Stem Cell Therapy for SARS-CoV-2 and Influenza Virus Infections
1Asutosh College – Department of Biochemistry, 92, Shyamaprasad Mukherjee Road, Kolkata, West Bengal 700026, India
*Correspondence to: Neelabh Datta, Asutosh College – Department of Biochemistry, 92, Shyamaprasad Mukherjee Road, Kolkata, West Bengal 700026, India. E-mail: neelabhdatta@gmail.com
Received: May 17 2024; Revised: July 4 2024; Accepted: July 17 2024; Published Online: July 29 2024
Cite this paper:
Datta N. Stem Cell Therapy for SARS-CoV-2 and Influenza Virus Infections. BIO Integration 2024; 5: 1–13.
DOI: 10.15212/bioi-2024-0016. Available at: https://bio-integration.org/
Download citation
© 2024 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
The emergence of infectious diseases, including viral zoonoses, has allowed intensive research into novel therapeutic approaches. Stem cell therapy, mostly using mesenchymal stem cells (MSCs), has garnered significant attention due to the immunomodulatory properties and tissue repair capabilities. MSCs have demonstrated promise in treating severe COVID-19 cases and several clinical trials have revealed that MSC therapy improves 28-day survival rates, reduces mortality, and accelerates recovery. These cells effectively mitigate a cytokine storm, relieve pulmonary symptoms, and positively influence organ recovery, including the liver and kidneys. Bioanalytical readings return to normal following MSC administration, emphasizing the potential in managing COVID-19-induced complications. MSC therapy offers a potential solution for infection with the influenza virus, which is responsible for historical pandemics and epidemics, and remains a global health concern. MSCs inhibit immune cell-mediated responses and reduce lung damage in animal models, and despite antiviral drugs, influenza-induced manifestations persist. MSCs, with an ability to counteract inflammation and promote lung tissue repair, hold promise for managing influenza infections. While MSCs offer therapeutic benefits, certain challenges remain. Specifically, ethical considerations, regulatory hurdles, and scalability are some of the challenges that hinder widespread adoption. However, ongoing systematic reviews and meta-analyses provide real-time insight that support the security and effectiveness of MSC therapy.
Keywords
Cytokine storm, immunomodulatory properties, stem cells, viral infections.
Introduction
In recent decades the emergence of infectious diseases has captured global attention [1, 2]. These diseases carry a significant threat to public health given the virulence, mortality rates, modes of transmission, and effect on patients’ quality of life [2]. Emerging infectious diseases are those infectious diseases that show signs of first occurring in a community or rapidly increasing in frequency or geographic spread according to the World Health Organization (WHO) [3]. Infectious diseases have remained the leading cause of morbidity and mortality throughout the 1970s, as demonstrated by the development of severe acute respiratory syndrome coronavirus (SARS-CoV-2) in 2019 and acquired immunodeficiency syndrome (AIDS) in 1978 [4]. Zoonotic transmission accounts for approximately 60% of emerging infectious diseases with 70% originating in wildlife [5]. Notably, some infectious agents that were once deemed inconsequential to public health have resurged, resulting in severe effects on the global population [6]. Moreover, some pathogens have exhibited an increased incidence and prevalence worldwide that are driven by factors, such as mutation, therapeutic resistance, and pathogen evolution [7].
While advances in medical science and technology have equipped us with potent tools to combat these diseases, several limitations and challenges persist. The rapid mutation rates of some pathogens, such as influenza virus and HIV, pose significant hurdles in the development of effective and long-lasting vaccines [8]. In addition, the rise in antimicrobial resistance (AMR) has emerged as a formidable challenge, rendering many standard treatments ineffective and complicating the management of common infectious diseases [9]. The socioeconomic disparities across different geographic regions further exacerbate infectious disease control because limited access to healthcare services and insufficient infrastructure hinder timely diagnosis and treatment [10]. Moreover, the global interconnectedness facilitated by modern travel and trade accelerates the spread of infectious agents, which complicates containment efforts [11]. Addressing these challenges requires a multidisciplinary approach involving advances in biomedical research and robust public health strategies, international collaboration, and policy interventions.
The ongoing challenge of emerging infectious diseases
The 21st century has witnessed the emergence of several epidemic- and pandemic-level viral infections without even including the disturbing SARS-CoV-2 (COVID-19) pandemic [12]. Despite the lessons learned from the lethal 1918 influenza outbreak, the world was ill-prepared for the SARS-CoV-2 outbreak given the prior warnings from the SARS-CoV (2003) and Middle East respiratory syndrome coronavirus [MERS-CoV] (2012) epidemics, which clearly indicated the heightened risk of such strains flowing in bats [1, 12, 13]. Nevertheless, remarkable progress in basic virology, cell biology, biochemistry, and immunology research has accelerated the development of antiviral products and novel vaccines, although the global distribution of these medical interventions remains a difficult task [14]. Concurrently, the swift spread of rumours and half-truths has created misperception and eroded public assurance in these interventions, resulting in more harm than good [15]. The current SARS-CoV-2 pandemic has underscored the necessity of investing in preparedness for global outbreaks and developing diagnostic and intervention technologies [16]. Indeed, these technologies are essential in the development of therapeutics tailored to specific viral infections with high heterogeneity [1, 16].
Mesenchymal stem cells as a promising therapeutic approach
Mesenchymal stem cell (MSC) therapy offers a favourable approach for mitigating the adverse effects of infectious viral diseases [7]. MSCs belong to a diverse subset of self-renewing progenitor cells that can be derived from many adult tissues, including umbilical cord blood, amniotic fluid, dental pulp, bone marrow, menstrual blood, foetal liver, Bichat’s fat pad, abdominal fat pad, and endometrium [1, 17]. This amazing versatility allows researchers and clinicians to harness the regenerative potential of these cells, making MSCs a suitable candidate for treating a wide spectrum of diseases. Upon invasion of a virus, MSCs respond to damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) by transforming into a pro-inflammatory phenotype (MSC1), which produces inflammatory chemokines that attract circulating leukocytes to the inflamed tissues. MSCs thereby regulate the activities of various immune cells involved in the antiviral response, such as dendritic cells (DCs), macrophages, natural killer (NK) cells, B lymphocytes, CD4+ T helper cells, and cytotoxic T lymphocytes (CTLs). Interferons (IFNs) produced by MSCs influence the cytotoxic functions of NK cells and CTLs, enhance the antigen-presenting capabilities of DCs and macrophages, regulate cytokine production in CD4+ T helper cells, and control antibody production in B cells, all of which are crucial for the effective elimination of virus-infected cells. During the tissue repair phase, MSCs adopt anti-inflammatory phenotypes and release a variety of immunoregulatory molecules, such as transforming growth factor-beta (TGF-β), indoleamine 2,3-dioxygenase (IDO), interleukin (IL)-10, interleukin-1 receptor antagonist (IL-1Ra), and prostaglandin E2 (PGE2). These molecules help suppress the overactivation of immune cells, thus preventing the development of a cytokine storm and the subsequent harmful systemic inflammatory response.
Because of the strong immunomodulatory properties, MSCs are being extensively studied in numerous experimental settings as a potential new therapeutic approach for treating viral diseases. Furthermore, the insightful immunomodulatory capabilities not only allow tissue repair but also offer exciting prospects for mitigating inflammatory responses and enhancing immune regulation, underscoring a focal role for MSCs in the future of regenerative medicine and disease management.
Understanding emerging viral zoonoses
Some viral zoonoses are categorized as “evolving infectious diseases” due to the recent recognition and substantial changes in epidemiologic features and geographic distribution of these zoonoses [1, 18]. The primary basis for virus emergence is the increased interaction between viruses and wild animals, which results in the transmission of viruses from non-human hosts to new human hosts [19, 20]. Climate changes also contribute to the rise in viruses. Variants of newly evolving viruses can cause severe epidemics and appear in drug-resistant forms, which are referred to as “re-emerging viruses.” Indeed, the relationship between climate changes and the emergence of viruses is a subject of intense significance in the virology domain. Evidentiary strands have unravelled over the years, underscoring the unquestionable influence of shifting environmental dynamics on the viral landscape. One cannot overlook the research that elucidates how alterations in temperature, humidity, and precipitation patterns can provide fertile ground for the resurgence of dormant viruses or the amplification of existing viruses. Furthermore, the ramifications extend beyond mere viral reactivation because these climatic variations can also catalyse the evolution of viral strains into hitherto unseen variants. Such variants with genetic novelties possess an alarming potential to unleash severe epidemics. This finding was exemplified by the emergence of the SARS-CoV-2 virus pandemic, which is believed to have zoonotic origins influenced by changing environmental conditions that brought humans into closer contact with animal reservoirs [1]. The spectre of drug-resistant “re-emerging viruses” looms ominously on the horizon as well and the selective pressures exerted by a changing climate can hasten the development of resistance mechanisms in viruses against antiviral drugs. The ongoing battle against antibiotic-resistant bacteria highlights the possibility that a similar scenario is plausible in the virosphere, which has been further substantiated by the growing body of evidence documenting the evolution of drug-resistant strains of HIV, and influenza and hepatitis viruses [1].
Versatility of stem cell therapy
Stem cell therapy has tremendous potential for treating a wide range of human diseases, including neurodegenerative disorders, amyotrophic lateral sclerosis, Alzheimer’s disease, Parkinson’s disease, autoimmune diseases, rheumatoid arthritis, type 1 diabetes mellitus, cardiovascular diseases, and cancers [21, 22]. A major increase in registered clinical studies using MSCs has occurred over the past 10 years. East Asia, especially China, has been leading this trend, followed by North America and Europe [6]. The distinctive feature of stem cells is in the permanent self-renewal and ability to differentiate into specialized adult cell types. MSCs can be categorized as pluripotent stem cells, which have the capacity to develop into any cell type, and multipotent stem cells, which have limitations in differentiation potential [23]. The ease with which MSCs are isolated from various tissues and the capacity to differentiate into multiple lineages make MSCs a smart option for treating various clinical conditions, including viral infections [24]. The vast stem cell therapeutic array is a testament to the potential stem cells hold in the field of regenerative medicine. Pluripotent stem cells, represented by embryonic stem cells and induced pluripotent stem cells, constitute the pinnacle of versatility within this spectrum because pluripotent stem cells possess the remarkable ability to criss-cross the developmental continuum, following the trajectory of embryogenesis itself [23]. With the potential to regenerate damaged or degenerated tissues and organs, pluripotent stem cells have ignited an enthusiasm for scientific exploration and ventured into uncharted territories of curing previously intractable diseases. Moreover, pluripotent stem cells offer a glimmer of hope to individuals afflicted by a myriad of medical conditions, including neurodegenerative disorders and heart diseases. In contrast, multipotent stem cells, although somewhat less all-inclusive in differentiation capabilities when juxtaposed with pluripotent counterparts, remain an option in the biomedical field. These cells, found in various tissues, such as bone marrow, adipose tissue, and the umbilical cord, harbour the inherent potential to transform into a more limited repertoire of cell types, typically within a specific tissue or organ system. The importance of this differentiation potential should not be underestimated because multipotent stem cells are endowed with the capacity to replenish and regenerate tissues, thereby having pivotal roles in maintaining homeostasis and repairing local injuries [23]. Clinically, multipotent stem cells have shown great promise in the domain of tissue engineering and regenerative therapies and offer practical solutions for ailments, such as orthopaedic injuries and haematologic disorders.
MSC mechanisms of action in viral infections
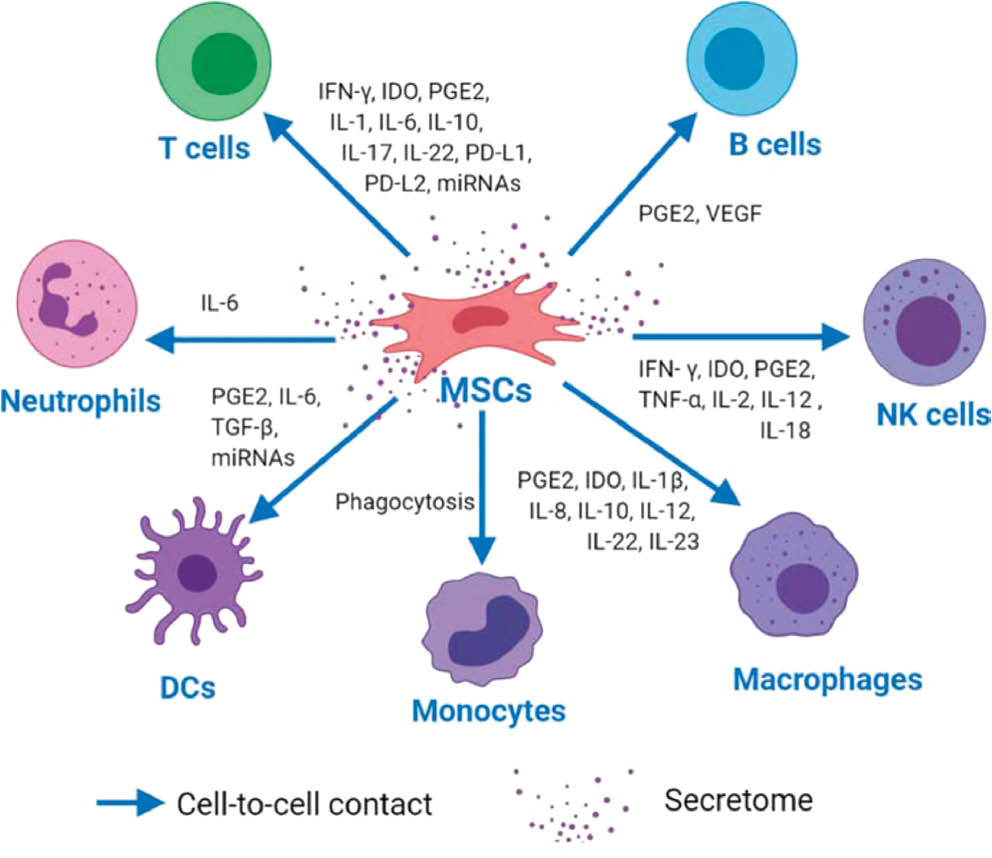
Human MSCs are non-hematopoietic stem cells identified by unique cell surface markers and differentiation clusters (CD29, CD44, CD73, and CD90) [25]. These cells can develop into three lineages, as follows: endodermal (hepatocytes); mesodermal (osteocytes, adipocytes, and chondrocytes); and ectodermal (neurocytes). MSCs have a crucial role in the innate and adaptive immune systems. MSCs exert immunomodulatory effects primarily through interactions with immune cells via direct cell-to-cell contact and the release of paracrine factors (Figure 1) [26]. MSCs interact with various immune cells, including T cells, B cells, NK cells, and macrophages [27]. In vitro studies have demonstrated that MSCs suppress naive and memory T-cell responses by interacting with antigen-presenting cells (APCs) [27]. This interaction involves the upregulation of intercellular adhesion molecule (ICAM)-) and vascular cell adhesion molecule (VCAM)-1, which are essential for T-cell activation and recruitment of leukocytes (WBCs) to sites of inflammation [28]. Further research has shown that co-culturing bone marrow-derived (BM)-MSCs with activated T cells induces lymphocytes that produce IL-17A [29]. MSCs co-cultured with CD4+ T cells have been shown to activate the Notch1/forkhead box P3 (FOXP3) pathway, increasing the percentage of CD4+CD25+FOXP3+ regulatory T cells (Tregs) [30]. Knockdown of Galectin-1, a protein abundantly expressed in MSCs that affects T lymphocytes and cytokine secretion, results in the loss of MSC immunomodulatory properties and restores CD4+ and CD8+ T-cell proliferation [31]. BM-MSCs express high levels of Toll-like receptors (TLRs) 3 and 4, which are responsible for nuclear factor kappa B (NF-κB) activity and cytokine production. Expression of these TLRs in MSCs can restore efficient T-cell responses during infection [26].
Human placenta MSCs (PMSCs) have been shown to express high levels of programmed-death ligand (PD)-1 and -2, which inhibit T-cell proliferation by arresting the cell cycle [32]. In vivo mouse models have provided further insight into immune regulation between MSCs and T cells. For example, in a syngeneic orthotopic mouse model of ovarian cancer, compact bone (CB)-derived MSCs have shown anti-tumor effects when combined with a fusion protein (VIC-008) that activates CD4+ and CD8+ T cells and inhibits Tregs in the tumor microenvironment (TME) [33]. In foetal abortion models, MSCs have enhanced the suppressive regulation of T cells and macrophages [34]. Conversely, MSCs primed by activated T cells derived from interferon-gamma knockout (IFN-γ −/−) mice exhibit a significantly reduced ability to suppress T-cell proliferation, which highlights the importance of cell-to-cell contact in MSC immunosuppressive function [35].
In addition to T cells, MSCs also impact B cells through direct contact. Adipose (A)-derived MSCs have been shown to enhance the survival of quiescent B cells via contact-dependent mechanisms and facilitate B-cell differentiation independently of T cells [36]. A-MSCs also inhibit caspase 3-mediated apoptosis of B cells by upregulating vascular endothelial growth factor (VEGF) [37] and inhibit B-cell proliferation by blocking the cell cycle in the G0/G1 phase through activation of the p38 mitogen-activated protein kinase (MAPK) pathway [38]. MSCs also interact with cells of the innate immune system through direct contact. Tracking studies have revealed that infused umbilical cord (UC)-derived MSCs briefly reside in the lungs before being rapidly phagocytosed by monocytes, which then migrate to other body sites. This phagocytosis induces phenotypic and functional changes in monocytes, which subsequently modulate adaptive immune cells and have a crucial role in mediating the immunomodulatory effects of MSCs [26]. Co-culture studies with different NK cell lines (KHYG-1 and NK-92) have shown differential crosstalk between MSCs and cytotoxic NK cells with granule polarization either suppressed or induced [39]. A-MSCs can also switch activated M1-like inflammatory macrophages to an M2-like phenotype via PGE2 and prevent neutrophil death through an ICAM-1-dependent mechanism, thereby exerting tissue-protective effects [26, 40].
MSCs also exert immunomodulatory properties through secretion of multifunctional molecules via paracrine mechanisms [41]. This secretome includes a diverse array of cytokines, growth factors, and chemokines, such as TGF-β1, tumor necrosis factor-alpha (TNF-α), PGE2, interferon-gamma (IFN-γ), hepatocyte growth factor (HGF), fibroblast growth factor (FGF), IDO, and nitric oxide (NO) [26]. These paracrine factors are encapsulated in extracellular vesicles (EVs) secreted by cells, which are classified into exosomes, micro-vesicles (MVs), and apoptotic bodies based on size and origin [42, 43]. MSCs influence the adaptive immune system, particularly T cells, through paracrine secretion. MSCs inhibit T helper (Th)17 cell differentiation by inducing IL-10 and PGE2 production while inhibiting IL-17, IL-22, and IFN-γ [43]. However, the mechanisms underlying MSC-Th17 interactions are not fully understood. In vitro and in vivo studies using IL-25 knockdown have shown that MSCs suppress Th17 responses through the IL-25/STAT3/PD-L1 axis [44]. MSC-secreted IDO induces Tregs, which are responsible for kidney allograft tolerance [45]. Additionally, exosomes derived from BM-MSCs transfected with plasmids encoding shFas and anti-miR-375 and co-cultured with peripheral blood mononuclear cells (PBMCs) suppress immune responses in immunodeficient mouse models by inhibiting PBMC proliferation and enhancing Treg function [46]. MSCs also secrete PD-1 ligands, including PD-L1 and PD-L2, to exert immunosuppressive effects directly on T-cell behaviour by suppressing CD4+ T-cell activation [47].
Figure 1 The figure depicts MSCs modulating immune cells through direct contact and secreted factors. MSCs influence T cells by secreting molecules, such as IFN-γ, IDO, PGE2, various interleukins, PD-L1, PD-L2, and miRNAs. For B cells, MSCs release PGE2 and VEGF to aid in proliferation and antibody production. NK cells are regulated by MSCs through factors, such as IFN-γ, IDO, PGE2, TNF-α, IL-2, IL-12, and IL-18, to enhance cytotoxicity. MSCs also modulate macrophages, dendritic cells (DCs), monocytes, and neutrophils with factors, including PGE2, IDO, IL-1β, IL-6, IL-8, IL-10, TGF-β, and miRNAs, which balance immune responses and prevent excessive inflammation. Source [26].
Within the innate immune system, MSCs interact with NK cells by inhibiting IL-2-induced NK cell proliferation [48] and modulating cytotoxic activity or cytokine production via IDO and PGE2 secretion [49]. MSCs enhance the ability of IL-12- and IL-18-stimulated NK cells to secrete IFN-γ, potentially improving infection defence at injury sites and influencing tissue regeneration [50]. MSC-derived IL-6 protects neutrophils from apoptosis, preserving neutrophils within the bone marrow niche [51]. MSC-derived exosomes augment neutrophil viability, while MSC-conditioned media (CM) increase neutrophil function, demonstrating the usefulness of MSC-derived exosomes and CM in enhancing immunity by modulating neutrophils [52]. Lipopolysaccharide (LPS)-stimulated MSCs enhance neutrophil anti-microbial functions by releasing IL-8 and macrophage migration inhibitory factor (MIF), which contributes to the resolution of infections and inflammation [53]. MSC-EVs also have a crucial role in macrophage polarization, enhancing the formation of anti-inflammatory M2 macrophages over M1-like inflammatory macrophages by downregulating IL-23 and IL-22 [54]. BM-MSCs activated by LPS or TNF-α reprogram macrophages by releasing PGE2, which acts on macrophages through PG EP2 and EP4 receptors [55]. Human PMSCs transform macrophages from an inflammatory M1 into an anti-inflammatory M2 phenotype via soluble IL-10, IL-1β, IL-12, MIP-1α, and glucocorticoid and progesterone receptors [56].
Importantly, IFN-stimulated gene (ISG) expression equips these cells with resistance to viral entrance by aiming at various stages of the viral lifecycle, including genome integration, transcription, and translation [1, 57]. Notable ISGs include PMAIP1, ISG15, IFI6, IFITM3, SAT1, p21/CDKN1A, SERPINE1, and CCL2 [57]. ISGs have been shown to suppress the infection of various viruses in vitro and some directly affect the susceptibility of specific viruses [58]. MSCs regulate the host tissue microenvironment by modulating immune responses, inhibiting NK and T cells, and downregulating inflammatory cytokines while upregulating regulatory cytokines [22]. TLRs, especially TLR3 and TLR4 on the surface of MSCs, have a crucial role in immunomodulatory action in response to RNA viruses, thereby preventing a cytokine storm [59, 60]. MSCs release anti-inflammatory chemicals, such as NO, PGE2, TGF-β1, HGF, IDO, and IL-10 in response to this hyperimmune response [61, 62].
Efficacy of MSCs in treating COVID-19
Clinical trials have recognised the safety and efficacy of MSCs in the treatment of COVID-19, which demonstrated that MSCs possess the remarkable ability to mitigate inflammation, release protective substances, offer antioxidant effects, reduce cell mortality, and enhance the overall immune response. As of June 2022, 104 clinical trials involving stem cells were registered and documented for COVID-19 therapy. Several studies have validated and shown that MSC therapy considerably lowers the incidence, frequency, and mortality of critically ill patients [63]. For example, Shu et al. [63] reported no mortality in the MSC therapy group, while Xu et al. [64] found significantly higher survival rates in MSC-treated patients compared to those receiving standard treatment. The harm inflicted on the respiratory system by COVID-19 arises from a combination of the inherent pathogenicity of the virus and the immune reaction to virus. A study piloted by Shi et al. [65] discovered that COVID-19-infected patients exhibit elevated levels of proinflammatory cytokines [65]. However, patients who required admission to intensive care units (ICUs) displayed a decrease in the levels of MCP1, MIP-1α, GCSF, IP10, and TNF-α when compared to non-admitted individuals. This shift in cytokine levels points to the occurrence of a cytokine storm within the lungs of infected individuals. Such alterations in cytokine levels have been linked to a cascade of immune responses and extensive lung damage following pulmonary inflammation. This combined effect ultimately contributes to a heightened risk of mortality. Patients treated with MSCs exhibit significantly improved 28-day survival rates and a reduced likelihood of death compared to the control group [66]. Moreover, MSC therapy accelerates COVID-19 recovery and improves pulmonary function [65]. After MSC delivery, several analytical parameters reverted to normal, including C-reactive protein (CRP), alanine aminotransferase (ALT), creatinine, and serum ferritin (SF) levels, as well as platelet counts [67]. MSCs improved the healing of other organs, such as the liver and kidneys, in addition to reducing pulmonary symptoms [67]. MSCs have a vital role in mitigating lung injuries caused by Coronaviridae family members by targeting the cytokine storm and promoting lung tissue repair and restoration [62].
Influenza viruses and the potential of MSC therapy
Influenza viruses are frequent culprits leading to pandemics and epidemics, with the emergence of H1N1 influenza in 1918 setting a historical precedent [68]. The 21st century has witnessed sporadic outbreaks of avian influenza virus (AIV) subtypes, such as H5N1, H7N9, and H9N2, which have caused high mortality rates in humans [69]. In spite of antiviral drugs as the primary therapeutic approach against influenza-induced manifestations, antiviral drugs often fail to repair damaged lung tissues, which has led researchers to explore alternative treatments involving human bone marrow-derived mesenchymal stromal cells (BM-MSCs) [70]. These cells have demonstrated promise in countering the impact of H5N1 virus infection on human alveolar epithelial cells. Specifically, BM-MSCs contribute to a reduction in enhanced alveolar protein permeability (APP) and an elevation in alveolar fluid clearance (AFC). The mechanisms underlying this process involve the secretion of human keratinocyte growth factor (KGF) and angiopoietin (Ang)-1 by BM-MSCs and in vivo experiments have underscored the significant anti-inflammatory properties of BM-MSCs, highlighting the ability of BM-MSCs to increase the population of M2 macrophages [1]. These specialized immune cells have a vital role in producing various cytokines and chemokines, including IL-1β, IL-4, IL-6, IL-8, and IL-17 [1]. MSCs have also shown promise in ameliorating lung injury caused by influenza viruses by restoring alveolar epithelial cell function, decreasing inflammation, and controlling the immune response [71, 72]. Furthermore, MSCs have been shown to inhibit the release of proinflammatory cytokines and multiplication of virus-specific CD8 + T cells, ultimately improving survival of influenza virus patients [73].
Comparison of efficacy of MSCs in treating COVID-19 versus influenza
Numerous clinical trials have demonstrated the promising potential of MSCs in addressing COVID-19 and its complications (Table 1) [74]. Over 100 registered clinical trials have explored MSCs for COVID-19 treatment, with many focusing on severely ill patients [75]. A phase I clinical study confirmed the safety of high and low doses of DW-MSC infusion in non-severe COVID-19 patients [76]. However, to firmly establish the therapeutic efficacy of MSCs, large-scale randomized controlled trials are necessary. Shu et al. [63] reported that patients treated with UC-MSCs had improvement in clinical symptoms, such as weakness, fatigue, shortness of breath, and the oxygenation index, within 3 days of treatment. Prenatal MSCs from UC-MSCs or placental (PL-MSC) tissues have been used in critically ill COVID-19 patients with ARDS, resulting in reduced dyspnoea and an increased SpO2 within 2–4 d after an initial infusion in 64% of the patients [74, 67]. A multicentre randomized, double-blind trial revealed a significant increase in the PaO2:FiO2 ratio in the UC-MSC group compared to the placebo group in COVID-19-associated acute respiratory distress syndrome (ARDS) [77]. A clinical trial using human menstrual blood-derived mesenchymal stromal cells for severe and critically ill COVID-19 patients showed significant improvement in dyspnoea, the SpO2, and the PaO2, and notably lower mortality in the MSC group compared to the control group [74, 64]. Farkhad et al. [78] found that mesenchymal stromal cell therapy improved the SPO2:FIO2 ratio in COVID-19-induced ARDS patients. Lanzoni et al. [66] reported improved patient survival and faster recovery after two rounds of intravenous allogeneic UC-MSC administration in ARDS patients. In a phase I/II study, survival rates were higher in the MSC group 28 and 60 d post BM-MSC treatment [79]. An Indonesian randomized controlled trial showed a survival rate 2.5-fold higher in the UC-MSC group than the control group among critically ill COVID-19 patients [80]. Fathi-Kazerooni et al. [81] reported a significantly higher survival rate in severe COVID-19 patients treated with human mesenchymal stromal cells compared to placebo treatment.
Table 1 Clinical Trials Exploring the Potential of MSC Infusions in COVID-19 Patients (Adapted from [74])
| No | Study Title | Trial ID NO | Phase | Indications | Source of MSCs | Country |
|---|---|---|---|---|---|---|
| 1 | Safety of DW-MSC infusion in patients with low clinical risk COVID-19 infection: a randomized, double-blind, placebo-controlled trial | NCT04535856 | Phase 1 | Low clinical risk COVID-19 | Allogenic UC-MSC | Indonesia |
| 2 | Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells | ChiCTR2000031494 | Phase 1 | Severe/critical COVID-19 | Human umbilical cord | China |
| 3 | Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series | IRCT20200217046526N2 | Phase 1 | ARDS in COVID-19 | Allogeneic umbilical cord/placenta | Iran |
| 4 | Treatment of COVID-19-associated ARDS with mesenchymal stromal cells: a multicenter randomized double-blind trial | NCT04333368 | Phase 2 | ARDS in COVID-19 | Umbilical cord | France |
| 5 | Evaluation of the safety and efficacy of using human menstrual blood-derived mesenchymal stromal cells in treating severe and critically ill COVID-19 patients: An exploratory clinical trial | ChiCTR2000029606 | Phase 1 | Severe and critical COVID-19 | Allogenic menstrual blood | China |
| 6 | Mesenchymal stromal cell therapy for COVID-19-induced ARDS patients: a successful phase 1, control-placebo group, clinical trial | IRCT20160809029275N1 | Phase 1 | ARDS in COVID-19 | Allogenic Umbilical cord | Iran |
| 7 | Conquering the cytokine storm in COVID-19-induced ARDS using placenta-derived decidua stromal cells | IRCT2017010531786N1 | Phase 1/2 | ARDS in COVID-19 | Placenta | Iran |
| 8 | Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial | NCT04355728 | Phase 1/2a | ARDS in COVID-19 | Allogeneic umbilical cord | USA |
| 9 | Bone marrow-derived mesenchymal stromal cell therapy in severe COVID-19: preliminary results of a phase I/II clinical trial | NCT04445454 | Phase 1/2 | Severe COVID-19 | Bone marrow | Belgium |
| 10 | Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: A randomized controlled trial | NCT04457609 | Phase 1 | ARDS in COVID-19 | Allogenic umbilical cord | Indonesia |
| 11 | Safety and efficacy study of allogeneic human menstrual blood stromal cell secretome to treat severe COVID-19 patients: clinical trial phase I & II | IRCT20180619040147N6 | Phase 1/2 | Severe COVID-19 | Allogeneic human menstrual blood | Iran |
| 12 | Human mesenchymal stem cell therapy in severe COVID-19 patients: 2-year follow-up results of a randomized, double- blind, placebo-controlled trial | NCT04288102 | Phase 2 | Severe COVID-19 | Allogenic umbilical cord | China |
| 13 | Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial | NCT04252118 | Phase 1 | Moderate and severe COVID-19 | Allogeneic umbilical cord | China |
| 14 | Nebulized exosomes derived from allogenic adipose tissue mesenchymal stromal cells in patients with severe COVID-19: a pilot study | NCT04276987. | Phase 2a | Severe COVID-19 | Allogeneic adipose tissue | China |
| 15 | Safety and long-term improvement of mesenchymal stromal cell infusion in critically COVID-19 patients: a randomized clinical trial | U1111-1254-9819 | Phase 1/2 | Critical COVID-19 | Allogeneic umbilical cord | Brazil |
| 16 | Allogenic mesenchymal stromal cells and their extracellular vesicles in COVID-19 induced ARDS: a randomized controlled trial | IRCT20200217046526N2 | Phase 2 | ARDS in COVID-19 | Allogenic perinatal tissue | Iran |
| 17 | The systematic effect of mesenchymal stem cell therapy in critical COVID-19 patients: a prospective double controlled trial | NCT04392778 | Phase 1/2 | Critical COVID-19 | Umbilical cord | Turkey |
| 18 | Cell therapy in patients with COVID-19 using Wharton’s jelly mesenchymal stem cells: a phase 1 clinical trial | IRCT20190717044241N2 | Phase 1 | Severe COVID-19 | Umbilical cord | Iran |
| 19 | Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial | NCT04288102 | Phase 2 | Severe COVID-19 | Umbilical cord | China |
| 20 | Safety and efficacy assessment of allogeneic human dental pulp stem cells to treat patients with severe COVID-19: structured summary of a study protocol for a randomized controlled trial (phase I/II) | NCT04336254 | Phase 1/2 | Severe COVID-19 | Allogeneic dental pulp | China |
| 21 | Human placenta-derived mesenchymal stem cells transplantation in patients with acute respiratory distress syndrome (ARDS) caused by COVID-19 (phase I clinical trial): safety profile assessment | IRCT20200621047859N4. | Phase 1 | ARDS in COVID-19 | Allogenic placenta | Iran |
| 22 | Nebulization therapy with umbilical cord mesenchymal stem cell-derived exosomes for COVID-19 pneumonia | ChiCTR2000030261 | Phase 1 | Pneumonia in COVID-19 | Umbilical cord | China |
| 23 | A randomized trial of mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome from COVID-19 | NCT04371393 | Phase 3 | ARDS in COVID-19 | Allogenic bone marrow | USA |
Many inflammatory biomarkers and cytokines are closely associated with severe and critical COVID-19. Meng et al. [82] reported improved PaO2:FiO2 ratios and a decline in inflammatory cytokines, such as IL-6, IFN-γ, TNF-α, MCP-1, IP-10, IL-22, IL-1RA, IL-18, IL-8, and MIP-1, after three rounds of UC-MSC treatment. Fathi-Kazerooni et al. [81] reported notably lower CRP levels on day 5 in severe COVID-19 patients treated with human mesenchymal stromal cells compared to placebo, with significant reductions in CRP, LDH, D-dimer, and SE levels in the treatment group. Sadeghi et al. [83] demonstrated significant reductions in IL-6 and CRP levels in COVID-19-induced ARDS patients treated with placenta-derived decidual stromal cells. A pilot study showed that aerosol inhalation of exosomes from human adipose-derived MSCs (haMSC-Exos) in COVID-19 patients increased lymphocyte counts and decreased CRP, LDH, and IL-6 levels [74, 84].
In COVID-19 patients, common CT findings include ground-glass opacification, infiltration, consolidation, pneumonia, and emphysematous changes [85]. Many clinical trials have shown that MSC treatment improves lung changes in COVID-19 patients. Shu et al. [63] reported faster lung inflammation absorption on CT imaging in severe COVID-19 patients treated with UC-MSC compared to the control group. A case series trial showed significant recovery indications, such as reduced ground-glass opacities or consolidations, in COVID-19-induced ARDS patients [74]. Meng et al. [82] observed complete fading of lung lesions within 2 weeks in moderate COVID-19 patients after a UC-MSC transfusion. A phase 2 randomized, double-blind, placebo-controlled trial showed a significant reduction in solid component lesion volume proportions in the UC-MSC group compared to the placebo group in COVID-19-induced ARDS patients [74]. A randomized clinical trial showed a decrease in lung damage extent 4 months after 3 rounds of a mesenchymal stromal cell infusion in critically ill COVID-19 patients [86]. A pilot study reported varying degrees of pulmonary lesion resolution after aerosol inhalation of haMSC-Exos in COVID-19 patients [84]. Xu et al. [64] detected a significant difference in the improvement rate of chest CT findings between experimental and control groups in the first month following MSC infusion in severe and critically ill COVID-19 patients. Fathi-Kazerooni et al. [81] demonstrated significant pulmonary involvement improvement in severe COVID-19 patients treated with human mesenchymal stromal cells secretome. Sadeghi et al. [83] showed complete disappearance of pulmonary infiltrates in COVID-19-induced ARDS patients treated with placenta-derived decidual stromal cells.
In the case of influenza virus-induced lung injury, despite substantial evidence indicating the beneficial effects of MSC administration in preclinical models, some studies challenge the efficacy of MSCs as a therapeutic or prophylactic option for reducing pulmonary inflammation (Table 2) [87]. Research conducted by Darwish et al. [88] showed that MSC therapy does not lead to improved outcomes in severe experimental influenza. Similarly, Gotts et al. [89] reported no significant beneficial effects of MSCs on weight loss, survival, or lung injury. These findings highlight the importance of considering the timing, dose, route, and frequency of MSC administration when evaluating treatment efficiency. One potential reason for the observed lack of efficacy is that MSCs may have difficulty accessing the injured epithelial barrier and could potentially become infected by the influenza virus. Moreover, the short duration of preclinical models limits the ability to thoroughly investigate lung recovery processes following influenza-induced injury [88, 89].
Table 2 Clinical Trials Exploring the Potential of MSC Infusions in Influenza Patients
| No | Study Title | Trial ID NO | Phase | Indications | Source of MSCs | Country |
|---|---|---|---|---|---|---|
| 1 | Clinical study of mesenchymal stem cell treatment for acute respiratory distress syndrome induced by epidemic influenza A (H7N9) infection: A hint for COVID-19 treatment | ChiCTR-OCC-15006355 | Completed | ARDS in Influenza | Menstrual blood derived MSCs | China |
| 2 | Using human menstrual blood cells to treat acute lung injury caused by H7N9 bird flu virus infection | NCT02095444 | Unknown | No result posted | Menstrual blood stem cells | China |
Nevertheless, these experimental findings do not entirely rule out the potential of MSC therapy to contribute to long-term lung repair and the restoration of full lung function post-influenza infection. In addition to exogenously administered MSCs, tissue-resident MSCs have a significant role in tissue repair and regeneration [87]. Adult pulmonary tissue-resident MSCs exhibit similar phenotypes and functions to BM-MSCs [90]. Although few studies have examined the status of resident lung MSCs after virus-induced lung injury, there is some evidence suggesting that altered lung MSC function may have a role in bleomycin-induced pulmonary arterial hypertension (PAH). In mouse models, bleomycin treatment has been shown to lead to the loss of endogenous lung MSCs, which are critical for modulating the severity of the injury through an influence on the T cell response [91]. Transplantation of isolated lung MSCs has been shown to mitigate bleomycin-induced PAH, reduce the number of inflammatory cells, and inhibit T cell proliferation. These findings imply that lung MSCs are crucial for maintaining lung integrity post-injury, but the loss compromises this protective function [92]. Ye et al. [93] demonstrated that BM-MSCs influence endogenous lung stem cells (club cells) through the release of cytokines and vesicles, activating the Notch signalling pathway and affecting the proliferation of club cells in phosgene-induced lung injury. Another study showed that BM-MSCs protect against LPS-induced lung injury by restoring alveolar bioenergetics through Cx43-dependent alveolar attachment and mitochondrial transfer [94]. These findings suggest that transplanted exogenous MSCs may create an important niche for various types of lung cells via different pathways.
The recent emergence of SARS-CoV-2, which has caused an outbreak of atypical viral pneumonia in patients, underscores the global public health risks posed by coronaviruses. Despite multiple occurrences of such viruses attacking humans, there are still limited specific strategies to address these virus-induced injuries [87]. Stem cells, including MSCs and LSCs, offer a potential therapeutic approach for treating virus-induced lung injuries. This potential has been explored from the perspectives of immune regulation and lung repair, although challenges remain. The number of clinical trials investigating stem cell therapy for virus-induced lung injury has been gradually increasing. Looking ahead, there is an expectation that stem cell therapy will become a viable treatment option for virus-induced lung injury.
Challenges and future directions in MSC therapy
The potential of MSCs in various pre-clinical disease models is well-documented, yet the exact mechanisms underlying many observed effects remain elusive [95, 96]. Over the past 10 years, clinical trials for MSC-based therapies have been initiated for numerous medical conditions [97]. These clinical studies have consistently demonstrated that MSC applications are safe and feasible, although proving their efficacy has often been challenging as therapies progressed through clinical development stages. This challenge is reflected in the absence of MSC-based products in the European market, with only a few such products approved globally. South Korea is at the forefront, having registered two MSC products, with the first authorization granted in 2011 [95]. This noteworthy MSC approval is possibly due to the South Korean regulatory framework, which permits conditional marketing approval, allowing commercial sales under specific conditions while pivotal trials are ongoing. The European Union has a similar procedure under Regulation (EC) No 507/2006, although there are differences in the regulatory systems. In North America, Health Canada’s Notice of Compliance with Conditions (NOC/c) allows for conditional marketing of MSC products, which is similar to the South Korean approach [95]. In the United States, the Food and Drug Administration (FDA) has stringent requirements for MSC-based therapies, requiring extensive pre-clinical and clinical data to demonstrate safety and efficacy [95]. Many studies involving MSC clinical applications often lack pre-clinical screening because only one active substance candidate is typically used. Evaluating the rationale behind using specific MSC cultures is essential if the clinical application does not require the same function as in the tissue of origin. MSC trials frequently aim to promote tissue regeneration or immunomodulation. Incorporating pre-clinical screening and selection, a standard part of conventional medicinal product development, should also apply to cell therapy. Techniques, such as modifying MSC populations with small compounds followed by expression or secretome profile screening, genetic modifications to enhance therapeutic effects, or comparing subpopulations from different origins, could be considered to improve study design and efficacy [98, 99].
Though costly and perhaps less attractive for academic research, pre-clinical screening can be a cost-effective improvement for discovering new MSC therapy candidates, which would offer benefits, such as novel intellectual property acquisition. MSC product developers should start with the EMA scientific guidelines detailing quality, safety, efficacy, and pharmacovigilance for Cell Therapy Medicinal Products (CTMPs). Despite these guidelines, the variety of cell therapy products means a risk-based approach is necessary for development and evaluation. Directive 2001/83/EC encourages a risk-based approach for ATMPs to determine the extent of quality, non-clinical, and clinical data required for marketing authorization applications [95]. The risk analysis must cover the entire development process with relevant biological parameters, including cell viability and biodistribution investigation, in place of conventional pharmacologic tests. Given the lack of relevant animal models for cell therapy products, the regulatory evaluation will likely follow a risk-based approach until more products reach the market. Understanding these ethical issues and regulatory frameworks, which differ globally, is crucial for advancing MSC-based therapies. Solutions, such as adaptive licensing and a risk-based approach, could improve the efficiency and accessibility of these promising treatments. Nevertheless, the prolonged culture of stem cells may lead to chromosomal abnormalities and epigenetic changes, necessitating adherence to “Good Manufacturing Practices” (GMP), in which the standards for sustainable cell production can compromise the immune response and increase the susceptibility to infections [100, 101]. Moreover, concerns have arisen regarding the role of MSCs in promoting viral transmission and tumor growth [102, 103]. Therefore, it is imperative to homogenise therapeutic protocols, ex vivo preparations, and MSC isolation methods to enhance clinical outcomes. To address these ethical and regulatory challenges, a harmonized global regulatory framework could be developed incorporating best practices from different regions. Adaptive licensing, which allows for conditional approvals based on iterative data gathering and regulatory re-evaluation could be widely adopted among regulatory boards because this approach balances the need for timely access to therapies with the requirement for thorough safety and efficacy evaluations. Enhancing international collaboration and sharing of clinical data could also improve the regulatory process, ensuring that MSC-based therapies meet high standards of safety and efficacy, while accelerating the availability to patients in need. Establishing clear guidelines for the ethical sourcing and use of MSCs, coupled with robust patient consent processes, can address ethical concerns and build public trust in these therapies.
Conclusion and future directions
MSCs offer a multitude of advantages that make them attractive for therapeutic applications. First, MSCs can be harvested from various tissues, including BM, which is considered the most preferable source. MSCs can also be isolated from peripheral blood, AD tissues, oral tissues, and menstrual blood. Neonatal birth-related tissues, such as the PL, UC, Wharton’s jelly, and amniotic membrane or fluid, are effective sources of MSCs [104]. Once isolated, MSCs can be preserved for future therapeutic use. Second, MSCs are multipotent stem cells that possess the ability to self-renew and differentiate into multiple specialized cell types [104]. Third, MSCs can be expanded to large quantities in a relatively short time, allowing MSCs to be stored for repeated therapeutic interventions [104]. Importantly, clinical studies involving MSCs have not reported severe adverse reactions to allogeneic MSCs to date. Lastly, various clinical trials have confirmed the safety of MSCs in therapeutic applications [105].
Using MSCs for COVID-19 treatment presents several challenges that must be addressed to ensure efficacy and safety. Because MSC therapy is classified under stem cell treatments, it is imperative to adhere to the guidelines set by the International Society for Stem Cell Research (ISSCR). The ISSCR “Guidelines for the Clinical Translation of Stem Cells” outline the criteria for advancing stem cell-based treatments to ensure that the therapeutic potential is realized [104]. Key challenges include optimizing the expansion period, determining the required cell dose, and addressing issues related to cell culture and exposure to animal-derived products, which can significantly impact safety and efficacy. Long-term in vitro culture can lead to a loss of essential stem cell characteristics, such as stemness and plasticity, or even induce malignant transformation [106]. Another significant challenge is determining patient eligibility for MSC-based treatments, particularly for those with chronic conditions, cancer, autoimmune diseases, allergies, or those who are pregnant or breastfeeding. Many clinical trials using MSCs or derived exosomes for COVID-19 treatment are still in phase I/II trials and have yielded unsatisfactory results [104]. It is crucial to have robust evidence and verification of MSC safety and efficacy, which involves assessing potential adverse events and long-term consequences [107]. Another major challenge is the significant heterogeneity within MSC populations, which may contribute to inconsistent research findings. Moreover, recent studies indicate that MSCs might not be as immunologically inert as previously believed [7]. This discovery necessitates further investigation to identify and isolate the “immune privileged” subpopulations within the diverse MSC pool for more reliable clinical applications. An alternative strategy to circumvent these issues is to explore cell-free therapies, such as MSC-derived exosomes [7]. These exosomes could offer significant advantages over MSCs, especially because the therapeutic effects of MSCs are largely attributed to paracrine activity. Given the limited research on MSC applications in virus-related diseases and the fact that most studies are still in early clinical phases, it is premature to draw definitive conclusions about efficacy. Therefore, it is essential to conduct well-designed, randomized controlled trials with larger sample sizes to thoroughly assess the safety and therapeutic effectiveness of MSC treatments over both short- and long-term periods [7].
Although the BM is the most common source of MSCs, the harvesting process is invasive and the yield is limited. ARDS can impair the immunomodulatory efficacy of BM-MSCs, complicating use for autologous transplantation [104]. While studies have explored the therapeutic efficacy of MSCs from other sources in ARDS, it remains unclear which source provides the best outcomes. Determining the optimal cell injection dose is critical for clinical MSC treatments. Emerging and re-emerging infectious diseases have continued to challenge global healthcare systems, emphasizing the urgent need for pioneering therapeutic methods. Stem cell therapy, particularly with MSCs, holds immense promise in moderating the impact of these viral infections by exerting antiviral, anti-inflammatory, immunomodulatory, anti-fibrotic, anti-apoptotic, and angiogenic effects. Future clinical trials should focus on understanding these characteristics of stem cells in infectious environments and explore innate recognition mechanisms to enhance their antiviral potential. Standardization of MSC protocols and quality improvements are essential for addressing the challenges associated with graft rejection, graft-versus-host disease, and delayed immune reconstitution. The interface between viruses and stem cells thus remains a dynamic field ripe for further exploration, offering hope in the ongoing scuffle against infectious diseases.
Conflict of Interest
The authors have no competing interest to declare.
References
- Khandelwal V, Sharma T, Gupta S, Singh S, Sharma MK, et al. Stem cell therapy: a novel approach against emerging and re-emerging viral infections with special reference to SARS-CoV-2. Mol Biol Rep 2023;50(3):2663-83. [DOI: 10.1007/s11033-022-07957-2]
- Löscher T, Prüfer-Krämer L. Emerging and re-emerging infectious diseases. In: Krämer A, Kretzschmar M, Krickeberg K, editors. Modern infectious disease epidemiology. Statistics for biology and health. New York, NY: Springer; 2009. [DOI: https://doi.org/10.1007/978-0-387-93835-6_3]
- Ogden NH, AbdelMalik P, Pulliam J. Emerging infectious diseases: prediction and detection. Can Commun Dis Rep 2017;43:206-11. [PMID: 29770047 DOI: 10.14745/ccdr.v43i10a03]
- Wilder-Smith A. COVID-19 incomparison with other emerging viral diseases: risk of geographic spread viatravel. Trop Dis Travel Med Vaccines 2021;7:3 [PMID: 33517914 DOI: 10.1186/s40794-020-00129-9]
- Haider N, Rothman-Ostrow P, Osman AY, Arruda LB, Macfarlane-Berry L, et al. COVID-19-zoonosis or emerging infectious disease? Front Public Health 2020;8:763. [PMID: 33324602 DOI: 10.3389/fpubh.2020.596944]
- Tulchinsky TH, Varavikova EA. A history of public health. In New public health. Cambridge, MA: Academic Press; 2014. [DOI: 10.1016/B978-0-12-415766-8.00001-X]
- Sleem A, Saleh F. Mesenchymal stem cells in the fight against viruses: face to face with the invisible enemy. Curr Res Transl Med 2020;68:105-10. [PMID: 32616467 DOI: 10.1016/j.retram.2020.04.003]
- Cascalho M, Balin SJ, Platt JL. The mutable vaccine for mutable viruses. Immunotherapy 2017;9(8):659-67. [PMID: 28653569 DOI: 10.2217/imt-2017-0030]
- Salam MA, Al-Amin MY, Salam MT, Pawar JS, Akhter N, et al. Antimicrobial resistance: a growing serious threat for global public health. Healthcare 2023;11(13):1946. [PMID: 37444780 DOI: 10.3390/healthcare11131946]
- McMaughan DJ, Oloruntoba O, Smith ML. Socioeconomic status and access to healthcare: interrelated drivers for healthy aging. Front Public Health 2020;8:231. [PMID: 32626678 DOI: 10.3389/fpubh.2020.00231]
- Baker RE, Mahmud AS, Miller IF, Rajeev M, Rasambainarivo F, et al. Infectious disease in an era of global change. Nat Rev Microbiol 2022;20(4):193-205. [PMID: 34646006 DOI: 10.1038/s41579-021-00639-z]
- Bell D, Brown GW, von Agris J, Tacheva B. Urgent pandemic messaging of WHO, World Bank, and G20 is inconsistent with their evidence base. Global Policy 2024;1-19. [DOI: 10.1111/1758-5899.13390]
- Elkhatib WF, Abdelkareem SS, Khalaf WS, Shahin MI, Elfadil D, et al. Narrative review on century of respiratory pandemics from Spanish flu to COVID-19 and impact of nanotechnology on COVID-19 diagnosis and immune system boosting. Virol J 2022;19(1):167. [PMID: 36280866 DOI: 10.1186/s12985-022-01902-2]
- Trovato M, Sartorius R, D’Apice L, Manco R, De Berardinis P. Viral emerging diseases: challenges in developing vaccination strategies. Front Immunol 2020;11:2130. [PMID: 33013898 DOI: 10.3389/fimmu.2020.02130]
- Zhou J, Ghose B, Wang R, Wu R, Li Z, et al. Health perceptions and misconceptions regarding COVID-19 in China: online survey study. J Medical Internet Res 2020;22(11):e21099. [PMID: 33027037 DOI: 10.2196/21099]
- Kashyap VK, Dhasmana A, Massey A, Kotnala S, Zafar N, et al. Smoking and COVID-19: adding fuel to the flame. Int J Mol Sci 2020;21:6581. [PMID: 32916821 DOI: 10.3390/ijms21186581]
- Kashyap VK, Dhasmana A, Yallapu MM, Chauhan SC, Jaggi M. Withania somnifera as a potential future drug molecule for COVID-19. Future Drug Discov 2020;2:FDD50-FDD. [PMID: 33269342 DOI: 10.4155/fdd-2020-0024]
- Gediz Erturk A, Sahin A, Bati Ay E, Pelit E, Bagdatli E, et al. A multidisciplinary approach to coronavirus disease (COVID-19). Molecules 2021;26(12):3526. [PMID: 34207756 DOI: 10.3390/molecules26123526]
- Swire-Thompson B, Lazer D. Public health and online misinformation: challenges and recommendations. Annu Rev Public Health 2020;41:433-51. [PMID: 31874069 DOI: 10.1146/annurev-publhealth-040119-094127]
- Liu C, Zhou Q, Li Y, Garner LV, Watkins SP, et al. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent Sci 2020;6:315-31. [PMID: 32226821 DOI: 10.1021/acscentsci.0c00272]
- Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther 2019;10:68. [PMID: 30808416 DOI: 10.1186/s13287-019-1165-5]
- Keatts LO, Robards M, Olson SH, Hueffer K, Insley SJ, et al. Implications of zoonoses from hunting and use of wildlife in North American arctic and boreal biomes: pandemic potential, monitoring, and mitigation. Front Public Health 2021;9:451. [PMID: 34026707 DOI: 10.3389/fpubh.2021.627654].
- Woolhouse M, Scott F, Hudson Z, Howey R, Chase-topping M. Human viruses: discovery and emergence. Philos Trans R Soc Lond B Biol Sci 2012;367:2864-71. [PMID: 22966141 DOI: 10.1098/rstb.2011.0354]
- Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells – current trends and future prospective. Biosci Rep 2015;35:e00191. [PMID: 25797907 DOI: 10.1042/BSR20150025]
- Sivandzade F, Cucullo L. Regenerative stem cell therapy for neurodegenerative diseases: an overview. Int J Mol Sci 2021;22(4):2153. [PMID: 33671500 DOI: 10.3390/ijms22042153]
- Song N, Scholtemeijer M, Shah K. Mesenchymal stem cell immunomodulation: mechanisms and therapeutic potential. Trends Pharmacol Sci 2020;41(9):653-64. [PMID: 32709406 DOI: 10.1016/j.tips.2020.06.009]
- Krampera M, Glennie S, Dyson J, Scott D, Laylor R, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003;101(9):3722-29. [PMID: 12506037 DOI: 10.1182/blood-2002-07-2104]
- Ren G, Zhao X, Zhang L, Zhang J, L’Huillier A, et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol 2010;184(5):2321-28. [PMID: 20130212 DOI: 10.4049/jimmunol.0902023]
- Najar M, Fayyad-Kazan H, Faour WH, Merimi M, Sokal EM, et al. Immunological modulation following bone marrow-derived mesenchymal stromal cells and Th17 lymphocyte co-cultures. Inflamm Res 2019;68(3):203-13. [PMID: 30506263 DOI: 10.1007/s00011-018-1205-0]
- Del Papa B, Sportoletti P, Cecchini D, Rosati E, Balucani C, et al. Notch1 modulates mesenchymal stem cells mediated regulatory T-cell induction. Eur J Immunol 2013;43(1):182-7. [PMID: 23161436 DOI: 10.1002/eji.201242643]
- Gieseke F, Böhringer J, Bussolari R, Dominici M, Handgretinger R, et al. Human multipotent mesenchymal stromal cells use galectin-1 to inhibit immune effector cells. Blood 2010;116(19):3770-9. [PMID: 20644118 DOI: 10.1182/blood-2010-02-270777]
- Wang G, Zhang S, Wang F, Li G, Zhang L, et al. Expression and biological function of programmed death ligands in human placenta mesenchymal stem cells. Cell Biol Int. 2013;37(2):137-48. [PMID: 23319413 DOI: 10.1002/cbin.10024]
- Zeng Y, Li B, Li T, Liu W, Ran C, et al. CD90low MSCs modulate intratumoral immunity to confer antitumor activity in a mouse model of ovarian cancer. Oncotarget 2019;10(43):4479. [PMID: 31320999 DOI: 10.18632/oncotarget.27065]
- Li Y, Zhang D, Xu L, Dong L, Zheng J, et al. Cell-cell contact with proinflammatory macrophages enhances the immunotherapeutic effect of mesenchymal stem cells in two abortion models. Cell Mol Immunol 2019;16(12):908-20. [PMID: 30778166 DOI: 10.1038/s41423-019-0204-6]
- Sheng H, Wang Y, Jin Y, Zhang Q, Zhang Y, et al. A critical role of IFNγ in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res 2008;18(8):846-57. [PMID: 18607390 DOI: 10.1038/cr.2008.80]
- Franquesa M, Mensah FK, Huizinga R, Strini T, Boon L, et al. Human adipose tissue-derived mesenchymal stem cells abrogate plasmablast formation and induce regulatory B cells independently of T helper cells. Stem cells 2015;33(3):880-91. [PMID: 25376628 DOI: 10.1002/stem.1881]
- Healy ME, Bergin R, Mahon BP, English K. Mesenchymal stromal cells protect against caspase 3-mediated apoptosis of CD19(+) peripheral B cells through contact-dependent upregulation of VEGF. Stem Cells Dev 2015;24(20):2391-402. [PMID: 26076727 DOI: 10.1089/scd.2015.0089]
- Tabera S, Pérez-Simón JA, Díez-Campelo M, Sánchez-Abarca LI, Blanco B, et al. The effect of mesenchymal stem cells on the viability, proliferation and differentiation of B-lymphocytes. Haematologica 2008;93(9):1301-9. [PMID: 18641017 DOI: 10.3324/haematol.12857]
- Hu C-HD, Kosaka Y, Marcus P, Rashedi I, Keating A. Differential immunomodulatory effects of human bone marrow-derived mesenchymal stromal cells on natural killer cells. Stem Cells Dev 2019;28(14):933-43. [PMID: 31122145 DOI: 10.1089/scd.2019.0059]
- Manferdini C, Paolella F, Gabusi E, Gambari L, Piacentini A, et al. Adipose stromal cells mediated switching of the pro-inflammatory profile of M1-like macrophages is facilitated by PGE2: in vitro evaluation. Osteoarthritis Cartilage 2017;25(7):1161-71. [PMID: 28153787 DOI: 10.1016/j.joca.2017.01.011]
- Zhou Y, Yamamoto Y, Xiao Z, Ochiya T. The immunomodulatory functions of mesenchymal stromal/stem cells mediated via paracrine activity. J Clin Med 2019;8(7):1025. [PMID: 31336889 DOI: 10.3390/jcm8071025]
- Ferreira JR, Teixeira GQ, Santos SG, Barbosa MA, Almeida-Porada G, et al. Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Front Immunol 2019;9:2837. [PMID: 30564236 DOI: 10.3389/fimmu.2018.02837]
- Mardpour S, Hamidieh AA, Taleahmad S, Sharifzad F, Taghikhani A, et al. Interaction between mesenchymal stromal cell-derived extracellular vesicles and immune cells by distinct protein content. J Cell Physiol 2019;234(6):8249-58. [PMID: 30378105 DOI: 10.1002/jcp.27669]
- Wang W-B, Yen ML, Liu KJ, Hsu PJ, Lin M-H, et al. Interleukin-25 mediates transcriptional control of PD-L1 via STAT3 in multipotent human mesenchymal stromal cells (hMSCs) to suppress Th17 responses. Stem Cell Rep 2015;5(3):392-404. [PMID: 26321145 DOI: 10.1016/j.stemcr.2015.07.013]
- Ge W, Jiang J, Arp J, Liu W, Garcia B, et al. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation 2010;90(12):1312-20. [PMID: 21042238 DOI: 10.1097/TP.0b013e3181fed001]
- Wen D, Peng Y, Liu D, Weizmann Y, Mahato RI. Mesenchymal stem cell and derived exosome as small RNA carrier and immunomodulator to improve islet transplantation. J Controlled Release 2016;238:166-75. [PMID: 27475298 DOI: 10.1016/j.jconrel.2016.07.044]
- Davies LC, Heldring N, Kadri N, Le Blanc K. Mesenchymal stromal cell secretion of programmed death-1 ligands regulates T cell mediated immunosuppression. Stem Cells 2017;35(3):766-76. [PMID: 27671847 DOI: 10.1002/stem.2509]
- Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 2006;107(4):1484-90. [PMID: 16239427 DOI: 10.1182/blood-2005-07-2775]
- Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, et al. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 2008;111(3):1327-33. [PMID: 17951526 DOI: 10.1182/blood-2007-02-074997]
- Thomas H, Jäger M, Mauel K, Brandau S, Lask S, et al. Interaction with mesenchymal stem cells provokes natural killer cells for enhanced IL-12/IL-18-induced interferon-gamma secretion. Mediat Inflamm 2014;2014:143463. [PMID: 24876666 DOI: 10.1155/2014/143463]
- Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, et al. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells 2008;26(1):151-62. [PMID: 17932421 DOI: 10.1634/stemcells.2007-0416]
- Mahmoudi M, Taghavi-Farahabadi M, Rezaei N, Hashemi SM. Comparison of the effects of adipose tissue mesenchymal stromal cell-derived exosomes with conditioned media on neutrophil function and apoptosis. Int Immunopharmacol 2019;74:105689. [PMID: 31207404 DOI: 10.1016/j.intimp.2019.105689]
- Brandau S, Jakob M, Bruderek K, Bootz F, Giebel B, et al. Mesenchymal stem cells augment the anti-bacterial activity of neutrophil granulocytes. PLoS One 2014;9(9):e106903. [PMID: 25238158 DOI: 10.1371/journal.pone.0106903]
- Sicco CL, Reverberi D, Balbi C, Ulivi V, Principi E, et al. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: endorsement of macrophage polarization. Stem Cells Trans Med 2017;6(3):1018-28. [PMID: 28186708 DOI: 10.1002/sctm.16-0363]
- Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 2009;15(1):42. [PMID: 19098906 DOI: 10.1038/nm.1905]
- Abumaree M, Al Jumah MA, Kalionis B, Jawdat D, Al Khaldi A, et al. Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell Rev Rep 2013;9(5):620-41. [PMID: 23812784 DOI: 10.1007/s12015-013-9455-2]
- Rahman MM, Islam MR, Islam MT, Harun-Or-Rashid M, Islam M, et al. Stem cell transplantation therapy and neurological disorders: current status and future perspectives. Biology 2022;11(1):147. [PMID: 35053145 DOI: 10.3390/biology11010147]
- Biehl JK, Russell B. Introduction to stem cell therapy. J Cardiovasc Nurs 2009;24:98-103(quiz 4-5). [PMID: 19242274 DOI: 10.1097/JCN.0b013e318197a6a5]
- Armstrong L, Collin J, Mostafa I, Queen R, Figueiredo FC, et al. The eye of the storm: SARS-CoV-2 infection and replication at the ocular surface? Stem Cells Transl Med 2021;10:976-86. [PMID: 33710758 DOI: 10.1002/sctm.20-0543]
- Ramos L, Sánchez-Abarca T, Muntión LI, Preciado S, Puig S, et al. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun Signal 2016;14:2. [PMID: 26754424 DOI: 10.1186/s12964-015-0124-8]
- Piras F, Kajaste-Rudnitski A. Antiviral immunity and nucleic acid sensing in haematopoietic stem cell gene engineering. Gene Ther 2021;28:16-28. [PMID: 32661282 DOI: 10.1038/s41434-020-0175-3]
- Rocha JLM, de Oliveira WCF, Noronha NC, Dos Santos NCD, Covas DT, et al. Mesenchymal stromal cells in viral infections: implications for COVID-19. Stem Cell Rev Rep 2021;17:71-93. [PMID: 32895900 DOI: 10.1007/s12015-020-10032-7]
- Shu L, Niu C, Li R, Huang T, Wang Y, et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther 2020;11:361. [PMID: 32811531 DOI: 10.1186/s13287-020-01875-5]
- Xu X, Jiang W, Chen L, Xu Z, Zhang Q, et al. Evaluation of the safety and efficacy of using human menstrual blood-derived mesenchymal stromal cells in treating severe and critically ill COVID-19 patients: an exploratory clinical trial. Clin Trans Med 2021;11:e297. [PMID: 33634996 DOI: 10.1002/ctm2.297]
- Shi L, Huang H, Lu X, Yan X, Jiang X, et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Trans Target Ther 2021;6:58. [PMID: 33568628 DOI: 10.1038/s41392-021-00488-5]
- Lanzoni G, Linetsky E, Correa D, Messinger Cayetano S, Alvarez RA, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med 2021;10:660-73. [PMID: 33400390 DOI: 10.1002/sctm.20-0472]
- Hashemian SR, Aliannejad R, Zarrabi M, Soleimani M, Vosough M, et al. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. Stem Cell Res Ther 2021;12:91. [PMID: 33514427 DOI: 10.1186/s13287-021-02165-4]
- Osterholm MT, Moore KA, Kelley NS, Brosseau LM, Wong G, et al. Transmission of Ebola viruses: what we know and what we do not know. mBio 2015;6:e00137. [PMID: 26199336 DOI: 10.1128/mBio.01154-15]
- Neumann G, Chen H, Gao GF, Shu Y, Kawaoka Y. H5N1 influenza viruses: outbreaks and biological properties. Cell Res 2010;20:51-61. [PMID: 19884910 DOI: 10.1038/cr.2009.124]
- Flerlage T, Boyd DF, Meliopoulos V, Thomas PG, Schultz-Cherry S. Influenza virus and SARS-CoV-2: pathogenesis and host responses in the respiratory tract. Nat Rev Microbiol 2021;19:425-41. [PMID: 33824495 DOI: 10.1038/s41579-021-00542-7]
- Yudhawati R, Amin M, Rantam FA, Prasetya RR, Dewantari JR, et al. Bone marrow-derived mesenchymal stem cells attenuate pulmonary inflammation and lung damage caused by highly pathogenic avian influenza A/H5N1 virus in BALB/c mice. BMC Infect Dis 2020;20(1):823. [PMID: 33176722 DOI: 10.1186/s12879-020-05525-2]
- Chan MC, Kuok DI, Leung CY, Hui KP, Valkenburg SA, et al. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc Natl Acad Sci U S A 2016;113:3621-26. [PMID: 26976597 DOI: 10.1073/pnas.1601911113]
- Malcherek G, Jin N, Hückelhoven AG, Mani J, Wang L, et al. Mesenchymal stromal cells inhibit proliferation of virus-specific CD8(+) T cells. Leukemia 2014;28:2388-94. [PMID: 25227910 DOI: 10.1038/leu.2014.273]
- Guo BC, Wu KH, Chen CY, Lin WY, Chang YJ, et al. Mesenchymal stem cells in the treatment of COVID-19. Int J Mol Sci 2023;24(19):14800. [PMID: 37834246 DOI: 10.3390/ijms241914800]
- Clinicaltrials.com. (Accessed on 13 July 2024); Available online: https://classic.clinicaltrials.gov/ct2/results?cond=COVID19&term=mesenchymal+stem+Cell+&cntry=&state=&city=&dist=
- Karyana M, Djaharuddin I, Rif’ati L, Arif M, Choi MK, et al. Safety of DW-MSC infusion in patients with low clinical risk COVID-19 infection: a randomized, double-blind, placebo-controlled trial. Stem Cell Res Ther 2022;13(1):134. [PMID: 35365239 DOI: 10.1186/s13287-022-02812-4]
- Monsel A, Hauw-Berlemont C, Mebarki M, Heming N, Mayaux J, et al. Treatment of COVID-19-associated ARDS with mesenchymal stromal cells: a multicenter randomized double-blind trial. Crit Care 2022;26(1):48. [PMID: 35189925 DOI: 10.1186/s13054-022-03930-4]
- Kaffash Farkhad N, Sedaghat A, Reihani H, Adhami Moghadam A, Bagheri Moghadam A, et al. Mesenchymal stromal cell therapy for COVID-19-induced ARDS patients: a successful phase 1, control-placebo group, clinical trial. Stem Cell Res Ther 2022;13(1):283. [PMID: 35765103 DOI: 10.1186/s13287-022-02920-1]
- Grégoire C, Layios N, Lambermont B, Lechanteur C, Briquet A, et al. Bone marrow-derived mesenchymal stromal cell therapy in severe COVID-19: preliminary results of a phase I/II clinical trial. Front Immunol 2022;13:932360. [PMID: 35860245 DOI: 10.3389/fimmu.2022.932360]
- Dilogo IH, Aditianingsih D, Sugiarto A, Burhan E, Damayanti T, et al. Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: a randomized controlled trial. Stem Cells Trans Med 2021;10(9):1279-87. [PMID: 34102020 DOI: 10.1002/sctm.21-0046]
- Fathi-Kazerooni M, Fattah-Ghazi S, Darzi M, Makarem J, Nasiri R, et al. Safety and efficacy study of allogeneic human menstrual blood stromal cells secretome to treat severe COVID-19 patients: clinical trial phase I & II. Stem Cell Res Ther 2022;13(1):96. [PMID: 35255966 DOI: 10.1186/s13287-022-02771-w]
- Meng F, Xu R, Wang S, Xu Z, Zhang C, et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Sig Trans Targeted Ther 2020;5(1):172. [PMID: 32855385 DOI: 10.1038/s41392-020-00286-5]
- Sadeghi B, Roshandel E, Pirsalehi A, Kazemi S, Sankanian G, et al. Conquering the cytokine storm in COVID-19-induced ARDS using placenta-derived decidua stromal cells. J Cell Mol Med 2021;25(22):10554-64. [PMID: 34632708 DOI: 10.1111/jcmm.16986]
- Zhu YG, Shi MM, Monsel A, Dai CX, Dong X. Nebulized exosomes derived from allogenic adipose tissue mesenchymal stromal cells in patients with severe COVID-19: a pilot study. Stem Cell Res Ther 2022;13(1):220. [PMID: 35619189 DOI: 10.1186/s13287-022-02900-5]
- Al-Shudifat AE, Al-Radaideh A, Hammad S, Hijjawi N, Abu-Baker S, et al. Association of lung CT findings in coronavirus disease 2019(COVID-19) with patients’ age, body weight, vital signs, and medical regimen. Front Med 2022;9:912752. [PMID: 35847782 DOI: 10.3389/fmed.2022.912752]
- Rebelatto CLK, Senegaglia AC, Franck CL, Daga DR, Shigunov P, et al. Safety and long-term improvement of mesenchymal stromal cell infusion in critically COVID-19 patients: a randomized clinical trial. Stem Cell Res Ther 2022;13(1):122. [PMID: 35313959 DOI: 10.1186/s13287-022-02796-1]
- Du J, Li H, Lian J, Zhu X, Qiao L, et al. Stem cell therapy: a potential approach for treatment of influenza virus and coronavirus-induced acute lung injury. Stem Cell Res Ther 2020;11(1):192. [PMID: 32448377 DOI: 10.1186/s13287-020-01699-3]
- Darwish I, Banner D, Mubareka S, Kim H, Besla R, et al. Mesenchymal stromal (stem) cell therapy fails to improve outcomes in experimental severe influenza. PLoS ONE, 2013;8(8):e71761. [PMID: 23967240 DOI: 10.1371/journal.pone.0071761]
- Gotts JE, Abbott J, Matthay MA. Influenza causes prolonged disruption of the alveolar-capillary barrier in mice unresponsive to mesenchymal stem cell therapy. Am J Physiol Lung Cell Mol Physiol 2014;307(5):L395-L406. [PMID: 25038188 DOI: 10.1152/ajplung.00110.2014]
- Martin J, Helm K, Ruegg P, Varella-Garcia M, Burnham E, et al. Adult lung side population cells have mesenchymal stem cell potential. Cytotherapy 2008;10(2):140-51. [PMID: 18368593 DOI: 10.1080/14653240801895296]
- Jun D, Garat C, West J, Thorn N, Chow K, et al. The pathology of bleomycin-induced fibrosis is associated with loss of resident lung mesenchymal stem cells that regulate effector T-cell proliferation. Stem Cells 2011;29(4):725-35. [PMID: 21312316 DOI: 10.1002/stem.604]
- Foronjy RF, Majka SM. The potential for resident lung mesenchymal stem cells to promote functional tissue regeneration: understanding microenvironmental cues. Cells 2012;1(4):874. [PMID: 23626909 DOI: 10.3390/cells1040874]
- Ye K, He D, Shao Y, Xu N, Jin C, et al. Exogenous mesenchymal stem cells affect the function of endogenous lung stem cells (club cells) in phosgene-induced lung injury. Biochem Biophys Res Commun 2019;514(3):586-92. [PMID: 31064653 DOI: 10.1016/j.bbrc.2019.04.182]
- Islam MN, Das SR, Emin MT, Wei M, Sun L, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med 2012;18(5):759-65. [PMID: 22504485 DOI: 10.1038/nm.2736]
- Ancans J. Cell therapy medicinal product regulatory framework in Europe and its application for MSC-based therapy development. Front Immunol 2012;3:253. [PMID: 22912639 DOI: 10.3389/fimmu.2012.00253]
- Prockop DJ, Oh JY. Medical therapies with adult stem/progenitor cells (MSCs): a backward journey from dramatic results in vivo to the cellular and molecular explanations. J Cell Biochem 2012;113(5):1460-69. [PMID: 22213121 DOI: 10.1002/jcb.24046]
- Wang S, Qu X, Zhao RC. Clinical applications of mesenchymal stem cells. J Hematol Oncol 2012;5:19. [PMID: 22546280 DOI: 10.1186/1756-8722-5-19]
- Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 2012;10:244-58. [PMID: 22385653 DOI: 10.1016/j.stem.2012.02.005]
- Olson SD, Pollock K, Kambal A, Cary W, Mitchell GM, et al. Genetically engineered mesenchymal stem cells as a proposed therapeutic for Huntington’s disease. Mol Neurobiol 2012;45:87-8. [PMID: 22161544 DOI: 10.1007/s12035-011-8219-8]
- Lukomska B, Stanaszek L, Zuba-Surma E, Legosz P, Sarzynska S, et al. Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells Int 2019:9628536. [PMID: 31093291 DOI: 10.1155/2019/9628536]
- Ning H, Yang F, Jiang M, Hu L, Feng K, et al. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia 2008;22:593-99. [PMID: 18185520 DOI: 10.1038/sj.leu.2405090]
- Thanunchai M, Hongeng S, Thitithanyanont A. Mesenchymal stromal cells and viral infection. Stem Cells Int 2015;2015:860950. [PMID: 26294919 DOI: 10.1155/2015/860950]
- Djouad F, Plence P, Bony C, Tropel P, Apparailly F, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 2003;102:3837-44. [PMID: 12881305 DOI: 10.1182/blood-2003-04-1193]
- Yasamineh S, Kalajahi HG, Yasamineh P, Gholizadeh O, Youshanlouei HR, et al. Spotlight on therapeutic efficiency of mesenchymal stem cells in viral infections with a focus on COVID-19. Stem Cell Res Ther 2022;13:257. [PMID: 35715852 DOI: 10.1186/s13287-022-02944-7]
- Basiri A, Mansouri F, Azari A, Ranjbarvan P, Zarein F, et al. Stem cell therapy potency in personalizing severe COVID-19 treatment. Stem Cell Rev Rep 2021;17(1):193-213. [PMID: 33511518 DOI: 10.1007/s12015-020-10110-w]
- Mallis P, Michalopoulos E, Chatzistamatiou T, Stavropoulos-Giokas C. Mesenchymal stromal cells as potential immunomodulatory players in severe acute respiratory distress syndrome induced by SARS-CoV-2 infection. World J Stem Cells 2020;12(8):731-51. [PMID: 32952855 DOI: 10.4252/wjsc.v12.i8.731]
- Jamshidi E, Babajani A, Soltani P, Niknejad H. Proposed mechanisms of targeting COVID-19 by delivering mesenchymal stem cells and their exosomes to damaged organs. Stem Cell Rev Rep 2021;17(1):176-92. [PMID: 33432484 DOI: 10.1007/s12015-020-10109-3]