Classification and Medical Applications of Biomaterials–A Mini Review
1Biotechnology Research Institute, Universiti Malaysia Sabah, Jalan UMS, 88400 Kota Kinabalu, Sabah, Malaysia
2Faculty of Science and Natural Resources, Universiti Malaysia Sabah, Jalan UMS, 88400 Kota Kinabalu, Sabah, Malaysia
*Correspondence to: Ping-Chin Lee, E-mail: leepc@ums.edu.my
Received: February 28 2022; Revised: March 18 2022; Accepted: April 1 2022; Published Online: April 20 2022
Cite this paper:
Eric Tzyy Jiann Chong, Jun Wei Ng and Ping-Chin Lee. Classification and Medical Applications of Biomaterials–A Mini Review. BIO Integration 2023; 4(2): 54–61.
DOI: 10.15212/bioi-2022-0009. Available at: https://bio-integration.org/
Download citation
© 2023 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
Biomaterials are natural, synthetic, or hybrid materials, which are used in medical devices or implants that are placed in contact with the human biological system to compensate for or restore diminished functions of the body. The field of biomaterials has rapidly developed to meet the ever-expanding needs in healthcare and medicine practices. Advancements in science and technology have enabled the fabrication and reengineering of biomaterials into useful medical devices or implants, such as heart valves, bone plates, hip joints, and cardiac pacemakers. Because biomaterials are placed in continuous close contact with the recipient’s body fluids or tissues, the classification of available biomaterials is crucial for selecting safer and highly biocompatible materials. This review focuses on biomaterial classification, namely bioceramic, polymeric, and metallic biomaterials. Their medical applications, advantages, and disadvantages are discussed. Current trends in biomaterials involved in disease treatments, such as controlled drug delivery and cancer therapy, are additionally explored.
Keywords
Bioceramic biomaterial, classification, medical application, metallic biomaterial, polymeric biomaterial.
Introduction
Biomaterials function in close contact with living tissue and may replace parts of a living system to augment, repair, or restore body function [1]. These materials can be derived from (i) natural sources, such as starch, chitosan, collagen, and bone; (ii) synthetic sources, in which a range of chemical reactions can be generated in a laboratory to produce biomaterials from not only metallic components but also polymeric and ceramic materials; or (iii) semi-synthetic or hybrid sources consisting of both natural and synthetic materials [1]. Biomaterials have diverse mechanical, biological, physical, and chemical properties that help them function properly and enable applications in or on the human body.
The field of biomaterials, particularly the development of biomedical devices and tissue engineering for human benefits, was established rapidly. Currently, thousands of biomedical devices and diagnostic products are being applied to facilitate the capability of human tissues or organs to regenerate after deterioration and restore normal bodily function [2]. More than 6000 types of medical devices have been listed in the Medical Device Product Classification Database regulated by the Food & Drug Administration’s Center for Medical Devices and Radiological Health, to ensure their safety and effectiveness [3]. A variety of devices and materials, such as cardiac pacemakers, bone plates, artificial heart valves, nerve stimulators, and artificial knee joints, are currently being applied in the treatment of human disease or injury.
During the past two centuries, evidence of the use of biomaterials as implants and prostheses has been discovered on various Roman, Egyptian, Greek, and Etruscan human body parts, such as in skeletons or skulls, thus indicating that biomaterials have been used in or on the human body since ancient times [4]. The use of biomaterials dramatically accelerated after World War II. Many newly developed high-performance metal, ceramic, and particularly polymeric materials were developed, and have been used to construct medical devices to repair or replace damaged body parts or tissues [5]. Biomaterials can be classified in many ways according to their functionality in the human body and their material properties. This review highlights the classification of biomaterials and their applications in the medical field. Current trends in the use of biomaterials for disease treatments, such as drug delivery and cancer immunotherapy, are also discussed.
Classification and medical applications of biomaterials
The functions of biomaterials in the medical field have markedly changed with advances in science and technology. The continual and ever-expanding practical needs in healthcare and medicine practices have significantly driven developments in the biomaterial field and its applications. Biomaterials can be classified in several ways, often according to their human body functionality and material properties [6]. First, biomaterials can be classified at the organ and system levels of the human body. For example, at the system level, the skeletal system can be repaired and restored with a joint replacement and bone plate; at the organ level, the human heart can be repaired and replaced by an artificial heart valve, total valve, and cardiac pacemaker. Second, biomaterials may be categorized according to the body parts treated. For example, an artificial hip joint and a kidney dialysis machine can be used to replace damaged or diseased body parts, whereas screws, sutures, and bone plates can assist in wound healing. Table 1 reviews the classification of biomaterials in medical applications, according to organs, systems, and other parts of the body.
Table 1 Classification of Biomaterials in Medical Applications, on the Basis of Organs, Systems, and Other Parts of the Body
| Classification | Examples | References | |
|---|---|---|---|
| Classification of biomaterials in medical applications based on body organs | Eyes | Intraocular lenses | [7] |
| Ears | Artificial stapes and cochlear implants | [8] | |
| Kidneys | Kidney dialysis machines | [9] | |
| Bladder | Catheters and stents | [8] | |
| Classification of biomaterials in medical applications based on different body systems | Nervous | Nerve stimulators | [10] |
| Circulatory | Artificial blood vessels | [11] | |
| Skeletal | Joint replacement and bone plates | [12] | |
| Muscular | Muscle stimulators and sutures | [13, 14] | |
| Respiratory | Tracheal stents | [15] | |
| Urinary | Catheters, stents, and kidney dialysis machines | [8, 9] | |
| Integumentary | Sutures, burn dressings, and artificial skin | [16, 17] | |
| Classification of biomaterials in medical applications based on other body parts | Improve body parts’ functions | Cardiac pacemakers | [18] |
| Aid in healing | Sutures, screws, and bone plates | [12, 19, 20] | |
| Substitute for a broken part | Hip joint prostheses | [12] | |
| Assist in treatment | Catheters and drains | [8] | |
| Aid in diagnosis | Probes and catheters | [21] | |
The third classification of biomaterials is based on to their material properties in three categories: bioceramic, polymeric, and metallic [22]. The vast variety of available biomaterials enhances the choice of materials for specific treatment purposes; for example, chemically inert metals may be chosen for high electroconductivity as electrodes in artificial organs and long-lasting restoration of lost body function. Nevertheless, biodegradable materials, such as sutures, can be used as a temporary framework for patients in whom function or lost tissue can be regenerated [1, 23]. Furthermore, some biomaterials, such as coronary and peripheral stents, are bioabsorbable and are used in cardiovascular implants. They are slowly eliminated from the body after fulfilling a function [24].
Bioceramic biomaterials
Bioceramic biomaterials are fabricated from non-metallic and metallic elements held together by covalent and/or ionic bonds [25]. Oxides, such as aluminum oxide (Al2O3), magnesium oxide (MgO), and silicon dioxide (SiO2), contain both non-metallic and metallic components, whereas ionic salts can form polycrystalline aggregates (such as ZnS, CsCl, and NaCl). Other common examples of ceramic materials are diamond and carbonaceous structures, which are usually covalently bonded. The strong covalent and ionic bonds between the ceramic elements make them hard, brittle, and stiff [26]. Consequently, the planes of atoms/ions in the ceramics do not easily slip past one another.
Ceramics and their composites have the potential to be used as medical devices to enhance or restore various parts of the body, owing to advances in science and technology. Consequently, various bioceramic devices or implants, including hip prostheses, bone grafts, and artificial tendons, have been developed for medical use. To be designated as bioceramics, the materials must have several important features after being placed in the recipient’s body, including non-inflammatory, non-allergic, biofunctional, biocompatible, carcinogen-free, and non-toxic characteristics [27]. Furthermore, ceramics have been regularly used in dentistry applications, because they are relatively inert to bodily fluids such as saliva, and have aesthetically favorable appearance and excellent compressive strength [28]. Recently, bioceramics have shown immense medical applications in controlled drug delivery, gene therapies, and cancer therapies [29].
Bioceramics such as black pyrolytic carbons have been used in cardiovascular implants, particularly for blood interfacing applications such as heart valves. Although their unappealing color is a disadvantage, particularly in dental applications, pyrolytic carbons are simple to make and have acceptable biocompatibility in the human body [30]. They are also being used as composite implant materials and supporting components for tensile loading applications, such as artificial ligaments and tendons, mainly because of their highly biocompatibility with the human body and their high specific strength as fibers [30].
Three types of ceramics can be used to make implants: (i) resorbable or biodegradable (non-inert) ceramics, such as calcium phosphate and calcium aluminate, (ii) surface reactive or bioactive (semi-inert) ceramics, such as glass ceramics and hydroxyapatites, and (iii) non-absorbable (relatively inert) ceramics, such as alumina, zirconia, and carbons [31]. The medical applications of different bioceramics are listed in Table 2.
Table 2 Medical Applications of Bioceramic Biomaterials
| Types of Bioceramic Biomaterials | Medical Applications | References | |
|---|---|---|---|
| Resorbable or biodegradable (non-inert) | Calcium aluminate | Dental restorative products, orthopedic applications | [32] |
| Calcium phosphate | Artificial bones, teeth, knees, hips, tendons, ligaments | [31, 33] | |
| Surface reactive or bioactive (semi-inert) | Glass-ceramic | Bone augmentation and restoration | [34] |
| Hydroxyapatite | Fillers, bone grafts, coatings for metal implants | [35] | |
| Non-absorbable (inert) | Alumina | Dental and bone implants, hip prostheses | [36] |
| Carbon | Heart valves, bone scaffolds, cartilage regeneration | [37, 38] | |
| Silicon nitride | Spinal fusion implants | [39] | |
| Zirconia | Hip joint replacement, tooth implants | [28, 40] | |
Fabricating bioceramics as medical devices or implants provides several advantages. For instance, these materials are resistant to corrosion and can withstand high compression strength. They also demonstrate excellent benefits as bioactive/inert materials in the human body, such as in articulating surfaces subjected to loads and friction. However, the use of bioceramics as biomaterials is limited by their tendency to have low fracture toughness and low strength in tension. Therefore, a high force could cause them to shatter or crack [31]. In addition, their fabrication is challenging.
Polymeric biomaterials
Polymers applied in biomaterials comprise naturally derived polymers and synthetic polymers, which are either biodegradable or non-biodegradable [41]. Naturally occurring polymers such as starch, collagen, and chitin are frequently used as biomaterials because they are biodegradable and easily obtained. In contrast, synthetic polymers are a mainstream polymer biomaterial widely used in prosthetic materials, dental materials, disposable medical supplies, and medical implants.
Most non-biodegradable synthetic polymers were initially created for non-medical purposes. However, their physical-mechanical qualities essentially identical to those of human soft tissues have led to their wide application as biomedical materials in or on the human body. Currently, many medical applications use various synthetic polymeric materials, including polypropylene, polyethylene, polymethylmethacrylate, polyethylenterephthalate, and polyurethane [41, 42]. The medical applications of these polymeric biomaterials are listed in Table 3.
Table 3 Medical Applications of Polymeric Biomaterials
| Types of Polymeric Biomaterials | Medical Applications | References |
|---|---|---|
| Polypropylene | Hernia repair, blood oxygenator membranes, artificial vascular grafts, degradable sutures | [41, 43] |
| Polyethylene | Surgical implants, tendons, tubing for drains and catheters, acetabular liners | [41, 44, 45] |
| Polymethylmethacrylate | Artificial teeth, provisional crowns, bone cement | [41, 46] |
| Polyethylenterephthalate | Artificial vascular grafts, heart valves | [41] |
| Polyurethane | Wound dressings, breast implants, cardiac patches, drug delivery vehicles, vascular grafts, tracheal soft tissue | [41, 47] |
Polymers are better biomaterials than metals or ceramics because of their ease of manufacturability in diverse forms such as fibers, films, sheets, and synthetic latex. Beyond that, they can be easily processed, have reasonable costs, and are available with desired physical and mechanical properties [48]. Several disadvantages of polymeric biomaterials include that they absorb water and protein in the human body; their surfaces are easily contaminated and difficult to sterilize; they are leachable compounds; they undergo biodegradation; and they are prone to wear and breakdown. In addition, the massive use of non-biodegradable polymers also poses challenges regarding environmental pollution and waste management [49, 50].
Metallic biomaterials
Metals’ excellent thermal and electrical conductivity make them among the most extensively used biomaterials [51]. They have been widely applied in artificial heart valves, including pacemaker leads and vascular stents [51, 52]. Moreover, load-bearing implants, such as hip and knee replacements, mostly use metallic biomaterials, because of their exceptional corrosion resistance and mechanical properties.
Beyond pure metal, alloys of metals with two or more elements are also frequently applied in producing biomaterials. These alloys are usually generated by surface modification, such as coating with bioactive ceramics and polymeric thin films, or surface structuring, thereby enhancing corrosion resistance and increasing the material strength. Currently, three major material groups dominate the metallic biomaterials: pure titanium (Ti) or titanium alloys such as Ti-6Al-4V; stainless steel; and cobalt-chromium (Co-Cr) alloys [51, 53]. Several considerations that influence the selection of metals and alloys as biomaterials in medical applications are appropriate physical and mechanical properties, reasonable cost, corrosion resistance, and biocompatibility [54]. Table 4 summarizes the medicinal applications of these three types of metallic biomaterials.
Table 4 Medical Applications of Metallic Biomaterials
| Types of Metallic Biomaterials | Medical Applications | References |
|---|---|---|
| Pure titanium (Ti) and titanium alloys (Ti-6Al-4V) | Conductive leads, screws, joint prostheses | [55] |
| Stainless steel | Vascular stents, fracture plates, guide wires | [56] |
| Cobalt-chromium (Co-Cr) alloys | Dental apparatus, artificial cardiac valves, joint replacement, screws, fracture plates | [57] |
Ti-6Al-4V is currently one of the most broadly used and desirable metallic biomaterials in medical applications, because of its outstanding properties: it is stronger, lighter, and more resistant to corrosion in the human body than stainless steel and Co-Cr alloys. However, Ti-6Al-4V has been reported to have issues in articulation surfaces in human bones, because it is less elastic and prone to wear and tear [58]. Moreover, the vanadium present in the alloy has the potential for adverse tissue and cytotoxicity reactions [59]. Over time, leached vanadium and aluminum can result in long-term neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease [60, 61]. The leached vanadium and aluminum ions in the human body also affect the respiratory and reproductive systems [59, 62]. Several recent studies have synthesized different coatings on Ti-6Al-4V alloys to increase their biocompatibility and corrosion resistance to the human body [63, 64].
Metals are beneficial as biomaterials because they possess corrosion resistance, wear resistance, and high strength. Furthermore, their ease of sterilization and fabrication, and their shape memory capabilities have led to their extensive use as biomaterials in medical applications. However, the drawbacks of using metallic biomaterials in the human body are their high modulus, cytotoxicity, easy corrosion, and metal ion sensitivity.
Current trends in biomaterials in medical applications
The advancements in science and technology have shifted trends in biomaterial functions. Biomaterials have recently been applied in disease treatments including drug delivery into cells, cancer immunotherapy, cell regeneration, and antimicrobial treatment, among many others. Several types of biomaterials have been used in these applications, including polymer-based biomaterials, lipid-based biomaterials, and inorganic biomaterials.
Polymer-based biomaterials
Hydrogels are polymer-based biomaterials widely used in disease treatment. Hydrogels may exist naturally or may be derived synthetically. Chitosan, fibrin, and alginate are examples of hydrogels of natural origin, whereas poly(vinyl alcohol) is an example of a synthetic hydrogel. Owing to their gelation properties, hydrogels have been used to carry DNAs, mRNA, proteins, or cytokines for disease treatments, such as in cancer immunotherapy and chemotherapy [65–70]. Interestingly, hydrogels’ clinical potential in treating systemic sclerosis and inflammatory airway disease have recently been reported [71, 72], thus illustrating their promise in medical applications for treating other human diseases.
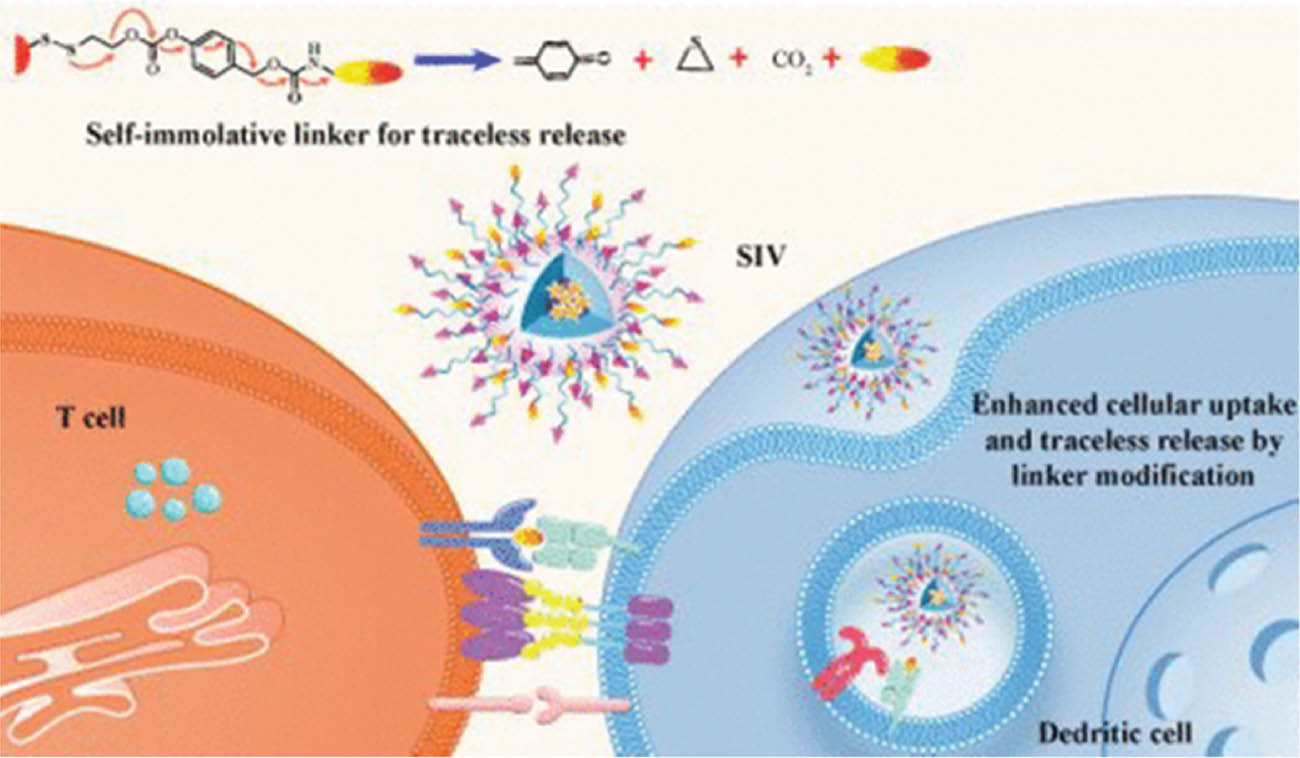
Micelles are another polymer-based biomaterial with important roles in drug delivery and cancer immunotherapy. Micelles are amphiphilic polymers assembled as nanosized particles that can deliver drugs to draining lymph nodes and therefore promote systemic drug administration. A recent study has constructed polypeptide-based micelles that regulate the tumor microenvironment and assist in inhibiting tumor cell metastasis [73]. Ren et al. [74] have also reported that micelles can be covalently bonded with chemically modified short peptide antigens to allow for effective delivery into dendritic cells for robust cellular immune responses (Figure 1), thus suggesting their potential in anti-cancer vaccine development and supporting their exploration as a component of cancer immunotherapy. Intriguingly, using micelles as a vehicle for ligand delivery in combination with chemotherapy drugs has recently been found to significantly increase selective immunogenic cell death in triple-negative breast cancer [75], an aggressive and deadly breast cancer type that lacks targeted therapy and has a poor prognosis because of high metastasis.
Figure 1 A system for delivery of short peptide antigens to dendritic cells for strong T-cell responses, on the basis of block copolymers chemically modified with a hydrophobic and self-immolative linker. The micelles effectively capture antigens and adjuvants via a covalent bond after modification [74]. Reproduced with permission from the American Chemical Society, 2022.
The blood-brain barrier (BBB) is a major challenge preventing effective systemic drug administration in the treatment of brain diseases. Lammers et al. [76] have demonstrated that poly(butylcyanoacrylate)-based microbubbles encased in ultrasmall superparamagnetic iron oxide nanoparticles can mediate and monitor BBB permeability in a mouse model. Another study in canines has reported that polymeric magnetite nanoparticles encapsulating chemotherapy drugs bypass[ing] the BBB and facilitate the delivery to intracranial tumors after infusion by convection-enhanced delivery [77]. The spatial control and bypassing of the BBB are critical for drug delivery in treating brain disorders such as brain tumors, Alzheimer’s disease, and Parkinson’s disease.
Lipid-based biomaterials
Liposomes are lipid-based biomaterials that are highly successful and commonly highlighted in disease treatments. They are spherical vesicles made of phospholipid bilayers that encapsulate various types of therapeutic drugs. Hydrophilic drugs are enclosed within the center aqueous region, and hydrophobic drugs are entrapped within the lipid bilayers [78]. These features make liposomes an effective biomaterials for disease treatments. Since 1986, many liposome products have been licensed for medical applications, such as delivering chemotherapy drugs for cancer treatments, encapsulating inactivated viruses for vaccination purposes, delivering antibiotics for antimicrobial therapy, delivering painkiller drugs for pain management, and even hormone therapy [79]. Furthermore, several investigations have shown that liposomes might be used in cancer immunotherapy and as nanocarriers of imaging agents to improve clinical diagnosis and treatment [80–84]. In a rat glioma model, targeted ultrasound technology has been used to temporarily permeabilize the BBB with doxorubicin hydrochloride drugs contained in long-circulating pegylated liposomes [85]. This exciting finding provides insights into future drug delivery into the brain. Recently, liposome-based mRNA vaccines for COVID-19 have been developed by Moderna and Pfizer/BioNTech, exploiting the excellent ability of liposomes to protect mRNAs against degradation by nucleases in the blood circulation and allow the mRNAs to easily enter the cytoplasm of cells through endocytosis [86].
Inorganic biomaterials
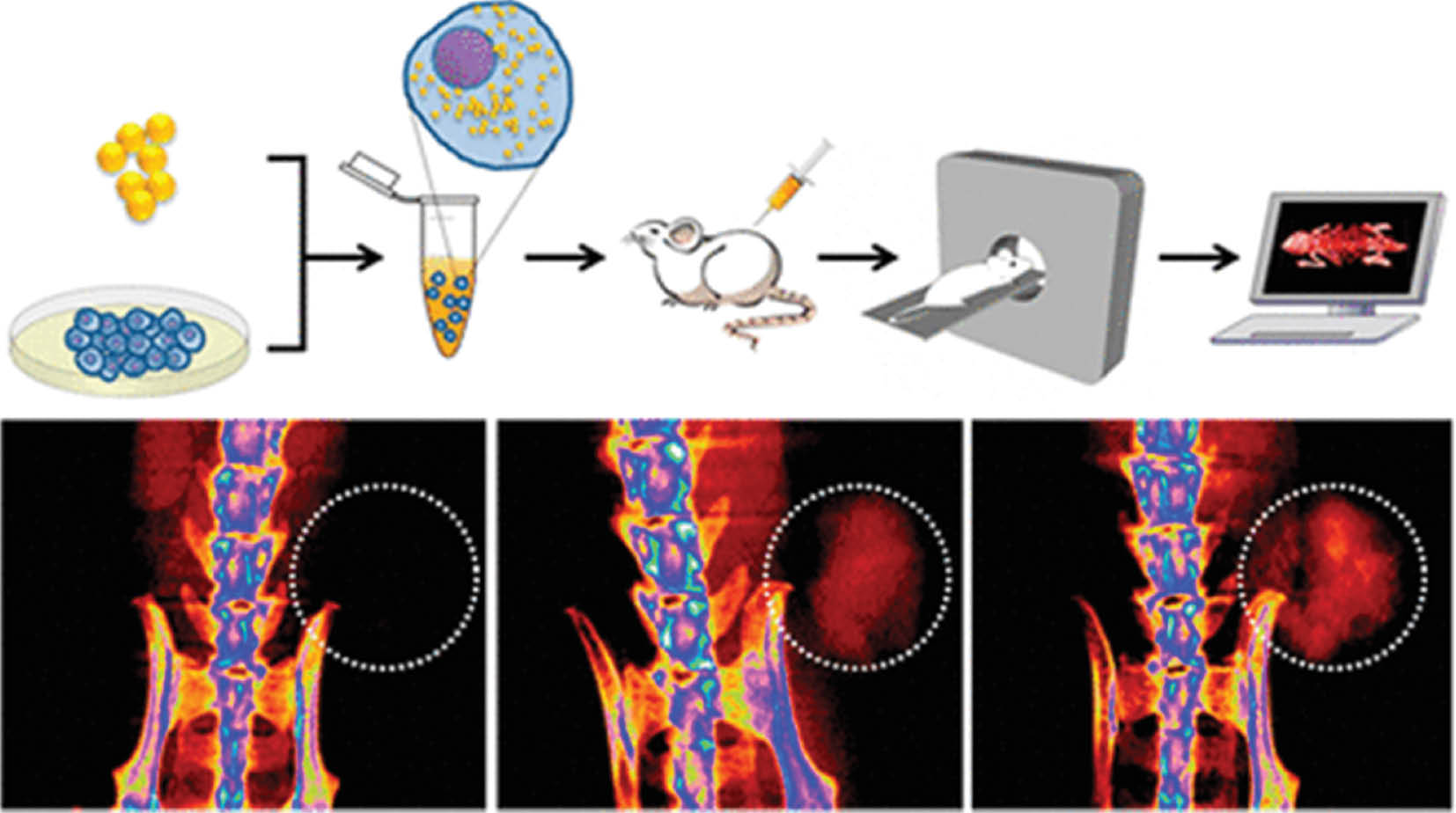
Gold nanoparticles are an inorganic biomaterial that has been extensively studied in disease treatments. According to Li et al. [87], the use of gold nanorods in photothermal therapy in combination with chemotherapy enhances cancer treatment efficiency and modulates the tumor microenvironment. Another study has found that radioisotope-labeled gold nanoclusters allow for the activation of dendritic cells and subsequently induce long-term anti-cancer immunity in a mouse model, by eliminating primary tumors and suppressing distant-tumor development [88]. Furthermore, gold nanoparticles have been found to aid in effective imaging by coating with cancer-specific T-cell receptors as a computed tomography contrast agent, thereby allowing for easy observation of T-cell migration, distribution, and kinetics under imaging (Figure 2) [89]. Although most research remains in animal-trial stages, gold nanoparticles appear to have great potential in human cancer treatment.
Figure 2 T-cells were transduced to express a melanoma-specific T-cell receptor and labeled with gold nanoparticles as a computed tomography contrast agent to examine the distribution, migration, and kinetics of T-cells [89]. Reproduced with permission from the American Chemical Society, 2015.
Another inorganic biomaterial, silica nanoparticles, also have anti-cancer properties after being doped with elements such as calcium, magnesium, and zinc [90]. These doped mesoporous silica nanospheres have been found to increase CD4+ and CD8+ T-cells in the spleen and stimulate an anti-cancer immune response. In addition, Kakizawa et al. [91] have found that silica nanoparticles coated with specific amino acids and incubated with dendritic cells and ligands induce the production of crucial cytokines, such as IL-1 and IFN, thereby implying that silica nanoparticles might be used as a carrier for cellular immunotherapy. Moreover, silica nanoparticles have been widely used in creating vaccines against bacteria and viruses such as Mycoplasma hyopneumoniae, hepatitis B, and most recently, SARS-CoV-2 [92–95]. These vaccinations, however, remain in pre-clinical stages.
Conclusions and outlook
This review discussed the classification of biomaterials and their medical applications. Developments in biomaterials have led to the fabrication and reengineering of various highly promising medical devices or implants to restore the functions of the human body. Bioceramic, polymeric, and metallic biomaterials are beneficial to humankind. Nonetheless, their application poses challenges, such as environmental pollution and substantial waste disposal resulting from the massive use of non-biodegradable synthetic polymeric biomaterials. Most recently developed biomaterials used in drug delivery and cancer therapy remain in early stages of development, facing hurdles relating to biocompatibility, biosafety, and toxicity. Thus, new biomaterials that are environmentally friendly and highly biocompatible with the human body will have enormous potential in medical applications.
Acknowledgement
This work was partly supported by the Universiti Malaysia Sabah Research Collaboration Grant Scheme (GKP0024-2018).
Competing interests
The authors declare that they have no competing interests.
References
- Bhat S, Kumar A. Biomaterials and bioengineering tomorrow’s healthcare. Biomatter 2013;3:e24717. [PMID: 23628868 DOI: 10.4161/biom.24717]
- Chen FM, Liu XH. Advancing biomaterials of human origin for tissue engineering. Prog Polym Sci 2016;53:86-168. [PMID: 27022202 DOI: 10.1016/j.progpolymsci.2015.02.004]
- Food & Drug Administration. Products and medical procedures. Available from: https://www.fda.gov/medical-devices/products-and-medical-procedures. [Last accessed on 22 Mar 2022].
- Migonney V. History of biomaterials. In: Migonney V, editor. Biomaterials. London: John Wiley & Sons, Inc; 2014. pp. 1-10.
- Ratner BD. History of biomaterials. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. Biomaterials science: an introduction to materials in medicine. 3rd ed. Oxford: Academic Press; 2013. pp. xli.
- Arjunan A, Baroutaji A, Preveen AS, Robinson J, Wang C. Classification of biomaterial functionality. Encycl Smart Mater 2022;1:86-102. [DOI: 10.1016/b978-0-12-815732-9.00027-9]
- Uwaezuoke OJ, Kumar P, Pillay V, Choonara YE. Fouling in ocular devices: implications for drug delivery, bioactive surface immobilization, and biomaterial design. Drug Deliv Transl Res 2021;11:1903-23. [PMID: 33454927 DOI: 10.1007/s13346-020-00879-1]
- Zare M, Ghomi ER, Venkatraman PD, Ramakrishna S. Silicone-based biomaterial applications: antimicrobial strategies and 3D printing technologies. J Appl Polym Sci 2021;138:50969. [DOI: 10.1002/app.50969]
- Eswari JS, Naik S. A critical analysis on various technologies and functionalized materials for manufacturing dialysis membranes. Mater Sci Energy Technol 2020;3:116-26. [DOI: 10.1016/j.mset.2019.10.011]
- Farokhi M, Mottaghitalab F, Saeb MR, Shojaei S, Zarrin NK, et al. Conductive biomaterials as substrates for neural stem cells differentiation towards neuronal lineage cells. Macromol Biosci 2020;21:2000123. [DOI: 10.1002/mabi.202000123]
- Wissing TB, Bonito V, Bouten CVC, Smits AIPM. Biomaterial-driven in situ cardiovascular tissue engineering – a multi-disciplinary perspective. NPJ Regen Med 2017;2:18. [PMID: 29302354 DOI: 10.1038/s41536-017-0023-2]
- Singh RK, Gangwar S. An assessment of biomaterials for hip joint replacement. Int J Eng Sci Technol 2021;13:25-31. [DOI: 10.4314/ijest.v13i1.4S]
- Dunn A, Talovic M, Patel K, Patel A, Marcinczyk M, et al. Biomaterial and stem cell-based strategies for skeletal muscle regeneration. J Orthop Res 2019;37:1246-62. [PMID: 30604468 DOI: 10.1002/jor.24212]
- Dong R, Ma PX, Guo B. Conductive biomaterials for muscle tissue engineering. Biomaterials 2020;229:119584. [PMID: 31704468 DOI: 10.1016/j.biomaterials.2019.119584]
- O’Leary C, Soriano L, Fagan-Murphy A, Ivankovic I, Cavanagh B, et al. The fabrication and in vitro evaluation of retinoic acid-loaded electrospun composite biomaterials for tracheal tissue regeneration. Front Bioeng Biotechnol 2020; Epub ahead of print [PMID: 32266229 DOI: 10.3389/fbioe.2020.00190]
- Flynn LE, Woodhouse KA. Burn dressing biomaterials and tissue engineering. In: Narayan R, editor. Biomedical materials. New York: Springer, Cham; 2021. pp. 537–80.
- Momin M, Mishra V, Gharat S, Omri A. Recent advancements in cellulose-based biomaterials for management of infected wounds. Expert Opin Drug Deliv 2021;11:1741-60. [PMID: 34605347 DOI: 10.1080/17425247.2021.1989407]
- Li Y, Wei L, Lan L, Gao Y, Zhang Q, et al. Conductive biomaterials for cardiac repair: a review. Acta Biomater 2022;139:157-78. [PMID: 34605347 DOI: 10.1016/j.actbio.2021.04.018]
- Li J, Qin L, Yang K, Ma Z, Wang Y, et al. Materials evolution of bone plates for internal fixation of bone fractures: a review. J Mater Sci Technol 2020;36:190-208. [DOI: 10.1016/j.jmst.2019.07.024]
- Dhandapani R, Krishnan PD, Zennifer A, Kannan V, Manigandan A, et al. Additive manufacturing of biodegradable porous orthopaedic screw. Bioact Mater 2020;5:458-67. [PMID: 32280835 DOI: 10.1016/j.bioactmat.2020.03.009]
- Shan D, Gerhard E, Zhang C, Tierney JW, Xie D, et al. Polymeric biomaterials for biophotonic applications. Bioact Mater 2018;3:434-45. [PMID: 30151431 DOI: 10.1016/j.bioactmat.2018.07.001]
- Biswal T, Badjena SK, Pradhan D. Sustainable biomaterials and their applications: a short review. Mater Today Proc 2020;30:274-82.
- Henkel J, Woodruff MA, Epari DR, Steck R, Glatt V, et al. Bone regeneration based on tissue engineering conceptions – a 21st century perspective. Bone Res 2013;1:216-48. [DOI: 10.4248/BR201303002]
- Venkatraman S, Huang Y, Wong YS. Bio-absorbable cardiovascular implants: status and prognosis. JOM 2020;72:1833-44. [DOI: 10.1007/s11837-020-04070-2]
- Subedi MM. Ceramics and its importance. Himal Phys 2013;4:80-2. [DOI: 10.3126/hj.v4i0.9433]
- Jodati H, Yilmaz B, Evis Z. A review of bioceramic porous scaffolds for hard tissue applications: effects of structural features. Ceram Int 2020;46:15725-39. [DOI: 10.1016/j.ceramint.2020.03.192]
- Shekhawat D, Singh A, Banerjee MK, Singh T, Patnaik A. Bioceramic composites for orthopaedic applications: a comprehensive review of mechanical, biological, and microstructural properties. Ceram Int 2021;47:3013-30. [DOI: 10.1016/j.ceramint.2020.09.214]
- Sharanraj V, Ramesha CM, Kavya K, Kumar V, Sadashiva M, et al. Zirconia: as a biocompatible used in dental implants. Adv Appl Ceram 2021;120:63-8. [DOI: 10.1080/17436753.2020.1865094]
- Ghiasi B, Sefidbakht Y, Mozaffari-Jovin S, Gharehcheloo B, Mehrarya M, et al. Hydroxyapatite as a biomaterial – a gift that keeps on giving. Drug Dev Ind Pharm 2020;46(7):1035-62. [PMID: 32476496 DOI: 10.1080/03639045.2020.1776321]
- More RB, Haubold AD, Bokros JC. Pyrolytic carbon for long-term medical implants. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. Biomaterials science: an introduction to materials in medicine. 3rd ed. Oxford: Academic Press; 2013. pp. 209–22.
- Kumar P, Dehiya BS, Sindhu A. Bioceramics for hard tissue engineering applications: a review. Int J Appl Eng Res 2018;13:2744-52.
- Parreira RM, Andrade TL, Luz AP, Pandolfelli VC, Oliveira IR. Calcium aluminate cement-based compositions for biomaterial applications. Ceram Int 2016;42:11732-8. [DOI: 10.1016/j.ceramint.2016.04.092]
- Eliaz N, Metoki N. Calcium phosphate bioceramics: a review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017;10:334. [PMID: 28772697 DOI: 10.3390/ma10040334]
- Ben-Nissan B, Choi AH, Macha I. Advances in bioglass and glass ceramics for biomedical applications. In: Li Q, Mai YW, editors. Biomaterials for implants and scaffolds. Berlin, Heidelberg: Springer; 2017. pp. 133-61.
- Bose S, Tarafder S, Bandyopadhyay A. Hydroxyapatite coatings for metallic implants. In: Mucalo M, editor. Hydroxyapatite (HAp) for biomedical applications. Oxford: Woodhead Publishing; 2015. pp. 143–57.
- Davoodi E, Zhainmanesh M, Montazerian H, Milani AS, Hoofar M. Nano-porous anodic alumina: fundamentals and applications in tissue engineering. J Mater Sci Mater Med 2020;31:60. [PMID: 32642974 DOI: 10.1007/s10856-020-06398-2]
- Aoki K, Ogihara N, Tanaka M, Haniu H, Saito N. Carbon nanotube-based biomaterials for orthopaedic applications. J Mater Chem B 2020;8:9227-38. [DOI: 10.1039/D0TB01440K]
- Oveissi F, Naficy S, Lee A, Winlaw DS, Dehghani F. Materials and manufacturing perspective in engineering heart valves: a review. Mater Today Bio 2020;5:100038. [PMID: 32211604 DOI: 10.1016/j.mtbio.2019.100038]
- Rahaman M, Xiao W. Silicon nitride bioceramics in healthcare. Int J Appl Ceram Technol 2017;15:861-72. [DOI: 10.1111/ijac.12836]
- Chen YW, Moussi J, Drury JL, Wataha JC. Zirconia in biomedical applications. Expert Rev Med Devices 2016;13:945-63. [PMID: 27635794 DOI: 10.1080/17434440.2016.1230017]
- Shirvan AR, Nouri A, Wen C. Structural polymer biomaterials. In: Wen C, editor. Structural biomaterials: properties, characteristics and selection. Amsterdam: Elsevier B.V.; 2021. pp. 395-439
- Teo AJT, Mishra A, Park I, Kim YJ, Park WT, et al. Polymeric biomaterials for medical implants and devices. ACS Biomater Sci Eng 2016;2:454-72. [DOI: 10.1021/acsbiomaterials.5b00429]
- Lanzalaco S, del Valle LJ, Turon P, Weis C, Estrany F, et al. Polypropylene mesh for hernia repair with controllable cell adhesion/de-adhesion properties. J Mater Chem B 2020;8:1049-59. [DOI: 10.1039/C9TB02537E]
- Paxton NC, Albenby MC, Lewis PM, Woodruff MA. Biomedical applications of polyethylene. Eur Polym J 2019;118:412-28. [DOI: 10.1016/j.eurpolymj.2019.05.037]
- Hasegawa M, Tone S, Naito Y Sudo A. Reconstruction of patellar tendon rupture after total knee arthroplasty using polyethylene cable. Knee 2021;29:63-7. [PMID: 33578282 DOI: 10.1016/j.knee.2021.01.008]
- Zafar MS. Prosthodontic applications of polymethyl methacrylate (PMMA): an update. Polymers 2020;12:2299. [PMID: 33049984 DOI: 10.3390/polym12102299]
- Joseph J, Patel RM, Wenham A, Smith JR. Biomedical applications of polyurethane materials and coatings. Int J Surf Eng Coat 2018;3:121-9. [DOI: 10.1080/00202967.2018.1450209]
- Parida P, Behera A, Mishra SC. Classification of biomaterials used in medicine. Int J Advances in Appl Sci 2012;1:31-5. [DOI: 10.11591/ijaas.v1i3.882]
- Zhang S, Wang J, Yan P, Hao X, Xu B, et al. Non-biodegradable microplastics in soil: a brief review and challenge. J Hazard Mater 2021;409:124525. [DOI: 10.1016/j.jhazmat.2020.124525]
- Morganti P, Morganti G. Surgical & beauty facial masks: the new waste problem of post covid-19. Biomed J Sci Tech Res 2020;29:22945-50. [DOI: 10.26717/BJSTR.2020.29.004878]
- Pilliar RM. Metallic biomaterials. In: Narayan R, editor. Biomedical materials. New York: Springer, Cham.; 2021. pp. 1–47.
- Pandey A, Awasthi A, Saxena K. Metallic implants with properties and latest production techniques: a review. Adv Mater Process Technol 2020;6:405-40. [DOI: 10.1080/2374068X.2020.1731236]
- Morsiya C. A review on parameters affecting properties of biomaterial SS 316L. Aust J Mech Eng 2020; Epub ahead of print [DOI: 10.1080/14484846.2020.1752975]
- Prasad K, Bazaka O, Chua M, Rochford M, Fedrick L, et al. Metallic biomaterials: current challenges and opportunities. Materials 2017;10:884. [PMID: 28773240 DOI: 10.3390/ma10080884]
- Biesiekierski A, Munir K, Li Y, Wen C. Titanium alloy. In: Wen C, editor. Structural biomaterials: properties, characteristics and selection. Elsevier B.V.: Amsterdam; 2021. pp. 157–87.
- Fu J, Su Y, Qin YX, Zheng Y, Wang Y, et al. Evolution of metallic cardiovascular stent materials: a comparative study among stainless steel, magnesium and zinc. Biomaterials 2020;230:119641. [DOI: 10.1016/j.biomaterials.2019.119641]
- Niinomi M. Co-Cr-based alloys. In: Wen C, editor. Structural biomaterials: properties, characteristics and selection. Elsevier B.V.: Amsterdam; 2021. pp. 103–26.
- Campanelli LC. A review on the recent advances concerning the fatigue performance of titanium alloys for orthopaedic applications. J Mater Res 2021;36:151-65. [DOI: 10.1557/s43578-020-00087-0]
- Xi WS, Li JB, Liu YY, Wu H, Cao A, et al. Cytotoxicity and genotoxicity of low-dose vanadium dioxide nanoparticles to lung cells following long-term exposure. Toxicology 2021;459:152859. [DOI: 10.1016/j.tox.2021.152859]
- Fatola OI, Olaolorun FA, Olopade FE, Olopade JO. Trends in vanadium neurotoxicity. Brain Res Bull 2019;145:75-80. [PMID: 29577939 DOI: 10.1016/j.brainresbull.2018.03.010]
- Raj K, Kaur P, Gupta GD, Singh S. Metals associated neurodegeneration in Parkinson’s disease: insight to physiological, pathological mechanisms and management. Neurosci Lett 2021;753:135873. [DOI: 10.1016/j.neulet.2021.135873]
- Bojanić N, Milenković J, Stojanović D, Milojković M, Djindjić N, et al. Pathophysiological mechanisms of aluminium toxicity. Acta Med Median 2020;59:100-9.
- Hamdi DA, Jiang ZT, No K, Rahman MM, Lee PC, et al. Biocompatibility study of multi-layered hydroxyapatite coatings synthesized on Ti-6Al-4V alloys by RF magnetron sputtering for prosthetic-orthopaedic implant applications. Appl Surf Sci 2019;463:292-9. [DOI: 10.1016/j.apsusc.2018.08.157]
- Machethe KE, Popoola API, Adebiyi DI. Foyami OSI. Influence of SiC-Ti/Al on the microstructural and mechanical properties of deposited Ti-6V-4Al alloy with cold spray technique. Procedia Manuf 2017;7:549-55. [DOI: 10.1016/j.promfg.2016.12.069]
- Yin Y, Li X, Ma H, Zhang J, Yu D, et al. In situ transforming RNA nanovaccines from polyethylenimine functionalized graphene oxide hydrogel from durable cancer immunotherapy. Nano Lett 2021;21:2224-31. [DOI: 10.1021/acs.nanolett.0c05039]
- Zhou Y, Ye T, Ye C, Wan C, Yuan S, et al. Secretions from hypochlorous acid-treated tumor cells delivered in a melittin hydrogel potentiate cancer immunotherapy. Bioact Mater 2022;9:541-53. [DOI: 10.1016/j.biomaterials.2021.121272]
- He H, Fei Z, Guo T, Hou Y, Li D, et al. Bioadhesive injectable hydrogel with phenolic carbon quantum dot supported Pd single atom nanozymes as a localized immunomodulation niche for cancer catalytic immunotherapy. Biomaterials 2022;280:121272. [PMID: 34864428 DOI: 10.1016/j.biomaterials.2021.121272]
- Liu Y, Han YY, Lu S, Wu Y, Li J, et al. Injectable hydrogel platform with biodegradable Dawson-type polyoxometalate and R848 for combinational photothermal-immunotherapy of cancer. Biomater Sci 2022;10:1257-66. [DOI: 10.1039/D1BM01835C]
- Rezk AI, Obiweluozor FO, Choukrani G, Park CH, Kim CS. Drug release and kinetic models of anticancer drug (BTZ) from a PH-responsive alginate polydopamine hydrogel: towards cancer chemotherapy. Int J Biol Macromol 2019;141:388-400. [DOI: 10.1016/j.ijbiomac.2019.09.013]
- Song Q, Zhang R, Lei L, Li X. Self-assembly of succinated paclitaxel into supramoleculer hydrogel for local cancer chemotherapy. J Biomed Nanotechnol 2018;14:1471-6. [PMID: 29903061 DOI: 10.1166/jbn.2018.2595]
- Nie M, Kong B, Chen G, Xie Y, Zhao Y, et al. MSCs-laden injectable self-healing hydrogel for systemic sclerosis treatment. Bioact Mater 2022;17:369-78 Epub ahead of print. [DOI: 10.1016/j.bioactmat.2022.01.006]
- Ousingsawat J, Centeio R, Cabrita I, Talbi K, Zimmer O, et al. Airway delivery of hydrogel-encapsulated niclosamide for the treatment of inflammatory airway disease. Int J Mol Sci 2022;23:1085. [DOI: 10.3390/ijms23031085]
- Han Z, Gong C, Li J, Guo H, Chen X, et al. Immunological modified enzyme-responsive micelles regulate the tumor microenvironment for cancer immunotherapy. Mater Today Bio 2022;13:100170. [DOI: 10.1016/j.mtbio.2021.100170]
- Ren H, Li J, Liu G, Sun Y, Yang X, et al. Anticancer vaccination with immunogenic micelles that capture an release pristine CD8+ T-cell epitopes and adjuvants. ACS Appl Mater Interfaces 2022;14:2510-21. [DOI: 10.1021/acsami.1c18117]
- Qiu X, Qu Y, Guo B, Zheng H, Meng F, et al. Micellar paclitaxel boosts ICD and chemo-immunotherapy of metastatic triple negative breast cancer. J Control Release 2022;341:498-510. [PMID: 34883139 DOI: 10.1016/j.jconrel.2021.12.002]
- Lammers T, Koczera P, Fokong S, Gremse F, Ehling J, et al. Theranostic USPIO-loaded microbubbles for mediating and monitoring blood-brain barrier permeation. Adv Funct Mater 2015;25:36-43. [PMID: 25729344 DOI: 10.1002/adfm.201401199]
- Young JS, Bernal G, Polster SP, Nunez L, Larsen GF, et al. Convection enhanced delivery of polymeric nanoparticles encapsulating chemotherapy in canines with spontaneous supratentorial tumours. World Neurosurg 2018;117:e698-e704. [PMID: 29960096 DOI: 10.1016/j.wneu.2018.06.114]
- Xing H, Hwang K, Lu Y. Recent developments of liposomes as nanocarriers for theranostic applications. Theranostics 2016;6:1336-52. [PMID: 27375783 DOI: 10.7150/thno.15464]
- Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev 2013;65:36-48. [PMID: 23036225 DOI: 10.1016/j.addr.2012.09.037]
- Yu J, Wang S, Qi J, Yu Z, Xian Y, et al. Mannose-modified liposome designed for epitope peptide drug delivery in cancer immunotherapy. Int Immunopharmacol 2021;101:108148. [PMID: 34653955 DOI: 10.1016/j.intimp.2021.108148]
- Zhang H, Tang WL, Kheirolomoom A, Fite BZ, Wu B, et al. Development of thermosensitive resiquimod-loaded liposomes for enhanced cancer immunotherapy. J Control Release 2021;330:1090-94. [PMID: 33189786 DOI: 10.1016/j.jconrel.2020.11.013]
- Watanabe S, Yuba E, Akazawa T, Wijewardana V, Kakihara Y, et al. Potent adjuvant effect elicited for tumor immunotherapy by a liposome conjugated pH-sensitive polymer and dendritic cell-targeting Toll-like receptor ligand. Vaccine 2022;40:1448-57. [DOI: 10.1016/j.vaccine.2022.01.048]
- Morse SV, Mishra A, Chan TG, de Rosales RTM, Choi JJ. Liposome delivery to the brain with rapid short-pulses of focused ultrasound and microbubbles. J Control Release 2022;341:605-15. [PMID: 34896448 DOI: 10.1016/j.jconrel.2021.12.005]
- Wood CA, Han S, Kim CS, Wen Y, Sampaio DRT, et al. Clinically translatable quantitative molecular photoacoustic imaging with liposome-encapsulated ICGJ-aggregates. Nat Commun 2021;12:5410. [PMID: 34518530 DOI: 10.1038/s41467-021-25452-3]
- Aryal M, Vykhodtseva N, Zhang YZ, Park J, McDannold N. Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood-tumor and blood-brain barriers improves outcomes in a rat glioma model. J Control Release 2013;169:103-11. [PMID: 23603615 DOI: 10.1016/j.jconrel.2013.04.007]
- Gregoriadis G. Liposomes and mRNA: two technologies together create a COVID-19 vaccine. Med Drug Dis 2021;12:100104. [DOI: 10.1016/j.medidd.2021.100104]
- Li D, Zhang M, Xu F, Chen Y, Chen B, et al. Biomimetic albumin-modified gold nanorods for photothermo-chemotherapy and macrophage polarization modulation. Acta Pharm Sin B 2018;8:74-84. [DOI: 10.1016/j.apsb.2017.09.005]
- Pei P, Shen W, Zhou H, Sun Y, Zhong J, et al. Radionuclide labelled gold nanoclusters boost effective anti-tumor immunity for augmented radio-immunotherapy of cancer. Nanotoday 2021;38:101144. [DOI: 10.1016/j.nantod.2021.101144]
- Meir R, Shamalov K, Betzer O, Motiei M, Horovitz-Fried M, et al. Nanomedicine for cancer immunotherapy: tracking cancer-specific T-cells in vivo with gold nanoparticles and CT imaging. ACS Nano 2015;9:6363-72. [PMID: 26039633 DOI: 10.1021/acsnano.5b01939]
- Wang X, Li X, Ito A, Sogo Y, Watanabe Y, et al. Biodegradable metal ion-doped mesoporous silica nanospheres stimulate anticancer Th1 immune response in vivo. ACS Appl Mater Interfaces 2017;9:43538-44. [PMID: 29192493 DOI: 10.1021/acsami.7b16118]
- Kakizawa Y, Lee JS, Bell B, Fahmy TM. Precies manipulation of biophysical particle parameters enables control of proinflammatory cytokine production in presence of TLR 3 and 4 ligands. Acta Biomater 2017;57:136-145. [PMID: 28069499 DOI: 10.1016/j.actbio.2017.01.025]
- Mechler-Dreibi ML, Almeida HMS, Sonalio K, Martines MAC, Petri FAM, et al. Oral vaccination of piglets against Mycoplasma hyopneumoniae using silica SBA-15 as an adjuvant effectively reduced consolidation lung lesions at slaughter. Sci Rep 2021;11:22377. [DOI: 10.1038/s41598-021-01883-2]
- Wang J, Zhu R, Gao B, Wu B, Li K, et al. The enhanced immune response of hepatitis B virus DNA vaccine using SiO2@LDH nanoparticles as an adjuvant. Biomaterials 2014;35:466-78. [PMID: 24099705 DOI: 10.1016/j.biomaterials.2013.09.060]
- Scaramuzzi K, Tanaka GD, Neto FM, Garcia PRAF, Gabrili JJM, et al. Nanostructured SBA-15 silica: an effective protective vehicle to oral hepatitis B vaccine immunization. Nanomedicine 2016;12:2241-50. [PMID: 27339784 DOI: 10.1016/j.nano.2016.06.003]
- Qiao L, Chen M, Li S, Hu J, Gong C, et al. A peptide-based subunit candidate vaccine against SARS-CoV-2 delivered by biodegradable mesoporous silica nanoparticles induced high humoral and cellular immunity in mice. Biomater Sci 2021;9:7287-96. [DOI: 10.1039/D1BM01060C]