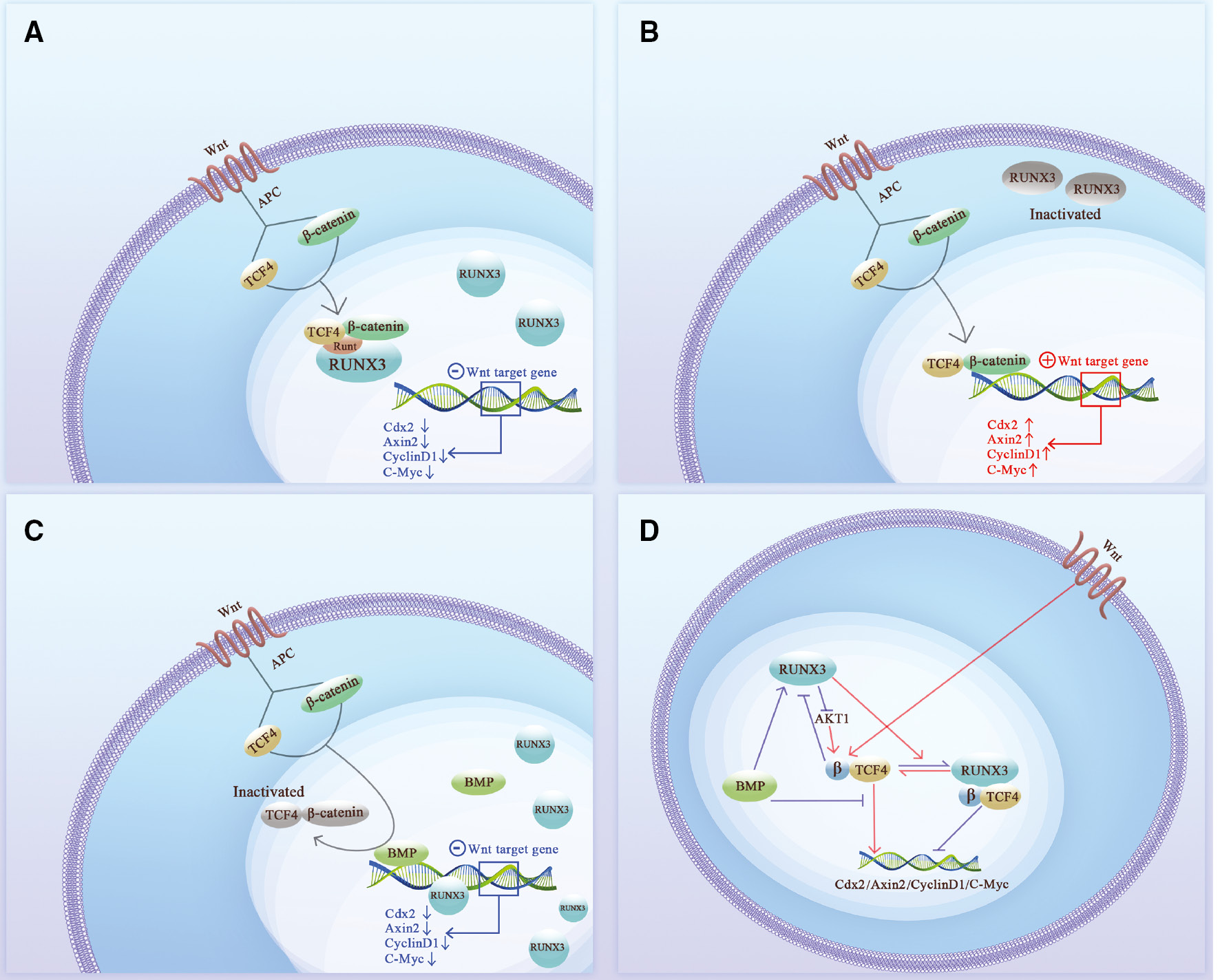
RUNX3 competitively inhibits oncogene transcription
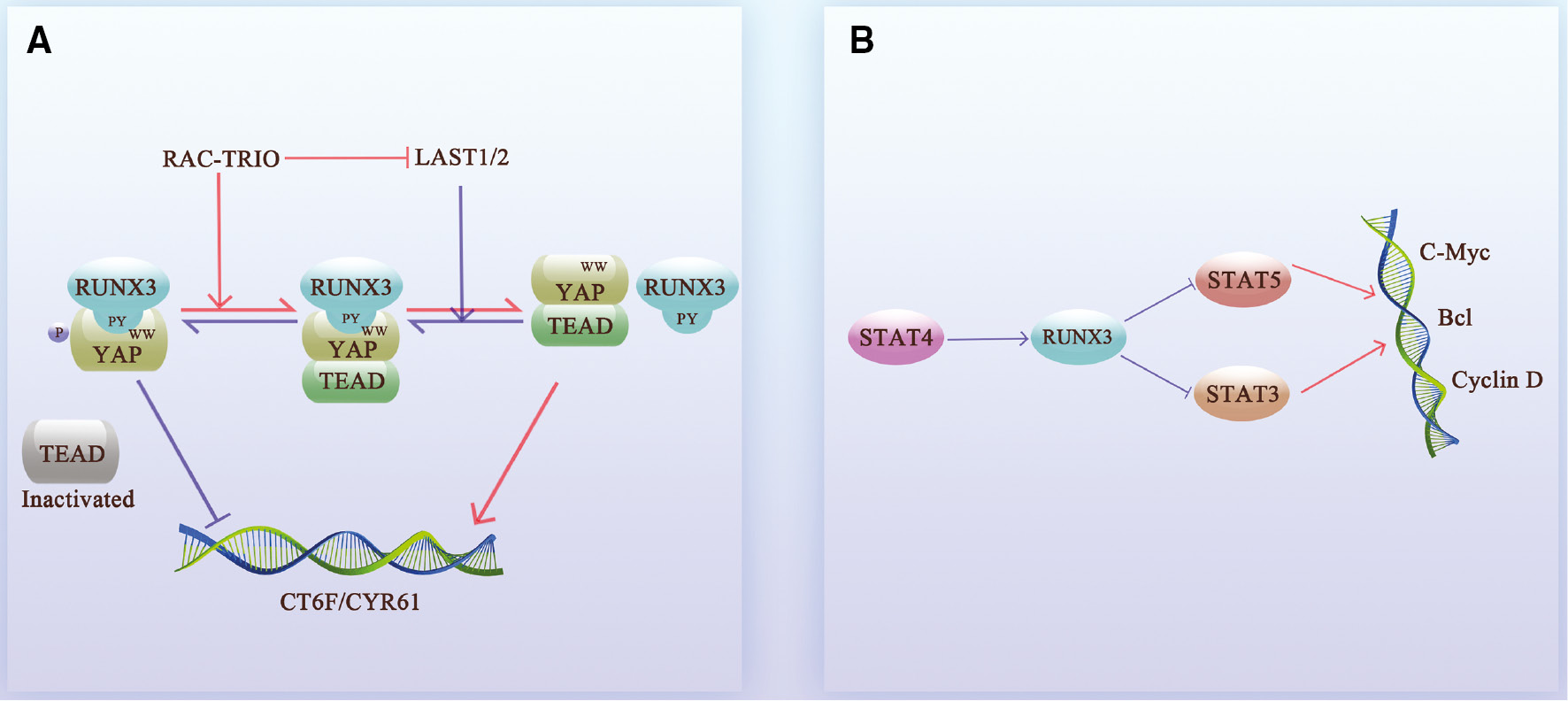
In addition to direct effects, RUNX3 also indirectly inhibits the transcriptional activity of cancer-promoting genes by competing for the DNA-binding sites of cancer-promoting factors, i.e., protein–protein interactions (PPIs). In fact, RUNX3 mostly affects the activity of cancer-promoting genes in an indirect way, blocking multiple cancer signaling pathways. RUNX3 inhibits the formation of the β-catenin/TCF4 complex in the Wnt signaling pathway [13, 28], thereby blocking the transcription of Cdx2, Axin2, Cyclin D1, and c-Myc, and inhibiting proliferation and invasion [14, 29, 30] (Figure 2A, C). RUNX3 also directly binds to the Akt1 promoter and inhibits the Akt1/β-catenin/cyclin D1 signaling axis [30], blocking the Wnt signaling pathway in an alternative way. In addition, BMP proteins cooperate with RUNX3 to bind to the c-Myc promoter, thus inhibiting transcriptional activity [31] (Figure 2D). Similarly, RUNX3 binds to the TEAD-YAP complex in the nucleus to form a YAP-TEAD-RUNX3 ternary complex in the Hippo pathway [32, 33], which accelerates the dissociation of TEAD-YAP; the RAC signal can even promote this process [34]. RUNX3 also blocks binding of TEAD-YAP to CTGF and CYR61, inhibiting tumor proliferation activity [33, 35–37] (Figure 3A).
Figure 2 The relationship between RUNX3 and the Wnt signaling pathway. A. Nuclear RUNX3 blocks the Wnt signaling pathway through “protein-protein interactions.” In the original state, TCF4 binds to β-catenin and translocates into the nucleus after Wnt signaling stimulation. The Runt domain of RUNX3 binds to the DNA-binding region of TCF4 in nucleus, thus preventing the TCF4-β-catenin complex from binding to DNA and inhibiting the target genes (Cdx2, Axin2, CyclinD1, and c-Myc) of the Wnt signaling pathway; B. Cytoplasmic RUNX3 fails to inhibit the Wnt signaling pathway. RUNX3 is localized in the cytoplasm in an inactive state. RUNX3 in the nucleus is absent or insufficient to counteract the TCF4-β-catenin complex, which subsequently binds to DNA and promotes the transcription of Cdx2, Axin2, CyclinD1, and c-Myc oncogenes. C. RUNX3 cooperates with the BMP family to suppress oncogenes. BMP occupies the DNA binding site of TCF and RUNX3 binds to the DNA binding site to jointly exert a tumor suppressor effect. D. The relationship between RUNX3 and Wnt signaling pathway. The Runt domain of nuclear RUNX3 combines with the DNA binding region of TCF4 to form a RUNX3-TCF4-β-catenin trimer, which blocks binding of the TCF4-β-catenin complex to the target gene promoter and inhibits the target genes (Cdx2, Axin2, CyclinD1, and c-Myc). When RUNX3 is localized in the cytoplasm and the nucleus RUNX3 is absent or deficient, the TCF4-β-catenin complex smoothly binds to the promoters of target genes to promote the transcription of Cdx2, Axin2, CyclinD1, and c-Myc oncogenes. RUNX3 can also inhibit the effect of AKT1 on CTNNB1 (β-catenin encoding gene) and indirectly inhibits the formation of TCF4-β-catenin complex. BMP interacts with the TCF4-β-catenin complex and upregulates the expression of RUNX3, directly or indirectly regulating Cdx2, Axin2, CyclinD1, and c-Myc transcription.
Figure 3 RUNX3 competitively inhibits oncogene transcription. A. RUNX3 inhibits the Hippo pathway. The TEAD-YAP complex of the Hippo signaling pathway promotes the transcription of the oncogenes, CTGF and CYR61, and promotes cancer progression. Nuclear NRUNX3 directly binds to YAP, thus preventing the formation of the TEAD-YAP complex, and forming a triple complex with TEAD-YAP for degradation. When RAC-TRIO is downregulated, the downstream LAST1/2 renders YAP more likely to interact with RUNX3 by phosphorylating YAP and prevents the formation of TEAD-YAP. B. Relationship between RUNX3 and the STAT family. STAT4 upregulates the expression of RUNX3. RUNX3 inhibits the promoting effect of STAT3 and STAT5 on c-Myc, Bcl, and Cyclin D.
Moreover, RUNX3 acts on the downstream target, STAT4, inhibits STAT5 [38] and JAK3/STAT3 signaling [39], and downregulates c-Myc [40–42], Bcl [43–45], and cyclin D [46, 47] (Figure 3B). With respect to DNA damage repair, RUNX3 recruits FANCD2-FANCI through the Fanconi anemia (FA) pathway to repair the DNA fork of interstrand crosslinks (ICLs) [48–50] and activates transcription of redox regulator heme oxygenase 1 (HO-1 or HMOX1), ameliorating the DNA damage caused by oxidative stress and thereby maintaining cellular homeostasis [47].
RUNX3 synergizes with P53 to exert antitumor effects
In addition to independent RUNX3-independent tumor suppressor regulation, DNA damage and activation of oncogenes (Myc and K-Ras) cause RUNX3 and p53 to exhibit a synergistic role [51–56]. In this context, RUNX3-p53 forms a genome surveillance system to cooperatively regulate gene transcription activity [57]. RUNX3 simultaneously recruits phosphorylated ATM and p300 to activate p53 downstream target genes [58, 59], execute DNA damage repair or promote cell cycle arrest [60], and apoptosis [51, 61]. In addition, p53 inhibits the overexpression of RUNX3, thereby forming a stable RUNX3-p53 negative feedback loop [62]. Therefore, the combination of RUNX3 and p53 is thought to be the gatekeeper and guardian of the genome, ensuring genomic stability. The synergistic effect of RUNX3 on p53, however, is destabilized by the p53 high mutation rate in many cancer types [63]. Mutant p53 (p53R175H-human/p53R172H-mouse) binds RUNX3 and promotes Myc transcription in osteosarcomas [64]. In summary, the RUNX3-p53 negative feedback loop monitors the genome, but the stability of p53 cannot be overlooked.
In general, under the action of various post-translational modifications, RUNX3 has an important role in the regulatory balance between oncogenes and tumor suppressor genes.
Cytoplasmic localization: a constraint of RUNX3
RUNX3 is normally localized and functions in the nucleus, but recent studies have shown that RUNX3 has an unusual cytoplasmic localization in many tumor cell lines, which is also called “cytoplasmic sequestration” or “mis-localization” of RUNX3 [11]. The cytoplasmic localization of RUNX3 is associated with the gastric epithelium in gastric cancer, suggesting a role in carcinogenesis [11, 65, 66]. Subsequent reports on RUNX3 mis-localization confirmed that cytoplasmic localization of RUNX3 is not a rare event [11], and cytoplasmic localization occurs in 80% of breast cancer patients [67]. Cytoplasmic localization of RUNX3 protein also exists in 46.0% and 30% of ovarian cancer and oral squamous cell carcinoma patients [68, 69], respectively. Cytoplasmic localization of RUNX3 has also been observed in colon cancer and is associated with tumorigenesis and metastasis [70]. The cytoplasmic localization of RUNX3 in lung small cell carcinoma is thought to lead to a higher probability of postoperative distant metastasis and is significantly associated with lymph node-positive involvement and margins indicating lymphatic invasion [71]. Cytoplasmic localization of RUNX3 greatly limits RUNX3 function, resulting in protein conversion to a tumor-promoting phenotype. Interestingly, although the three members of the RUNX family share a high degree of homology, only RUNX3 exhibits cytoplasmic localization that leads to functional differences.
Current studies suggest that the cytoplasmic sequestration of RUNX3 originates via two mechanisms: ① blockade of the RUNX3 nuclear import pathway (cytoplasmic retention); and ② abnormal activation of the nuclear RUNX3 nuclear export signal (nuclear exclusion). We will discuss the impact on tumor formation from the perspective of these two mechanisms.
Cytoplasmic retention of RUNX3: RUNX3 nuclear import pathway dysfunction
The nuclear import of RUNX3 can be mediated in two ways: ① TGFβ-SMAD signaling [11]; and ② DNA damage or proto-oncogene activation signaling [51]. TGFβ signaling is the initiation signal by which RUNX3 enters the nucleus to regulate transcription. Dysregulation of one or more molecules in the TGFβ pathway upstream of RUNX3, such as downregulated expression of Smad4 and TGF-βI/II receptors and mutation of TGF-βII receptors, may lead to plasma retention of RUNX3. Occupation of SMAD-binding sites can also lead to retention of RUNX3. This process leads to the continuous activation of intracellular TGF-β-SMAD signaling without negative feedback inhibition, stimulates cell proliferation, and induces tumorigenesis [11, 67] (Figure 1B). In addition, RUNX3 can also rapidly enter the nucleus and co-localize with p53 under the stimulation of DNA damage or proto-oncogene activation [51]. Given that RUNX proteins rely on nuclear localization signals (NLSs) [72] and nuclear matrix targeting signal (NMTS) sequences for nuclear import [73–75], cytoplasmic retention could be related to inactivation of these two nuclear import-related signals. The absence of effective repair methods after DNA damage makes it possible for the aggravation of abnormal DNA mutations and the occurrence of tumor signals [53, 64, 76, 77].
Nuclear exclusion of RUNX3: aberrant activation of nuclear export signaling
RUNX3, which is localized in the nucleus, can also be exported to the cytoplasm. After being exported, RUNX3 loses tumor suppressor regulatory function and DNA damage monitoring, and indirectly promotes tumorigenesis.
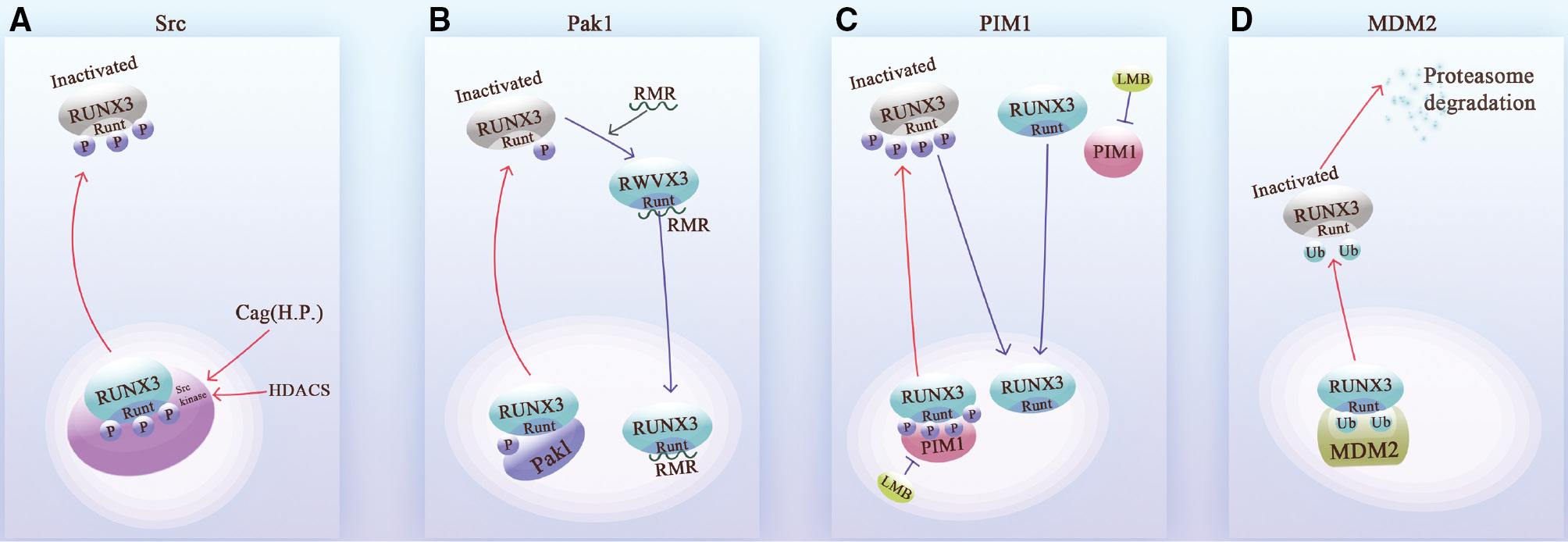
This nuclear export program is activated by phosphorylation- or ubiquitination-modifying enzymes or other types of molecules. Specifically, abnormal phosphorylation of RUNX3 is one of the main triggers of nuclear exportation, and this process can be mediated by phosphokinases, such as the Src kinase family [65, 78], Pak1 [79, 80], and Pim-1 [81, 82]. Histone deacetylase (HDAC) produced by oxidative stress or the Cag. oncoprotein produced by Helicobacter pylori (H.p.), can increase the level of Src in cells [70]. Src phosphorylates multiple tyrosine residues on the surface of the RHD of RUNX3, initiating the RUNX3 nuclear export program [65]. Subsequently, chromosome region maintenance protein 1 (CRM1) binds the RUNX3-containing nuclear export signal (NES) to the nuclear pore complex and mediates nuclear export [83, 84] (Figure 4).
Figure 4 The mechanisms underlying RUNX3 nuclear exportation. A. Src kinase phosphorylates the tyrosine residues of the Runt domain of RUNX 3, promoting RUNX3 nuclear exportation. Scr kinase can be induced by Helicobacter pylori (H.p.) and HDACs. B. Pak1 phosphorylates the serine and threonine sites of the Runt domain to promote inactivation of RUNX3 after nuclear exportation. The discovered RMR peptide competitively inhibits Pak1 and restores RUNX3 activity and nuclear localization. C. PIM-1 shuttles between the cytoplasmic and nucleus. PIM-1 phosphorylates and inactivates of RUNX3 after nuclear exportation. This process can be reversed by LMB, the Pim-1 inhibitor. D. The acidic domain of MDM2 interacts directly with Runt, and the RING finger domain of MDM2 is the active region for ubiquitination. After ubiquitination on the surface of the Runt domain, RUNX3 is exported from the nucleus and degraded by the proteasome.
Thus, tyrosine-phosphorylated RUNX3 is mainly present in the cytoplasm, while non-tyrosine-phosphorylated RUNX3 is present in the nucleus [85]. In addition, the Src kinase family members, Fyn and Lck, are also able to phosphorylate RUNX3, which shows that this family has multiple roles in RUNX3 nuclear export [65]. Moreover, Pak1 and PIM-1 kinases also promote the nuclear export of RUNX3 in a similar manner. The three phosphorylation sites are located on the surface of the RHD [80, 81]. Moreover, the nuclear export of ubiquitinated RUNX3 is mediated by MDM2. The acidic domain of MDM2 directly ubiquitinates lys94 and lys148 of the RHD of RUNX3 [52] (Figure 4).
Furthermore, the Jab1/CSN protein complex is also responsible for the nuclear export and degradation of RUNX3. Jun activation domain-binding protein 1 (Jab1) binds to RUNX3 through the MPN domain and initiates complex nuclear export via the NES of Jab1 [86]. The Mpr1p Pad1p N-terminal (MPN) domain of Jab1 has a role in the physical interaction between RUNX3 and Jab1, while the NES is the receptor of the CRM1 export substrate. Moreover, the COP9 signalosome complex (CSN complex) is regulated by CSN-associated kinases that degrade RUNX3 via the proteasomal pathway [86, 87].
Prospects
As a tumor suppressor in the RUNX family, RUNX3 attenuates multiple oncogenic signals. Restoring RUNX3 activity in nuclear RUNX3-negative cells significantly reverses the tumor phenotype [79, 88], suggesting that remobilization of cytoplasmic RUNX3 into the nucleus or restoring the level of nuclear RUNX3 expression exogenously may be a possible therapeutic strategy for the treatment of cancer.
It is worth mentioning that among the many mechanisms of protein dysregulation, only cytoplasmic localization causes a functional restriction through a change in the spatial physical position, rather than the common mechanism underlying changes in protein levels. Simply overexpressing RUNX3 to reverse the tumor phenotype will not completely overcome this challenge. This RUNX3 mislocalization undoubtedly increases the complexity of tumor progression and the difficulty of diagnosis and treatment. Overall, cytoplasmic localization of RUNX3 promotes oncogenesis. Thus, it is necessary to pay more attention to this unconventional phenomenon.
Abbreviations
RUNX3, Runt-related transcription factor 3; RHD, Runt homology domain; TADs, transactivation domains; IDs, Inhibitory domains; PY motifs, proline-tyrosine motifs; R-SMAD, reactive SMAD; Co-SMAD, cooperative SMAD; P21, cyclin-dependent kinase inhibitor 1A; Claudin1, senescence associated epithelial membrane protein 1; Trkb, tyrosine kinase receptor B; TGF-β, transforming growth factor-β; PPIs, protein–protein interactions; TCF4, transcription factor 4; Cdx2, caudal-type homeobox protein 2; Axin2, axis inhibition protein 2; cyclin D1, G1/S-specific cyclin-D1; c-Myc, Myc proto-oncogene protein; Akt1, v-akt murine thymoma viral oncogene homolog 1; BMP, bone morphogenetic protein; TEAD, TEA domain family member; YAP, Yes-associated protein; RAC protein, Ras-related C3 botulinum toxin substrate protein; CTGF, connective tissue growth factor; CYR61, 61 cysteine-rich, angiogenic inducer; STAT, signal transducer and activator of transcription; JAK3, Janus kinase 3; FA pathway, Fanconi anemia pathway; ICLs, interstrand crosslinks; HO-1 or HMOX1, heme oxygenase 1; K-Ras, Kirsten rat sarcoma viral oncogene homolog; ATM protein, ataxia telangiectasia-mutated protein; NLS, nuclear localization signal; NMTS, nuclear matrix targeting signal; Pak1, p21 activated kinase 1; Pim-1, provirus integration site for Moloney murine leukemia virus kinase-1; HDAC, histone deacetylase; CRM1, chromosome region maintenance protein 1; NES, nuclear export signal; MDM2, mouse double minute 2 homolog; CSN, constitutive photomorphogenic signalosome; Jab1, Jun activation domain-binding protein 1; MPN domain, Mpr1p Pad1p N-terminal domain.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (#81802700), the Science and Technology Program of Guangdong (#2021A1515111121), the Guangzhou Science and Technology Project (#202103000093), the Key Laboratory of Malignant Tumour Gene Regulation and Target Therapy of Guangdong Higher Education Institutes, Sun-Yat-Sen University (Grant KLB09001), and the Key Laboratory of Malignant Tumour Molecular Mechanism and Translational Medicine of Guangzhou Bureau of Science and Information Technology ([2013]163).
Conflict of Interest
This manuscript is a review article and does not involve a research protocol requiring approval by the relevant institutional review board or ethics committee.Tianshu Xu, Yancan Liang, Zhiquan Huang and Zixian Huang declare that they have no conflicts of interest.
References
- Liu C-F, Ni Y, Thachil V, Morley M, Moravec CS, Tang WHW. Differential expression of members of SOX family of transcription factors in failing human hearts. Transl Res 2022;242:66-78. [PMID: 34695607 DOI: 10.1016/j.trsl.2021.10.002]
- Li QL, Ito K, Sakakura C, Fukamachi H, Inoue Ki, et al. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell 2002;109:113-24. [PMID: 11955451 DOI: 10.1016/s0092-8674(02)00690-6]
- Brenner O, Levanon D, Negreanu V, Golubkov O, Fainaru O, et al. Loss of Runx3 function in leukocytes is associated with spontaneously developed colitis and gastric mucosal hyperplasia. Proc Natl Acad Sci U S A 2004;101:16016-21. [PMID: 15514019 DOI: 10.1073/pnas.0407180101]
- Inoue K, Ozaki S, Shiga T, Ito K, Masuda T, et al. Runx3 controls the axonal projection of proprioceptive dorsal root ganglion neurons. Nat Neurosci 2002;5:946-54. [PMID: 12352981 DOI: 10.1038/nn925]
- Taniuchi I, Osato M, Egawa T, Sunshine MJ, Bae SC, et al. Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development. Cell 2002;111:621-33. [PMID: 12464175 DOI: 10.1016/s0092-8674(02)01111-x]
- Woolf E, Brenner O, Goldenberg D, Levanon D, Groner Y. Runx3 regulates dendritic epidermal T cell development. Dev Biol 2007;303:703-14. [PMID: 17222403 DOI: 10.1016/j.ydbio.2006.12.005]
- Ebihara T, Song C, Ryu SH, Plougastel-Douglas B, Yang L, et al. Runx3 specifies lineage commitment of innate lymphoid cells. Nat Immunol 2015;16:1124-33. [PMID: 26414766 DOI: 10.1038/ni.3272]
- Ito Y. Molecular basis of tissue-specific gene expression mediated by the Runt domain transcription factor PEBP2/CBF. Genes Cells 1999;4:685-96. [PMID: 10620014 DOI: 10.1046/j.1365-2443.1999.00298.x]
- Manandhar S, Lee YM. Emerging role of RUNX3 in the regulation of tumor microenvironment. BMB Rep 2018;51:174-81. [PMID: 29429451 DOI: 10.5483/bmbrep.2018.51.4.033]
- Bangsow C, Rubins N, Glusman G, Bernstein Y, Negreanu V, et al. The RUNX3 gene–sequence, structure and regulated expression. Gene 2001;279:221-32. [PMID: 11733147 DOI: 10.1016/s0378-1119(01)00760-0]
- Ito K, Liu Q, Salto-Tellez M, Yano T, Tada K, et al. RUNX3, a novel tumor suppressor, is frequently inactivated in gastric cancer by protein mislocalization. Cancer Res 2005;65:7743-50. [PMID: 16140942 DOI: 10.1158/0008-5472.CAN-05-0743]
- Chi XZ, Yang JO, Lee KY, Ito K, Sakakura C, et al. RUNX3 suppresses gastric epithelial cell growth by inducing p21(WAF1/Cip1) expression in cooperation with transforming growth factor beta-activated SMAD. Mol Cell Biol 2005;25:8097-107. [PMID: 16135801 DOI: 10.1128/MCB.25.18.8097-8107.2005]
- Ito K, Lim AC, Salto-Tellez M, Motoda L, Osato M, et al. RUNX3 attenuates beta-catenin/T cell factors in intestinal tumorigenesis. Cancer Cell 2008;14:226-37. [PMID: 18772112 DOI: 10.1016/j.ccr.2008.08.004]
- Ito K, Chuang LS, Ito T, Chang TL, Fukamachi H, et al. Loss of Runx3 is a key event in inducing precancerous state of the stomach. Gastroenterology 2011;140:1536-46.e8. [PMID: 21277301 DOI: 10.1053/j.gastro.2011.01.043]
- Miyazono K, Maeda S, Imamura T. Coordinate regulation of cell growth and differentiation by TGF-beta superfamily and Runx proteins. Oncogene 2004;23:4232-7. [PMID: 15156178 DOI: 10.1038/sj.onc.1207131]
- Miyazawa K, Shinozaki M, Hara T, Furuya T, Miyazono K. Two major Smad pathways in TGF-beta superfamily signalling. Genes Cells 2002;7:1191-204. [PMID: 12485160 DOI: 10.1046/j.1365-2443.2002.00599.x]
- Wang Q, Stacy T, Miller JD, Lewis AF, Gu TL, et al. The CBFbeta subunit is essential for CBFalpha2 (AML1) function in vivo. Cell 1996;87:697-708. [PMID: 8929538 DOI: 10.1016/s0092-8674(00)81389-6]
- Wang S, Wang Q, Crute BE, Melnikova IN, Keller SR, et al. Cloning and characterization of subunits of the T-cell receptor and murine leukemia virus enhancer core-binding factor. Mol Cell Biol 1993;13:3324-39. [PMID: 8497254 DOI: 10.1128/mcb.13.6.3324-3339.1993]
- Hasegawa K, Yazumi S, Wada M, Sakurai T, Kida M, et al. Restoration of RUNX3 enhances transforming growth factor-beta-dependent p21 expression in a biliary tract cancer cell line. Cancer Sci 2007;98:838-43. [PMID: 17470130 DOI: 10.1111/j.1349-7006.2007.00460.x]
- Chang TL, Ito K, Ko TK, Liu Q, Salto-Tellez M, et al. Claudin-1 has tumor suppressive activity and is a direct target of RUNX3 in gastric epithelial cells. Gastroenterology 2010;138:255-65.e1-3. [PMID: 19706291 DOI: 10.1053/j.gastro.2009.08.044]
- Geiger TR, Peeper DS. Critical role for TrkB kinase function in anoikis suppression, tumorigenesis, and metastasis. Cancer Res 2007;67:6221-9. [PMID: 17616679 DOI: 10.1158/0008-5472.CAN-07-0121]
- Dedoni S, Marras L, Olianas MC, Ingianni A, Onali P. Downregulation of TrkB expression and signaling by valproic acid and other histone deacetylase inhibitors. J Pharmacol Exp Ther 2019;370:490-503. [PMID: 31308194 DOI: 10.1124/jpet.119.258129]
- Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, et al. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature 2004;430:1034-9. [PMID: 15329723 DOI: 10.1038/nature02765]
- Inoue K, Ito K, Osato M, Lee B, Bae SC, et al. The transcription factor Runx3 represses the neurotrophin receptor TrkB during lineage commitment of dorsal root ganglion neurons. J Biol Chem 2007;282:24175-84. [PMID: 17584746 DOI: 10.1074/jbc.M703746200]
- Kim MS, Lee WS, Jin W. TrkB promotes breast cancer metastasis via suppression of Runx3 and Keap1 expression. Mol Cells 2016;39:258-65. [PMID: 26657794 DOI: 10.14348/molcells.2016.2310]
- Voon DC, Wang H, Koo JK, Nguyen TA, Hor YT, et al. Runx3 protects gastric epithelial cells against epithelial-mesenchymal transition-induced cellular plasticity and tumorigenicity. Stem Cells 2012;30:2088-99. [PMID: 22899304 DOI: 10.1002/stem.1183]
- Zhang Y, Wang S, Lai Q, Fang Y, Wu C, et al. Cancer-associated fibroblasts-derived exosomal miR-17-5p promotes colorectal cancer aggressive phenotype by initiating a RUNX3/MYC/TGF-β1 positive feedback loop. Cancer Lett 2020;491:22-35. [PMID: 32730779 DOI: 10.1016/j.canlet.2020.07.023]
- Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science 2002;296:1644-6. [PMID: 12040179 DOI: 10.1126/science.1071549]
- Ju X, Ishikawa TO, Naka K, Ito K, Ito Y, et al. Context-dependent activation of Wnt signaling by tumor suppressor RUNX3 in gastric cancer cells. Cancer Sci 2014;105:418-24. [PMID: 24447505 DOI: 10.1111/cas.12356]
- Sun J, Li B, Jia Z, Zhang A, Wang G, et al. RUNX3 inhibits glioma survival and invasion via suppression of the β-catenin/TCF-4 signaling pathway. J Neurooncol 2018;140:15-26. [PMID: 29916101 DOI: 10.1007/s11060-018-2927-0]
- Lee CWL, Ito K, Ito Y. Role of RUNX3 in bone morphogenetic protein signaling in colorectal cancer. Cancer Res 2010;70:4243-52. [PMID: 20442291 DOI: 10.1158/0008-5472.CAN-09-3805]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 2007;21:2747-61. [PMID: 17974916 DOI: 10.1101/gad.1602907]
- Qiao Y, Lin SJ, Chen Y, Voon DC, Zhu F, et al. RUNX3 is a novel negative regulator of oncogenic TEAD-YAP complex in gastric cancer. Oncogene 2016;35:2664-74. [PMID: 26364597 DOI: 10.1038/onc.2015.338]
- Jang JW, Kim MK, Lee YS, Lee JW, Kim DM, et al. RAC-LATS1/2 signaling regulates YAP activity by switching between the YAP-binding partners TEAD4 and RUNX3. Oncogene 2017;36:999-1011. [PMID: 27425596 DOI: 10.1038/onc.2016.266]
- Jiang C-G, Lv L, Liu F-R, Wang ZN, Liu FN, et al. Downregulation of connective tissue growth factor inhibits the growth and invasion of gastric cancer cells and attenuates peritoneal dissemination. Mol Cancer 2011;10:122. [PMID: 21955589 DOI: 10.1186/1476-4598-10-122]
- Lai D, Ho KC, Hao Y, Yang X. Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res 2011;71:2728-38. [PMID: 21349946 DOI: 10.1158/0008-5472.CAN-10-2711]
- Lin MT, Zuon CY, Chang CC, Chen ST, Chen CP, et al. Cyr61 induces gastric cancer cell motility/invasion via activation of the integrin/nuclear factor-kappaB/cyclooxygenase-2 signaling pathway. Clin Cancer Res 2005;11:5809-20. [PMID: 16115920 DOI: 10.1158/1078-0432.CCR-04-2639]
- Ogawa S, Satake M, Ikuta K. Physical and functional interactions between STAT5 and Runx transcription factors. J Biochem 2008;143:695-709. [PMID: 18296717 DOI: 10.1093/jb/mvn022]
- Li S, Cui HZ, Xu CM, Sun ZW, Tang ZK, et al. RUNX3 protects against acute lung injury by inhibiting the JAK2/STAT3 pathway in rats with severe acute pancreatitis. Eur Rev Med Pharmacol Sci 2019;23:5382-91. [PMID: 31298391 DOI: 10.26355/eurrev_201906_18207]
- Chen Y, Han L, Bai L, Tang H, Zheng A. Trichosanthin inhibits the proliferation of cervical cancer cells and downregulates STAT-5/C-myc signaling pathway. Pathol Res Pract 2019;215:632-8. [PMID: 30567634 DOI: 10.1016/j.prp.2018.12.010]
- Matikainen S, Sareneva T, Ronni T, Lehtonen A, Koskinen PJ, et al. Interferon-alpha activates multiple STAT proteins and upregulates proliferation-associated IL-2Ralpha, c-myc, and pim-1 genes in human T cells. Blood 1999;93:1980-91. [PMID: 10068671]
- Subramaniam KS, Omar IS, Kwong SC, Mohamed Z, Woo YL, et al. Cancer-associated fibroblasts promote endometrial cancer growth via activation of interleukin-6/STAT-3/c-Myc pathway. Am J Cancer Res 2016;6:200-13. [PMID: 27186396]
- Vier J, Groth M, Sochalska M, Kirschnek S. The anti-apoptotic Bcl-2 family protein A1/Bfl-1 regulates neutrophil survival and homeostasis and is controlled via PI3K and JAK/STAT signaling. Cell Death Dis 2016;7:e2103. [PMID: 26890142 DOI: 10.1038/cddis.2016.23]
- Scheeren FA, Naspetti M, Diehl S, Schotte R, Nagasawa M, et al. STAT5 regulates the self-renewal capacity and differentiation of human memory B cells and controls Bcl-6 expression. Nat Immunol 2005;6:303-13. [PMID: 15711548 DOI: 10.1038/ni1172]
- Wingelhofer B, Neubauer HA, Valent P, Han X, Constantinescu SN, et al. Implications of STAT3 and STAT5 signaling on gene regulation and chromatin remodeling in hematopoietic cancer. Leukemia 2018;32:1713-26. [PMID: 29728695 DOI: 10.1038/s41375-018-0117-x]
- Lin FC, Liu YP, Lai CH, Shan YS, Cheng HC, et al. RUNX3-mediated transcriptional inhibition of Akt suppresses tumorigenesis of human gastric cancer cells. Oncogene 2012;31:4302-16. [PMID: 22231444 DOI: 10.1038/onc.2011.596]
- Krishnan V, Chong YL, Tan TZ, Kulkarni M, Bin Rahmat MB, et al. TGFβ Promotes genomic instability after loss of RUNX3. Cancer Res 2018;78:88-102. [PMID: 29074538 DOI: 10.1158/0008-5472.CAN-17-1178]
- Klein Douwel D, Boonen RA, Long DT, Szypowska AA, Räschle M, et al. XPF-ERCC1 acts in Unhooking DNA interstrand crosslinks in cooperation with FANCD2 and FANCP/SLX4. Mol Cell 2014;54:460-71. [PMID: 24726325 DOI: 10.1016/j.molcel.2014.03.015]
- Zhang J, Dewar JM, Budzowska M, Motnenko A, Cohn MA, et al. DNA interstrand cross-link repair requires replication-fork convergence. Nat Struct Mol Biol 2015;22:242-7. [PMID: 25643322 DOI: 10.1038/nsmb.2956]
- Tay LS, Krishnan V, Sankar H, Chong YL, Chuang LSH, et al. RUNX Poly(ADP-Ribosyl)ation and BLM Interaction Facilitate the Fanconi Anemia Pathway of DNA Repair. Cell Rep 2018;24:1747-55. [PMID: 30110632 DOI: 10.1016/j.celrep.2018.07.038]
- Yamada C, Ozaki T, Ando K, Suenaga Y, Inoue K, et al. RUNX3 modulates DNA damage-mediated phosphorylation of tumor suppressor p53 at Ser-15 and acts as a co-activator for p53. J Biol Chem 2010;285:16693-703. [PMID: 20353948 DOI: 10.1074/jbc.M109.055525]
- Chi XZ, Kim J, Lee YH, Lee JW, Lee KS, et al. Runt-related transcription factor RUNX3 is a target of MDM2-mediated ubiquitination. Cancer Res 2009;69:8111-9. [PMID: 19808967 DOI: 10.1158/0008-5472.CAN-09-1057]
- Lee Y-S, Lee J-Y, Song S-H, Kim DM, Lee JW, et al. K-Ras–activated cells can develop into lung tumors when Runx3-mediated tumor suppressor pathways are abrogated. Mol Cells 2020;43:889-97. [PMID: 33115981 DOI: 10.14348/molcells.2020.0182]
- Selvarajan V, Osato M, Nah GSS, Yan J, Chung TH, et al. RUNX3 is oncogenic in natural killer/T-cell lymphoma and is transcriptionally regulated by MYC. Leukemia 2017;31:2219-27. [PMID: 28119527 DOI: 10.1038/leu.2017.40]
- Sachdeva M, Zhu S, Wu F, Wu H, Walia V, et al. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc Natl Acad Sci U S A 2009;106:3207-12. [PMID: 19202062 DOI: 10.1073/pnas.0808042106]
- Porter JR, Fisher BE, Baranello L, Liu JC, Kambach DM, et al. Global inhibition with specific activation: how p53 and MYC redistribute the transcriptome in the DNA double-strand break response. Mol Cell 2017;67:1013-25.e9. [PMID: 28867293 DOI: 10.1016/j.molcel.2017.07.028]
- Ozaki T, Nakagawara A, Nagase H. RUNX family participates in the regulation of p53-dependent DNA damage response. Int J Genomics 2013;2013:271347. [PMID: 24078903 DOI: 10.1155/2013/271347]
- Chi X-Z, Lee J-W, Lee Y-S, Park IY, Ito Y, et al. Runx3 plays a critical role in restriction-point and defense against cellular transformation. Oncogene 2017;36:6884-94. [PMID: 28846108 DOI: 10.1038/onc.2017.290]
- Lee Y-S, Lee J-W, Jang J-W, Chi XZ, Kim JH, et al. Runx3 inactivation is a crucial early event in the development of lung adenocarcinoma. Cancer Cell 2013;24:603-16. [PMID: 24229708 DOI: 10.1016/j.ccr.2013.10.003]
- Ho JSL, Ma W, Mao DYL, Benchimol S. p53-Dependent transcriptional repression of c-myc is required for G1 cell cycle arrest. Mol Cell Biol 2005;25:7423-31. [PMID: 16107691 DOI: 10.1128/MCB.25.17.7423-7431.2005]
- Whittle MC, Hingorani SR. Runx3 and cell fate decisions in pancreas cancer. Adv Exp Med Biol 2017;962:333-52. [PMID: 28299667 DOI: 10.1007/978-981-10-3233-2_21]
- Whittle MC, Izeradjene K, Rani PG, Feng L, Carlson MA, et al. RUNX3 controls a metastatic switch in pancreatic ductal adenocarcinoma. Cell 2015;161:1345-60. [PMID: 26004068 DOI: 10.1016/j.cell.2015.04.048]
- 20 years studying p53 functions in genetically engineered mice – Search Results [Internet]. PubMed. [cited 31 January 2023]. Available from: https://pubmed.ncbi.nlm.nih.gov/?term=20+years+studying+p53+functions+in+genetically+engineered+mice.
- Otani S, Date Y, Ueno T, Ito T, Kajikawa S, et al. Runx3 is required for oncogenic Myc upregulation in p53-deficient osteosarcoma. Oncogene 2022;41:683-91. [PMID: 34803166 DOI: 10.1038/s41388-021-02120-w]
- Goh Y-M, Cinghu S, Hong ETH, Lee YS, Kim JH, et al. Src kinase phosphorylates RUNX3 at tyrosine residues and localizes the protein in the cytoplasm. J Biol Chem 2010;285:10122-9. [PMID: 20100835 DOI: 10.1074/jbc.M109.071381]
- Yano T, Ito K, Fukamachi H, Chi XZ, Wee HJ, et al. The RUNX3 tumor suppressor upregulates Bim in gastric epithelial cells undergoing transforming growth factor beta-induced apoptosis. Mol Cell Biol 2006;26:4474-88. [PMID: 16738314 DOI: 10.1128/MCB.01926-05]
- Lau QC, Raja E, Salto-Terez M, Liu Q, Ito K, et al. RUNX3 is frequently inactivated by dual mechanisms of protein mislocalization and promoter hypermethylation in breast cancer. Cancer Res 2006;66:6512-20. [PMID: 16818622 DOI: 10.1158/0008-5472.CAN-06-0369]
- Kawakami Y, Miyamoto K, Takehara K, Kumagai M, Samura O, et al. Downregulation of RUNX3 by protein mislocation and gene inactivation in human epithelial ovarian cancer cells. J Clin Oncol 2008;26:16545. [DOI: 10.1200/jco.2008.26.15_suppl.16545]
- Gao F, Huang C, Lin M, Wang Z, Shen J, et al. Frequent inactivation of RUNX3 by promoter hypermethylation and protein mislocalization in oral squamous cell carcinomas. J Cancer Res Clin Oncol 2009;135:739-47. [PMID: 19015875 DOI: 10.1007/s00432-008-0508-x]
- Kang KA, Piao MJ, Ryu YS, Maeng YH, Hyun JW. Cytoplasmic localization of RUNX3 via histone deacetylase-mediated SRC expression in oxidative-stressed colon cancer cells. J Cell Physiol 2017;232:1914-21. [PMID: 27990641 DOI: 10.1002/jcp.25746]
- Chen X, Deng Y, Shi Y, Zhu W, Cai Y, et al. Loss of expression rather than cytoplasmic mislocalization of RUNX3 predicts worse outcome in non-small cell lung cancer. Oncol Lett 2018;15:5043-55. [PMID: 29545901 DOI: 10.3892/ol.2018.7993]
- Kanno T, Takahashi T, Tsujisawa T, Ariyoshi W, Nishihara T. Mechanical stress-mediated Runx2 activation is dependent on Ras/ERK1/2 MAPK signaling in osteoblasts. J Cell Biochem 2007;101:1266-77. [PMID: 17265428 DOI: 10.1002/jcb.21249]
- Harrington KS, Javed A, Drissi H, McNeil S, Lian JB, et al. Transcription factors RUNX1/AML1 and RUNX2/Cbfa1 dynamically associate with stationary subnuclear domains. J Cell Sci 2002;115:4167-76. [PMID: 12356919 DOI: 10.1242/jcs.00095]
- Zeng C, van Wijnen AJ, Stein JL, Meyers S, Sun W, et al. Identification of a nuclear matrix targeting signal in the leukemia and bone-related AML/CBF-α transcription factors. Proc Natl Acad Sci U S A 1997;94:6746-51. [PMID: 9192636 DOI: 10.1073/pnas.94.13.6746]
- Tang L, Guo B, Javed A, Choi JY, Hiebert S, et al. Crystal structure of the nuclear matrix targeting signal of the transcription factor acute myelogenous leukemia-1/polyoma enhancer-binding protein 2αB/core binding factor α2*. J Biol Chem 1999;274:33580-6. [PMID: 10559245 DOI: 10.1074/jbc.274.47.33580]
- Wang CQ, Krishnan V, Tay LS, Chin DW, Koh CP, et al. Disruption of Runx1 and Runx3 leads to bone marrow failure and leukemia predisposition due to transcriptional and DNA repair defects. Cell Rep 2014;8:767-82. [PMID: 25066130 DOI: 10.1016/j.celrep.2014.06.046]
- Wu D, Ozaki T, Yoshihara Y, Kubo N, Nakagawara A. Runt-related transcription factor 1 (RUNX1) stimulates tumor suppressor p53 protein in response to DNA damage through complex formation and acetylation. J Biol Chem 2013;288:1353-64. [PMID: 23148227 DOI: 10.1074/jbc.M112.402594]
- Cinghu S, Goh Y-M, Oh B-C, Lee YS, Lee OJ, et al. Phosphorylation of the gastric tumor suppressor RUNX3 following H. pylori infection results in its localization to the cytoplasm. J Cell Physiol 2012;227:1071-80. [PMID: 21567391 DOI: 10.1002/jcp.22820]
- Kanumuri R, Chelluboyina AK, Biswal J, Vignesh R, Pandian J, et al. Small peptide inhibitor from the sequence of RUNX3 disrupts PAK1-RUNX3 interaction and abrogates its phosphorylation-dependent oncogenic function. Oncogene 2021;40:5327-41. [PMID: 34253860 DOI: 10.1038/s41388-021-01927-x]
- Kumar A, Singhal M, Chopra C, Srinivasan S, Surabhi RP, et al. Threonine 209 phosphorylation on RUNX3 by Pak1 is a molecular switch for its dualistic functions. Oncogene 2016;35:4857-65. [PMID: 26898755 DOI: 10.1038/onc.2016.18]
- Kim HR, Oh BC, Choi JK, Bae SC. Pim-1 kinase phosphorylates and stabilizes RUNX3 and alters its subcellular localization. J Cell Biochem 2008;105:1048-58. [PMID: 18767071 DOI: 10.1002/jcb.21906]
- Liu H, Chen C, Ma D, Li Y, Yin Q, et al. Inhibition of PIM1 attenuates the stem cell-like traits of breast cancer cells by promoting RUNX3 nuclear retention. J Cell Mol Med 2020;24:6308-23. [PMID: 32307917 DOI: 10.1111/jcmm.15272]
- Fornerod M, Ohno M, Yoshida M, Mattaj IW. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 1997;90:1051-60. [PMID: 9323133 DOI: 10.1016/s0092-8674(00)80371-2]
- Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, et al. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature 1997;390:308-11. [PMID: 9384386 DOI: 10.1038/36894]
- Chuang LS, Khor JM, Lai SK, Garg S, Krishnan V, et al. Aurora kinase-induced phosphorylation excludes transcription factor RUNX from the chromatin to facilitate proper mitotic progression. Proc Natl Acad Sci U S A 2016;113:6490-5. [PMID: 27217562 DOI: 10.1073/pnas.1523157113]
- Aumann K, Frey A-V, May AM, Hauschke D, Kreutz C, et al. Subcellular mislocalization of the transcription factor NF-E2 in erythroid cells discriminates prefibrotic primary myelofibrosis from essential thrombocythemia. Blood 2013;122:93-9. [PMID: 23670178 DOI: 10.1182/blood-2012-11-463257]
- Kim J-H, Choi J-K, Cinghu S, Jang JW, Lee YS, et al. Jab1/CSN5 induces the cytoplasmic localization and degradation of RUNX3. J Cell Biochem 2009;107:557-65. [PMID: 19350572 DOI: 10.1002/jcb.22157]
- Lim J, Duong T, Do N, Do P, Kim J, et al. Antitumor activity of cell-permeable RUNX3 protein in gastric cancer cells. Clin Cancer Res 2013;19:680-90. [PMID: 23230322 DOI: 10.1158/1078-0432.CCR-12-2692]