Current Applications of Organ-on-a-Chip: A Step Closer to Personalized Medicine
1Department of Biomedical Engineering, McMaster University, Hamilton, Ont., Canada
2Current affiliation: Arranta Bio, Watertown, MA, USA
*Correspondence to: Amanda Victorious, E-mail: victoriousamanda@gmail.com
Cite this paper:
Amanda Victorious. Current Applications of Organ-on-a-Chip: A Step Closer to Personalized Medicine. BIO Integration 2022; 3(4): 143–150.
DOI: 10.15212/bioi-2022-0027. Available at: https://bio-integration.org/
Download citation
© 2022 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
In the pharmaceutical industry, a critical need exists for effective drug development approaches that better account for factors imposed by the physiological microenvironment. Organ-on-a-chip (OOAC)—a revolutionary technology that simulates human organs’ physiological milieu and performance on a chip—has applications in curing illnesses and drug screening, and enormous potential to transform the drug discovery workflow. However, the effective integration of this unique engineering system into ordinary pharmacological and medical contexts remains in development. This Editorial summarizes current research on OOAC systems, and offers insight into future development prospects and the need for a next-generation OOAC framework.
Keywords
Clinical translation, drug discovery, drug screening, organ-on-a-chip.
Introduction
Patients with cancer show substantial inter-patient variability arising from differences in therapeutic by-products, prognosis, reactivity, or tolerance to treatments [1]. Consequently, predictive preclinical studies capable of identifying optimal therapy regimens for particular patients are increasingly in demand. This tailored strategy has the potential to augment the positive response to therapy while decreasing the frequency of adverse effects [2, 3]. The responsiveness to chemotherapy regimens is determined by the molecular subgroups of the tumors, the stage of cancer, the presence of comorbidities, genetic history, and patient susceptibility to treatments, which vary greatly among individuals. One successful method for developing tailored therapy involves duplicating illness in laboratory settings by using three-dimensional (3D) cellular models associated with patient-derived tumors after debulking surgery, and subsequently evaluating alternative treatment options for the unique cancer phenotype. Although this method can be used to assess treatment efficacy, it may fail to indicate unfavorable and off-target effects. The precision medicine paradigm does not rely on a “one-size-fits-all” model but instead uses unique treatment strategies for each individual; this paradigm cannot always be applied in traditional and generic in vivo and in vitro conditions. The development of tailored chemotherapeutic treatment programs is facilitated by advances in precision medicine through cell-based tumor organoids and xenografts derived from plants [4]. Research advances have promoted the use of bioprinting for antitumor drug screening through manufacturing of physiologically relevant 3D cancer or tumor models, thereby offering a platform presenting more stringently controlled environments and physiologically suitable models [5]. Herein, recent advances in organs-on-a-chip for various major organs are summarized, and their applicability in many diagnostic and research areas in the field of oncology is discussed.
Currently available organ-on-a-chip systems: an overview
Liver
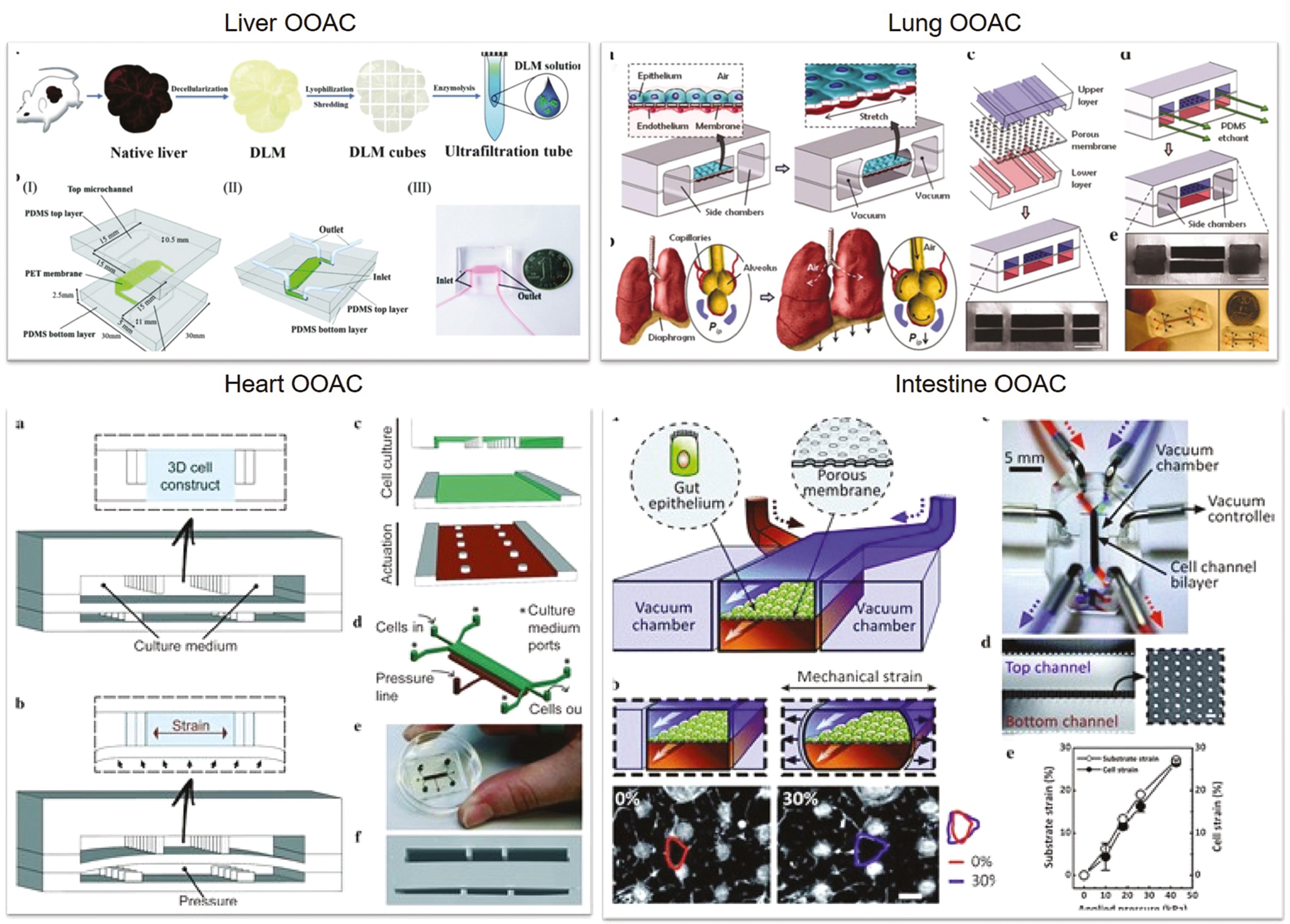
The hepatic organs are the primary loci of drug/toxin metabolism. The liver is made up of several complicated hepatic lobules that allow for multicellular functional interaction [6]. Hepatocyte physiology cannot easily be sustained for long durations [7]. Kane et al. devised the first liver-based model by using microfluidic perforations that constituted co-cultured 3T3-J2 fibroblasts and rat liver cells, which imitated an airway interface [8]. Rat hepatocytes cultivated on the chip were able to produce albumin and engage in metabolic activity indefinitely. Lee et al. [9] have designed a chip replicating the interstitial morphology of endothelial cells and cultivated primary hepatocytes, wherein culture medium infused extrinsic to the gap. Electrophoretically generated radial electric field gradients have been used by Ho et al. [10] to position cells on round polydimethylsiloxane (PDMS) chips, thus conferring the biomimic with programmable and directed cell localization capabilities. These innovative approaches replicate the anatomy of the hepatic lobule. Hegde et al. [11] have demonstrated an approach wherein a double-layered chip with a perforated polyethylene terephthalate layer splits the channels from continuously perfused collagen and fibronectin-polished rat primary hepatocytes in the bottom channel via the top chamber. Ma et al. [12] have created a biomimetic platform for in situ circulation of hepatic spheroids. Riahi et al. [13] have developed microfluidic electrochemical chip immunosensors to identify hepatotoxicity-derived biomarkers. Chong et al. [14] have developed tests to track drug surface sensitization by measuring metabolite synthesis and antigen-presenting-cell stimulation. This technology may potentially be used as a drug testing platform to identify chemicals that cause systemic skin hypersensitivity. Lu et al. [15] have used the integration of decellularized liver matrixes and gelatin methacryloyl to develop biomimetic liver tumors mimicking the 3D tumor milieu. This technology provides a disease model with enhanced precision for future anti-cancer pharmaceutical investigations (Figure 1).
Figure 1 Different methodologies in creating organ-on-a-chip in various organs (liver, lung, heart and intestine). All figures are reproduced with permission.
Lung
The alveoli govern gaseous exchange in the lungs. Cellular constructs are often challenging to replicate in vivo. Through precise liquid flow and continuous gaseous exchange, microfluidics may generate extracorporeal lung models of lung diseases. Huh et al. have used soft lithography to create a lung-on-a-chip model [16] by dividing the chip into zones split by 10 m PDMS sheaths containing an extracellular matrix. The upper PDMS sections contained alveolar epithelial cells, whereas the bottom areas contained human pulmonary microvascular endothelial cells, thereby replicating the alveolar-capillary obstacle. The membrane architectures were modulated under vacuum conditions to replicate the expansion and contraction of the alveoli during respiration [17]. In 2015, Stucki et al. [18] described a lung chip resembling the lung parenchyma. The model incorporated an alveolar shield and 3D cyclic strain to replicate respiration, and thus was the first-ever elastic membrane expansion model. Blume et al. [19] have created 3D airway culture prototypes that replicate pulmonary interstitial flow by initiating the inter-transmission of fluid and media. These prototypes have enabled detailed physiological examination of the epithelial layer. The device uses a porous filter as a chamber of single tissue culture; numerous chambers can be combined for enhanced integration. Peng et al. [20] have created lung assist devices to allow for increased gaseous exchange in the placenta in the event of respiratory arrest in preterm neonates. Large-diameter channels are created in the umbilical arteries and veins, thus providing substantial extracorporeal blood supply to the lung assist devices. Dabaghi et al. [21] have used dual-sided gas delivery to microfabricate microfluidic blood oxygenators for enhanced gaseous exchange, which have demonstrated 343% greater oxygen consumption than that in single-sided systems [22] (Figure 1).
Heart
Because the myocardium is a crucial part of the heart, cardiomyocyte (CM) pulsing can be used for direct monitoring of medication responses [23]. Grosberg et al. created an elastic PDMS membrane with surface roughness in 2012 and transplanted neonatal rat CMs onto the membrane to construct muscle membranes [24]. Later, in 2013, Zhang et al. used hydrogels to create self-assembled cardiac sheets in a PDMS model [25]. The CMs were created by separating distinctive myocardium. The technique was used to create micro-organ tissue chips enabling the coupling of cardiac and vascular systems [26]. The vascular endothelial cells in this model were used to build vascular networks in combination with CMs to bridge the vascular network void. Zhang et al. have introduced a heart-on-a-chip device using high-speed impedance detection to assess cardiac drug efficacy [27]. The device records the contraction of CMs to reveal drug effects. The chip enables preliminary evaluation of a drug’s cardiac effectiveness. A cardiac organ platform replicating the physiological and mechanical conditions of CMs has also been created [28]. This platform enables direct visualization and statistical analysis, which are not possible with conventional cell culture or animal models. This platform heralded a breakthrough in the field by providing standard functioning 3D heart replicas. Schneider created simple systematic chips based on human-derived pluripotent stem cells to grow cardiac tissue in a confined space [29] (Figure 1).
Intestine
To model the intestinal tract, Imura et al. [30] have created chips comprising a membrane with glass slide permeability and a PDMS sheet with channels to grow Caco-2 cells. Sung et al. have created the first 3D hydrogel construct imitating human intestinal villi [31], and Kim et al. have developed bionic equipment [32]. The intestine’s milieu was recapitulated by using shear force and cyclic stresses. Caco-2 cells have been shown to exhibit sustained proliferation and to aid in retention of the microbial flora in the human gut. The intestine’s complicated composition and function provide a platform for drug testing as well as research on the involvement of the intestinal microbiota, inflammatory cells, and peristalsis-based mechanical distortion during intestinal illness [33]. Intestinal cells may be cultured alone or with endothelial cells, including HUVECs [32]. Because of low genome fidelity, the chips imitate functions of the intestine. Through integration of intestinal tissue engineering [34] and organ-on-a-chip technology, Kasendra et al. [35] have created in vitro biological replicas of the human duodenum. Endoscopic biopsies or organ surgeries were used to grow intestinal epithelial cells in the chip. This chip provides the closest available approximation to a live duodenum and replicates essential aspects of the small intestine. Recent discoveries using this chip have expanded understanding of the gut microbiota [36] and intestinal structure [37] (Figure 1).
Kidneys
Jang et al. [38] have developed the first multi-layered microfluidic device using mouse kidney medullary collection duct cells to model renal filtration. The system provides a biomimetic setting that increases the polarity of the inner medullary collection duct in response to hormone activation, by encouraging cytoskeletal remodeling and molecular transport. In 2013, researchers used a similar microfluidic technique to cultivate human primary renal epithelial cells [39]. Musah et al. [40] have developed techniques for creating human glomerular chips with organ culture equipment, by using podocytes derived from pluripotent stem cells. These methods have been used to duplicate the structural and functional attributes of the glomerular capillary membrane—a feat previously deemed infeasible through existing approaches. Sakolish et al. [41] have devised a microfluidic device in human proximal tubules and glomeruli with an integrated reusability feature allowing renal epithelial cells to proliferate under diverse conditions. Shear tension is known to often lead to nephrotoxicity. Schutgens et al. [42] have created robust tubule culture techniques enabling extensive extension and examination of human kidney tissue. A multi-functional primary renal epithelial cell culture replica built with this system has enabled rapid personalized molecular and cellular investigation, disease modeling, and drug testing. Tao et al. have demonstrated a favorable approach for producing human islet organoids from pluripotent stem cells derived from humans [43].
Current applications of tumor modeling in oncology
Tumor models for drug screening
Monolayer cell models, which are the typical framework for numerous drug tests, cannot adequately reflect the intricacies of complicated 3D organs. Consequently, organ technology is frequently used in the identification of tumor drugs. In past years, scientists have created several tumor-on-a-chip prototypes for medication testing [44–46]. Continued optimization of the model has enhanced its ability to screen drugs with better efficiency and low toxicity. For example, a microphysiological system is being developed to overcome the complexities of in vivo physiology. Gervais et al. have incubated eight distinct types of micro-dissected tissues in a low-shear tensile environment by using a microfluidic technology that can easily and reliably capture samples. This platform has been used to examine micro-dissected tissue viability through confocal microscopy and flow cytometry, thereby yielding data on chemosensitivity tests and therapeutic response [45]. Phan et al. have created arrayed vascularized micro tumors and used them for blind-hole screening [46]. Anti-cancer medications can be successfully discovered through the evaluation of tiny chemical libraries, including FDA-approved compounds. This 3D platform is appropriate for assessing the effectiveness/toxicity of various tissues under more complicated settings than physiological surroundings. Polymethyl methacrylate (PMMA) based organ chips provide more reliable cytotoxicity findings than conventional PDMS chips, thus aiding in research interventions in drug testing. Nguyen et al. have used trimethoxysilane to join PMMA polyethylene terephthalate orbital etching film, which can be used in microfluidic systems that do not allow the passage of small molecules and can facilitate reliable cytotoxicity assessments, such as those for antitumor medicines [47]. The adhesion force between substrates is adequate for culture interchange; even at gauge pressures exceeding 135 kPa, the fluid may still flow through the system without leakage. PMMA organ chips have been shown to provide more reliable cytotoxicity findings for vincristine when the chips are used with human lung adenocarcinoma cells. This technology expands basic membrane production capabilities, produces a 3D microstructure physiological environment, and more precisely replicates organ levels by using a range of thermoplastics and permeable orbital etching. The multiorgan-chip device may be used to estimate preliminary target effectiveness, metabolic conversion rates, and target off-target toxicity in addition to drug testing [48]. Hickman et al. have used a pumpless four-organ system (liver, heart, nerve, and muscle) to assess human responsiveness to five medications over a 14-day period. The system is operated in a serum-free specified medium under uninterrupted fluid flow. This method offers a unique approach for improving preclinical efficacy/toxicity studies’ anticipatory potential [49]. Hickman’s group subsequently discovered that antileukemia medications can be studied in a chip system via co-culture of primary human hepatocytes and human bone marrow cell cultures from two forms of malignancy [50]. Imatinib and diclofenac have cytostatic effects on human bone marrow. Anti-imatinib has no effect on liver vitality; however, diclofenac diminishes liver vitality by 30%. Multiple medications have been tested in organ models of multidrug-resistant vulvar cancer strains in comparison to non-multidrug-tolerant breast cancer cells, native liver cells, and cardiomyocytes derived from pluripotent stem cells. Tamoxifen hinders the breast cancer cell activity only after its metabolites are generated, and it does not affect vulvar tumor cells. The combination of tamoxifen and verapamil has nontargeted cardiac consequences, such as decreased contractility, lower beating frequency, and decreased conduction speed, without affecting survival. These models have demonstrated that cell-based in vitro culture methods may be used to assess the target effectiveness and safety of parent medications and their metabolites, and increase drug assessment performance in preclinical investigations. The organ-chip model technique greatly decreases research and development expenditures between preclinical experiments and human trials by developing a better predictive model [51, 52].
Modeling cancer invasion and metastasis
Tumor proliferation, a key challenge currently limiting modern clinical anti-cancer therapy, is responsible for more than 90% of cancer-associated fatalities [53, 54]. Maximum tumor-on-a-chip models currently replicate only tumors in situ; hence, tumor cell metastasis remains unknown, particularly the causes of the initial activation of tumor cell tumorigenesis and metastasis (such as specialized signaling pathways) and the contribution of the microenvironment to controlling this phenomenon [52]. Consequently, using empirical methods is critical to effectively define the metastatic microenvironment [55, 56]. Skardal’s team has demonstrated the effectiveness of a two-organoid metastasis-on-a-chip platform [57]. When microfluidics is used to generate circulatory flow across the organoid system, tumor cells proliferate in the main focus and infiltrate the bloodstream, thus causing colorectal cancer (CRC) cells from the colon organoids to disperse into the circulation and deposit on the downstream liver organoids. This simulation was among the first in vitro models to mimic cancer cell metastasis, recapitulating metastasis from a 3D parent tissue in a 3D target tissue. The same researchers have improved the tumor metastasis system by incorporating new capabilities, such as increasing the downstream organoids from one to four locations. The authors have created a multicenter metastasis-on-a-chip system to evaluate cancer cells’ metastatic tendencies [56]. Researchers have created chips with several 3D organoids by using 3D photopatterning technologies. Cancer cells begin as CRC organoids in a single microfluidic chamber coupled with various downstream cavities containing liver, lung, and endothelial components. Under the continuous fluid flow in this model, fluorescence imaging tracking has revealed that HCT-116 CRC cells predominantly penetrate liver and lung tissues, which correspond to the organs with the highest CRC metastasis in humans. The platform has the potential to aid in the determination of intervention targets through better interpretation of metastasis [58]. The model is made up of four organs: one upstream lung and three downstream parallel organs comprising the brain, bone, and liver; consequently, it replicates the spread of lung cancer to the brain, bone, and liver.
Conclusion and future outlook
Over the past two decades, the initial design of organ-on-a-chip systems has undergone major changes with developments in fabrication technology, and their immense potential as a novel tool for drug design and development has been demonstrated. New organ-on-a-chip systems with major advances in functional performance, interfacing, automation, production, and customized precision therapy are beginning to emerge to address the increasing demand for improved preclinical studies for drug discovery. Future organ-on-a-chip platforms will be built on patient-induced materials including patient tissue, decellularized extracellular matrix, and other biological agents for individualized precision medicine, wherein patient sampling and stratification bioindicators will be crucial elements for drug discovery success. Recent research has revealed that patient-derived pluripotent stem cells (iPSCs), such as those obtained from skin fibroblasts, may provide a limitless option for manufacturing autologous target organs or tissues, thus enabling the development of patient-specific organ chips for customized disease modeling and drug testing. Moreover, the inclusion of iPSC-based organoids in organ-on-a-chip systems has led to the creation of organoids-on-a-chip, a potent hybrid tool comprising an ex vivo organotypic microtissue generated by self-structuring and segregation of stem cells in a 3D matrix. Efforts in developing patient iPSC-derived organ chips are expected to overcome the limitations of traditional “one-size-fits-all” therapeutics, thus offering an ideal remedy for individual patients within populations with the same disease.
References
- Al-Lamki RS, Bradley JR, Pober JS. Human organ culture: updating the approach to bridge the gap from in vitro to in vivo in inflammation, cancer, and stem cell biology. Front Med (Lausanne) 2017;4:148. [PMID: 28955710 DOI: 10.3389/fmed.2017.00148]
- Lee SH, Jun BH. Advances in dynamic microphysiological organ-on-a-chip: design principle and its biomedical application. J Ind Eng Chem 2019;71:65-77. [DOI: 10.1016/j.jiec.2018.11.041]
- Alépée N, Bahinski A, Daneshian M, De Wever B, Fritsche E, et al. State-of-the-art of 3D cultures (organs-on-a-chip) in safety testing and pathophysiology. ALTEX 2014;31:441-77. [PMID: 25027500 DOI: 10.14573/altex.1406111]
- Reardon S. ‘Organs-on-chips’ go mainstream. Nature 2015;523:266. [PMID: 26178942 DOI: 10.1038/523266a]
- Young EW, Beebe DJ. Fundamentals of microfluidic cell culture in controlled microenvironments. Chem Soc Rev 2010;39:1036-48. [PMID: 20179823 DOI: 10.1039/b909900j]
- McCuskey RS. The hepatic microvascular system in health and its response to toxicants. Anat Rec (Hoboken) 2008;291:661-71. [PMID: 18484612 DOI: 10.1002/ar.20663]
- Cho CH, Park J, Tilles AW, Berthiaume F, Toner M, et al. Layered patterning of hepatocytes in co-culture systems using microfabricated stencils. Biotechniques 2010;48:47-52. [PMID: 20078427 DOI: 10.2144/000113317]
- Kane BJ, Zinner MJ, Yarmush ML, Toner M. Liver-specific functional studies in a microfluidic array of primary mammalian hepatocytes. Anal Chem 2006;78:4291-8. [PMID: 16808435 DOI: 10.1021/ac051856v]
- Lee PJ, Hung PJ, Lee LP. An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture. Biotechnol Bioeng 2007;97:1340-6. [PMID: 17286266 DOI: 10.1002/bit.21360]
- Chong K, Ku T, Saw PE, Jon S, Park J-H, et al. Enhancement of the photocytotoxic efficiency of sub-12 nm therapeutic polymeric micelles with increased co-localisation in mitochondria. Chem Commun. 2013;49:11476-8. [PMID: 24064984 DOI: 10.1039/c3cc46166a]
- Hegde M, Jindal R, Bhushan A, Bale SS, McCarty WJ, et al. Dynamic interplay of flow and collagen stabilizes primary hepatocytes culture in a microfluidic platform. Lab Chip 2014;14:2033-9. [PMID: 24770663 DOI: 10.1039/c4lc00071d]
- Ma LD, Wang YT, Wang JR, Wu JL, Meng XS, et al. Design and fabrication of a liver-on-a-chip platform for convenient, highly efficient, and safe in situ perfusion culture of 3D hepatic spheroids. Lab Chip 2018;18:2547-62. [PMID: 30019731 DOI: 10.1039/c8lc00333e]
- Riahi R, Shaegh SA, Ghaderi M, Zhang YS, Shin SR, et al. Automated microfluidic platform of bead-based electrochemical immunosensor integrated with bioreactor for continual monitoring of cell secreted biomarkers. Sci Rep 2016;6:24598. [PMID: 27098564 DOI: 10.1038/srep24598]
- Chong LH, Li H, Wetzel I, Cho H, Toh YC. A liver-immune coculture array for predicting systemic drug-induced skin sensitization. Lab Chip 2018;18:3239-50. [PMID: 30252012 DOI: 10.1039/c8lc00790j]
- Lu S, Cuzzucoli F, Jiang J, Liang LG, Wang Y, et al. Development of a biomimetic liver tumor-on-a-chip model based on decellularized liver matrix for toxicity testing. Lab Chip 2018;18:3379-92. [PMID: 30298144 DOI: 10.1039/c8lc00852c]
- Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, et al. Reconstituting organ-level lung functions on a chip. Science 2010;328:1662-8. [PMID: 20576885 DOI: 10.1126/science.1188302]
- Huh D, Leslie DC, Matthews BD, Fraser JP, Jurek S, et al. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med 2012;4:159ra147. [PMID: 23136042 DOI: 10.1126/scitranslmed.3004249]
- Stucki AO, Stucki JD, Hall SR, Felder M, Mermoud Y, et al. A lung-on-a-chip array with an integrated bio-inspired respiration mechanism. Lab Chip 2015;15:1302-10. [PMID: 25521475 DOI: 10.1039/c4lc01252f]
- Blume C, Reale R, Held M, Millar TM, Collins JE, et al. Temporal monitoring of differentiated human airway epithelial cells using microfluidics. PLoS One 2015;10:e0139872. [PMID: 26436734 DOI: 10.1371/journal.pone.0139872]
- Peng J, Rochow N, Dabaghi M, Bozanovic R, Jansen J, et al. Postnatal dilatation of umbilical cord vessels and its impact on wall integrity: prerequisite for the artificial placenta. Int J Artif Organs 2018;41:393-9. [PMID: 29562805 DOI: 10.1177/0391398818763663]
- Dabaghi M, Fusch G, Saraei N, Rochow N, Brash JL, et al. An artificial placenta type microfluidic blood oxygenator with double-sided gas transfer microchannels and its integration as a neonatal lung assist device. Biomicrofluidics 2018;12:044101. [PMID: 30867861 DOI: 10.1063/1.5034791]
- Xu Z, Gao Y, Hao Y, Li E, Wang Y, et al. Application of a microfluidic chip-based 3D co-culture to test drug sensitivity for individualized treatment of lung cancer. Biomaterials 2013;34:4109-17. [PMID: 23473962 DOI: 10.1016/j.biomaterials.2013.02.045]
- Visone R, Gilardi M, Marsano A, Rasponi M, Bersini S, et al. Cardiac meets skeletal: what’s new in microfluidic models for muscle tissue engineering. Molecules 2016;21:1128. [PMID: 27571058 DOI: 10.3390/molecules21091128]
- Grosberg A, Nesmith AP, Goss JA, Brigham MD, McCain ML, et al. Muscle on a chip: in vitro contractility assays for smooth and striated muscle. J Pharmacol Toxicol Methods 2012;65:126-35. [PMID: 22521339 DOI: 10.1016/j.vascn.2012.04.001]
- Zhang D, Shadrin IY, Lam J, Xian HQ, Snodgrass HR, et al. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials 2013;34:5813-20. [PMID: 23642535 DOI: 10.1016/j.biomaterials.2013.04.026]
- Zhang YS, Arneri A, Bersini S, Shin SR, Zhu K, et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 2016;110:45-59. [PMID: 27710832 DOI: 10.1016/j.biomaterials.2016.09.003]
- Zhang X, Wang T, Wang P, Hu N. High-throughput assessment of drug cardiac safety using a high-speed impedance detection technology-based heart-on-a-chip. Micromachines (Basel) 2016;7:122. [PMID: 30404295 DOI: 10.3390/mi7070122]
- Marsano A, Conficconi C, Lemme M, Occhetta P, Gaudiello E, et al. Beating heart on a chip: a novel microfluidic platform to generate functional 3D cardiac microtissues. Lab Chip 2016;16:599-610. [PMID: 26758922 DOI: 10.1039/c5lc01356a]
- Schneider O, Zeifang L, Fuchs S, Sailer C, Loskill P. User-friendly and parallelized generation of human induced pluripotent stem cell-derived microtissues in a centrifugal heart-on-a-chip. Tissue Eng Part A 2019;25:786-98. [PMID: 30968738 DOI: 10.1089/ten.tea.2019.0002]
- Imura Y, Asano Y, Sato K, Yoshimura E. A microfluidic system to evaluate intestinal absorption. Anal Sci 2009;25:1403-7. [PMID: 20009325 DOI: 10.2116/analsci.25.1403]
- Sung JH, Yu J, Luo D, Shuler ML, March JC. Microscale 3-D hydrogel scaffold for biomimetic gastrointestinal (GI) tract model. Lab Chip 2011;11:389-92. [PMID: 21157619 DOI: 10.1039/c0lc00273a]
- Kim HJ, Huh D, Hamilton G, Ingber DE. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 2012;12:2165-74. [PMID: 22434367 DOI: 10.1039/c2lc40074j]
- Kim HJ, Li H, Collins JJ, Ingber DE. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc Natl Acad Sci U S A 2016;113:E7-15. [PMID: 26668389 DOI: 10.1073/pnas.1522193112]
- VanDussen KL, Marinshaw JM, Shaikh N, Miyoshi H, Moon C, et al. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut 2015;64:911-20. [PMID: 25007816 DOI: 10.1136/gutjnl-2013-306651]
- Kasendra M, Tovaglieri A, Sontheimer-Phelps A, Jalili-Firoozinezhad S, Bein A, et al. Development of a primary human Small Intestine-on-a-Chip using biopsy-derived organoids. Sci Rep 2018;8:2871. [PMID: 29440725 DOI: 10.1038/s41598-018-21201-7]
- Jalili-Firoozinezhad S, Gazzaniga FS, Calamari EL, Camacho DM, Fadel CW, et al. A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat Biomed Eng 2019;3:520-31. [PMID: 31086325 DOI: 10.1038/s41551-019-0397-0]
- Shin W, Hinojosa CD, Ingber DE, Kim HJ. Human Intestinal Morphogenesis Controlled by Transepithelial Morphogen Gradient and Flow-Dependent Physical Cues in a Microengineered Gut-on-a-Chip. iScience 2019;15:391-406. [PMID: 31108394 DOI: 10.1016/j.isci.2019.04.037]
- Jang KJ, Suh KY. A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab Chip 2010;10:36-42. [PMID: 20024048 DOI: 10.1039/b907515a]
- Kyung-Jin J, Poyan MA, Hamilton GA, Mcpartlin LA, Seyoon C, et al. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr Biol 2013:1119-29. [PMID: 23644926 DOI: 10.1039/c3ib40049b]
- Musah S, Dimitrakakis N, Camacho DM, Church GM, Ingber DE. Directed differentiation of human induced pluripotent stem cells into mature kidney podocytes and establishment of a glomerulus chip. Nat Protoc 2018;13:1662-85. [PMID: 29995874 DOI: 10.1038/s41596-018-0007-8]
- Sakolish CM, Philip B, Mahler GJ. A human proximal tubule-on-a-chip to study renal disease and toxicity. Biomicrofluidics 2019;13:014107. [PMID: 30867877 DOI: 10.1063/1.5083138]
- Schutgens F, Rookmaaker MB, Margaritis T, Rios A, Ammerlaan C, et al. Tubuloids derived from human adult kidney and urine for personalized disease modeling. Nat Biotechnol 2019;37:303-13. [PMID: 30833775 DOI: 10.1038/s41587-019-0048-8]
- Nieskens TTG, Sjögren AK. Emerging in vitro systems to screen and predict drug-induced kidney toxicity. Semin Nephrol 2019;39:215-26. [PMID: 30827343 DOI: 10.1016/j.semnephrol.2018.12.009]
- Domansky K, Inman W, Serdy J, Dash A, Lim MH, et al. Perfused multiwell plate for 3D liver tissue engineering. Lab Chip 2010;10:51-8. [PMID: 20024050 DOI: 10.1039/b913221j]
- Astolfi M, Péant B, Lateef MA, Rousset N, Kendall-Dupont J, et al. Micro-dissected tumor tissues on chip: an ex vivo method for drug testing and personalized therapy. Lab Chip 2016;16:312-25. [PMID: 26659477 DOI: 10.1039/c5lc01108f]
- Phan DTT, Wang X, Craver BM, Sobrino A, Zhao D, et al. A vascularized and perfused organ-on-a-chip platform for large-scale drug screening applications. Lab Chip 2017;17:511-20. [DOI: 10.1039/C6LC01422D]
- Nguyen T, Jung SH, Lee MS, Park TE, Ahn SK, et al. Robust chemical bonding of PMMA microfluidic devices to porous PETE membranes for reliable cytotoxicity testing of drugs. Lab Chip 2019;19:3706-13. [DOI: 10.1039/C9LC00338J]
- Abaci HE, Shuler ML. Human-on-a-chip design strategies and principles for physiologically based pharmacokinetics/pharmacodynamics modeling. Integr Biol (Camb) 2015;7:383-91. [PMID: 25739725 DOI: 10.1039/c4ib00292j]
- Oleaga C, Bernabini C, Smith AS, Srinivasan B, Jackson M, et al. Multi-Organ toxicity demonstration in a functional human in vitro system composed of four organs. Sci Rep 2016;6:20030. [PMID: 26837601 DOI: 10.1038/srep20030]
- McAleer CW, Long CJ, Elbrecht D, Sasserath T, Bridges LR, et al. Multi-organ system for the evaluation of efficacy and off-target toxicity of anticancer therapeutics. Sci Transl Med 2019;11:eaav1386. [PMID: 31217335 DOI: 10.1126/scitranslmed.aav1386]
- Franzen N, van Harten WH, Retèl VP, Loskill P, van den Eijnden-van Raaij J, et al. Impact of organ-on-a-chip technology on pharmaceutical R&D costs. Drug Discov Today 2019;24:1720-4. [PMID: 31185290 DOI: 10.1016/j.drudis.2019.06.003]
- Liu X, Fang J, Huang S, Wu X, Xie X, et al. Tumor-on-a-chip: from bioinspired design to biomedical application. Microsyst Nanoeng 2021;7:50. [PMID: 34567763 DOI: 10.1038/s41378-021-00277-8]
- Li M, Tang Z, Lv S, Song W, Hong H, et al. Cisplatin crosslinked pH-sensitive nanoparticles for efficient delivery of doxorubicin. Biomaterials 2014;35:3851-64. [PMID: 24495487 DOI: 10.1016/j.biomaterials.2014.01.018]
- Lei Q, Qiu WX, Hu JJ, Cao PX, Zhu CH, et al. Multifunctional mesoporous silica nanoparticles with thermal-responsive gatekeeper for NIR light-triggered chemo/photothermal-therapy. Small 2016;12:4286-98. [PMID: 27376247 DOI: 10.1002/smll.201601137]
- Han S, Kim J, Li R, Ma A, Kwan V, et al. Hydrophobic patterning-based 3D microfluidic cell culture assay. Adv Healthc Mater 2018;7:e1800122. [PMID: 29700986 DOI: 10.1002/adhm.201800122]
- Aleman J, Skardal A. A multi-site metastasis-on-a-chip microphysiological system for assessing metastatic preference of cancer cells. Biotechnol Bioeng 2019;116:936-44. [PMID: 30450540 DOI: 10.1002/bit.26871]
- Skardal A, Devarasetty M, Forsythe S, Atala A, Soker S. A reductionist metastasis-on-a-chip platform for in vitro tumor progression modeling and drug screening. Biotechnol Bioeng 2016;113:2020-32. [PMID: 26888480 DOI: 10.1002/bit.25950]
- Xu Z, Li E, Guo Z, Yu R, Hao H, et al. Design and construction of a multi-organ microfluidic chip mimicking the in vivo microenvironment of lung cancer metastasis. ACS Appl Mater Interfaces 2016;8:25840-7. [PMID: 27606718 DOI: 10.1021/acsami.6b08746]