Non-Invasive Physical Stimulation to Modulate the Tumor Microenvironment: Unveiling a New Frontier in Cancer Therapy
1Key Laboratory of Medical Imaging Precision Theranostics and Radiation Protection, University of South China, College of Hunan Province, Changsha, Hunan, China
2Institute of Medical Imaging, Hengyang Medical School, University of South China, Hengyang, Hunan, China
3The Seventh Affiliated Hospital, Hunan Veterans Administration Hospital, Hengyang Medical School, University of South China, Changsha, Hunan, China
4Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Guangdong-Hong Kong Joint Laboratory for RNA Medicine, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China
5Department of Biological Sciences, KAIST Institute for the BioCentury, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, Republic of Korea
*Correspondence to: Sangyong Jon, Department of Biological Sciences, KAIST Institute for the BioCentury, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, Republic of Korea. E-mail: syjon@kaist.ac.kr
Received: 31 March 2024; Revised: 16 April 2024; Accepted: 13 May 2024; Published Online: 17 July 2024
Cite this paper:
Guo Z, Saw PE, Jon S. Non-Invasive Physical Stimulation to Modulate the Tumor Microenvironment: Unveiling a New Frontier in Cancer Therapy. BIO Integration 2024; 5: 1–14.
DOI: 10.15212/bioi-2024-0012. Available at: https://bio-integration.org/
Download citation
© 2024 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
The tumor microenvironment (TME) has a crucial role in tumor development, metastasis, and recurrence. The chaotic and complex physical structure of the TME not only limits drug delivery but also contributes to the development of resistance to immunotherapy. Breaking the physical barrier limitation of the TME could further optimize the existing tumor treatment protocols. Physical stimulation, such as ionizing radiation, light, electricity, magnetic field, and ultrasound, modulate the TME by altering tumor vasculature, remodeling the extracellular matrix, and activating immune responses to achieve the goal of adjuvant to other tumor therapeutic approaches. In addition to adjuvant chemotherapy and immunotherapy, these physical stimulations also enhance the efficacy of other physical treatments for cancer. In this review we discuss the structural characteristics of TME and focus on the modulation of TME by different physical stimulations. We also analyze the adjuvant effects of these stimulations on other tumor therapies.
Keywords
Adjunctive approach, drug delivery, extracellular matrix, immune responses, immunotherapy, tumor vasculature.
Introduction
The tumor microenvironment (TME) is a crucial factor that influences the survival, invasion, and metastasis of tumor cells. The TME consists of various components, including immune cells, stromal cells, blood vessels, and the extracellular matrix [ECM] [1]. The TME has a significant role in promoting cancer growth, metastasis, and resistance to treatment [2]. The dense ECM structure of the tumor tissue, along with the compressive stress on blood vessels, lymphatic vessels, and other tissues, impede drug delivery [3, 4]. Additionally, the biophysical structure of the TME can hinder immunotherapy-related antibodies or activate downstream signaling through mechano-transduction, leading to upregulation of programmed cell death ligand 1 (PD-L1) expression and anti-apoptotic molecules, the massive secretion of immunosuppressive factors, and abnormal function of anti-tumor immune cells. These effects act synergistically to result in resistance to immunotherapy [5].
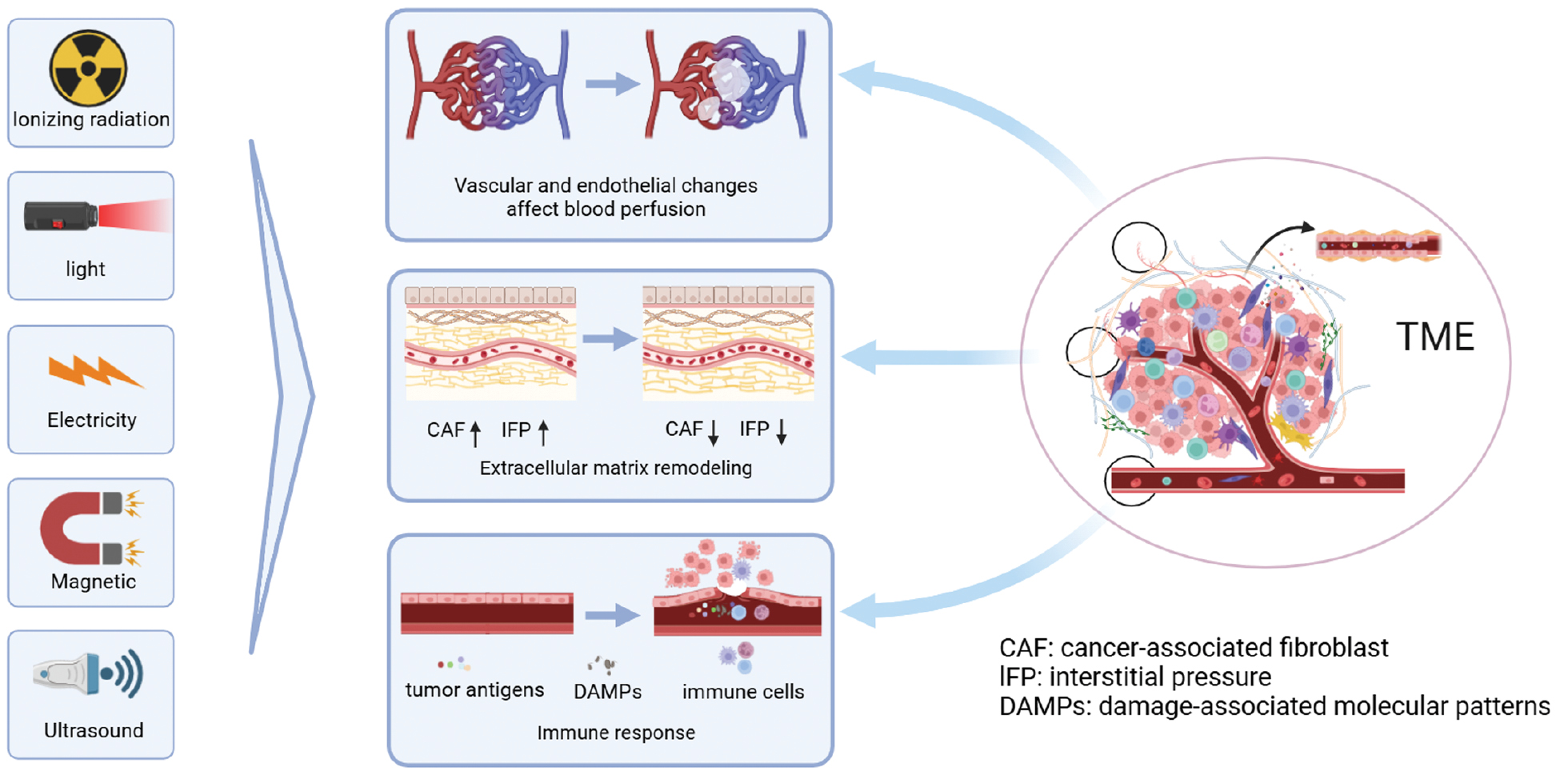
In conventional tumor treatment regimens, chemotherapeutic agents have low specificity [6, 7]. As a systemic therapy, the higher the dose of the chemotherapeutic drug, the greater the side effects [6, 7]. Physical stimulation, such as ionizing radiation, light, electricity, magnetic field, and ultrasound [8, 9], offer significant advantages in the treatment of TME limitations. Specifically, radiation or heat can break the physical barrier of the TME, which makes it easier for drugs to accumulate at the tumor site by modulating the structure of the TME and lowering the interstitial fluid pressure (IFP), thus reducing serious side effects and improving the efficacy of treatment [10]. In addition, emerging tumor treatments, such as cancer immunotherapy (CIT), have limited effects due to immune tolerance associated with the TME. Currently, the most successful and widely used immune checkpoint in clinical practice is PD-L1/programmed cell death protein 1 (PD-1). However, PD-L1/PD-1 monotherapy only has an effective rate of 10%–40% due to primary resistance [5]. In contrast, combining immunotherapy with physical stimulation therapies, such as radiation, photodynamic therapy (PDT), photothermal therapy (PTT), and sonodynamic therapy (SDT), significantly improves the efficacy of immunotherapy [11]. All of the combined therapeutic approaches demonstrate the advantages of physical stimulation in modulating the TME for drug delivery and adjuvant tumor therapy and show the future promise of the multidisciplinary intersection in medicine. In this review we explore the structural features of the TME and focus on the modulatory effects of different physical stimulations on the characteristic structures of the TME, such as blood vessels, the ECM, and immune cells (Figure 1). The adjuvant effects of these stimulations on other tumor therapies are also analyzed.
Figure 1 A schematic diagram of the tumor microenvironment (TME) modulation by physical stimulation. Under specific conditions, physical stimulation (ionizing radiation, light, electricity, magnetic field, and ultrasound) modulate the TME by altering the endothelium and vasculature to affect perfusion, reduce the interstitial fluid pressure (IFP), cause ECM remodeling, and activate immune responses to modulate the TME.
Characteristics of the TME
The TME refers to the internal physicochemical state that supports the survival of cancer cells. The TME encompasses various components, including blood vessels, the ECM, immune cells, stromal cells (e.g., fibroblasts), lymphatic vessels, soluble cytokines, mediators, and other non-cellular elements, such as extracellular vesicles [12, 13]. The characteristics of the TME include low acidity [14], H2O2 accumulation [15], hypoxia [16], low catalase activity [17], high reducibility, and immunosuppression [18]. The organizational structure and metabolic changes within the TME interact with one another. From the perspective of the tumor vasculature, compared to normal blood vessels, the vascular network at the tumor site is irregular and disorganized. Tumor cells are metabolically active, continuously consuming oxygen and nutrients, and secreting large quantities of pro-angiogenic factors, which further contribute to abnormal blood vessel growth. Simultaneously, the abnormal tumor vasculature contributes to the hypoxic environment within the TME. The aberrant tumor vessels also contribute to increased IFP, which promotes tumor progression and immune resistance. The walls of these abnormal vessels contain a large number of endothelial cells, which serve as a significant source of cancer-associated fibroblasts (CAFs). Hypoxic conditions stimulate CAFs, leading to CAF dispersal and exacerbating physical stress on the tumor. As a result, blood and lymphatic vessels can be compressed, leading to reduced perfusion [19]. CAFs also secrete factors that impede T lymphocyte infiltration, reduce immune cell activity, and promote the accumulation of immunosuppressive cells, thereby suppressing anti-tumor immunity [20]. Furthermore, CAFs secrete factors that promote tumor angiogenesis [21]. In conclusion, the components of the TME interact with each other and collectively establish the tumor survival environment. Modulating tumor vascular changes and remodeling the ECM through physical stimulation create favorable conditions for drug and nanoparticle delivery. Moreover, physical stimulation of the TME activates the immune response, laying the foundation for combining relevant tumor therapy with immunotherapy in a physically stimulated approach.
Application of physical stimulation to the TME
The abnormal tumor vasculature within the TME has a crucial role, displaying structural and functional abnormalities that result in hypoxia, acidity, elevated IFP, and increased permeability. These factors not only sustain the TME but also facilitate tumor cell invasion, metastasis, and immunosuppression, while hindering drug delivery [19]. Physical stimulation can be used to target the tumor vasculature and address these issues. For example, radiotherapy has the potential to normalize the tumor vasculature at specific dosage levels [22]. CAFs present within the ECM have a role in remodeling the ECM, which creates a supportive microenvironment for cancer cells and acts as a physical barrier that hinders drug penetration. Moreover, CAFs secrete matrix metalloproteinases (MMPs) that interfere with ECM degradation, which initiates cancer cell migration and invasion [12]. Innovative approaches, such as physical stimulation using nanoparticles that target CAFs and employing a low-frequency rotating magnetic field (RMF) with torque, have shown promising results in disrupting CAFs [23]. The remarkable efficacy of immune checkpoint inhibitors (ICIs) and chimeric antigen receptor T (CAR-T) cell therapy in solid tumors has prompted researchers to investigate the role of immune cells within the TME. Over the past decade, significant progress has been made in understanding the role of immune cells in the TME and developing strategies for immune cell-based tumor therapy [12]. PDT and PTT have been demonstrated to reprogram tumor-associated macrophages (TAMs), inducing activation of innate immunity by upregulating M1 macrophages and downregulating M2 macrophages [24]. The vasculature, ECM, and immune responses within the TME are modulated by several types of physical stimulations (ionizing radiation, light, electricity, magnetic field, and ultrasound; Table 1).
Table 1 Summary of the Effects of Different Physical Stimulations on the Vasculature, ECM, and Immune Responses
| Physical Stimulation | Effects on the TME | ||
|---|---|---|---|
| Vasculature | ECM | Immune Responses | |
| Ionizing radiation | Normalization, dilation, and collapse of tumor vasculature [22] | Matrix protein hydrolysis [34–37] | Induce ICD and reprogram TAMs [42, 43] |
| Light | Vasoconstriction/diastole and vascular collapse [56] | Denaturation of collagen [72] | Induce ICD and reprogram TAMs [24] |
| Electricity | Endothelial damage, vascular destruction, or vascular normalization [86, 87] | Activation of MMPs, a decrease in collagen production [89] | Induce ICD and re-program TAMs [90, 91] |
| Magnetic field | Inhibits tumor vasculature and promotes normal angiogenesis [96–98] | Collagen denaturation, CAFs death [23, 100] | Induce ICD and re-program TAMs [104, 105] |
| Ultrasound | Endothelial damage, vasodilation/constriction, vascular disruption, or generation [117, 121] | Decreased collagen content and decreased IFP by ultrasound combined with MBs [126, 127] | Induce ICD and re-program TAMs [130, 131] |
Abbreviations: MMPs: metalloproteinases; CAFs: cancer-associated fibroblasts; IFP: interstitial fluid pressure; ICD: immunogenic cell death; MBs: microbubbles; TAMs: tumor-associated macrophages.
Ionizing radiation
Radiotherapy is a crucial component of cancer treatment, with >50% of cancer patients undergoing at least one session of radiotherapy during treatment. Radiotherapy utilizes high-energy ionizing radiation, such as γ-, β-, and X-rays, to target and damage tumor cells. The primary objective of radiotherapy is to induce DNA damage directly in tumor cells or indirectly generate reactive oxygen species (ROS) by interacting with water molecules, which leads to the elimination of tumor cells [25].
Vasculature alteration
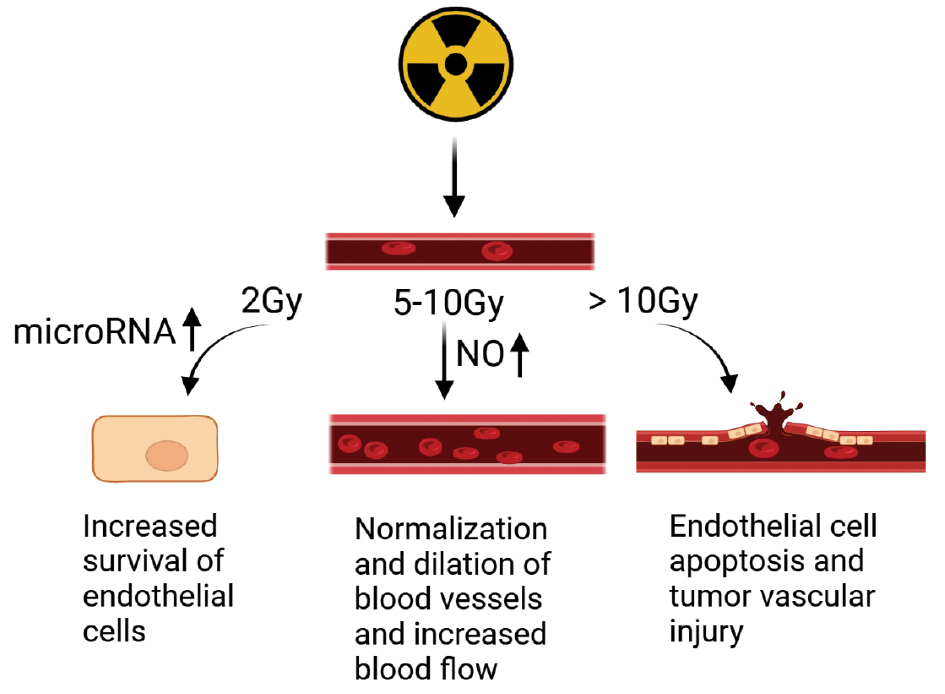
The TME exhibits distinct physical characteristics, including ECM structure and stiffness, solid stress, the IFP, and vascular shear stress [5]. These factors contribute to tumor progression and resistance to immunotherapy through various mechanisms. X-ray radiation remodels the stroma and alters tumor-associated blood flow, while also reducing the IFP by impacting tumor cell morphology, intra-tumoral microvasculature, and mesenchyme, thereby leading to vascular decompression and improved perfusion [26]. Pre-existing alterations in the tumor vasculature often hinder T cell infiltration into the TME due to the endothelial barrier. However, radiation induces increased expression of adhesion molecules, such as vascular cell adhesion molecule (VCAM)-1 and intercellular adhesion molecule (ICAM)-1 [27], which promotes activation of endothelial-type nitric oxide synthase (eNOS) and nitric oxide (NO) production [28]. This process induces angiogenesis, enhances tumor blood flow [29], and facilitates T cell homing by upregulating E- and P-selectin expression on endothelial cells [30]. The effects of radiation on endothelial cells and blood vessels are dose-dependent (Figure 2).
Figure 2 Effects of different radiation doses on tumor vasculature and endothelium. At a radiation dose of 2 Gy, microRNA upregulation increased endothelial cell survival [136], normalization and dilation of the tumor vasculature and increased perfusion were observed at radiation doses of 5-10 Gy [22], and apoptosis of endothelial cells and damage to the tumor vasculature were induced at radiation doses >10 Gy [22].
Extracellular matrix remodeling
It is generally believed that CAFs actively promote cancer invasiveness by modulating various processes, including angiogenesis, inflammation, and ECM remodeling [31, 32]. CAFs do not undergo apoptosis at a radiation dose of 30 Gray (Gy), but senescence occurs when the radiation dose exceeds 10-12 Gy [33]. Radiation can affect ECM remodeling in tumors by modulating protease activity. Radiation has the potential to induce hydrolysis of matrix proteins within the ECM, releasing stored active molecules, such as angiogenic factors, growth factors, and active matrix components. Tumor cell protease activity is altered after radiation with MMP-2 expression upregulated in different types of tumors, such as lung cancer [34], pancreatic cancer [35], colorectal cancer [36], and glioblastomas [37], potentially leading to increased tumor invasion. Inhibition of MMP-2 before radiation enhances the sensitivity of lung tumor cells to radiation therapy. Furthermore, radiation affects other proteases, such as MMP-9, which undergoes altered expression and activity in hepatocellular carcinoma cells through the PI3K/Akt/NF-κB cascade [38]. In non-small-cell lung cancer cells, a radiation dose of 2 Gy activates the SDF-1/CXCR-4 pathway, resulting in increased invasiveness through the PI3K/Akt and MAPK pathways, leading to MMP expression both in vitro and in vivo [39]. However, when administered at ablative doses, radiation upregulates MMP-3 and downregulates MMP-1, inducing premature senescence of CAFs and inhibiting proliferation, migration, and invasive capabilities [33]. Additionally, radiation-induced ECM remodeling involves lysyl oxidase (LOX), which enhances the soluble deposition and tensile strength of the ECM and correlated with tumor metastasis and invasion [40].
Overall, radiation may affect the invasive and metastatic capacity of tumors by modulating protease activity and ECM remodeling. Some differences exist as a function of tumor type and microenvironmental conditions, so further investigation is warranted to better understand the effects of radiation on the TME.
Immune response activation
Radiation can induce tumor-targeted immune responses, which are largely dependent on the antigenicity of tumor cells and the ability to generate adjuvant signals [41]. Specifically, radiation-induced immunogenic cell death (ICD) [42] contributes to immune cell dissemination. The effects of radiation dose on the TME are diverse. For example, low-dose radiation (2 Gy) stimulates TAMs of the M1 phenotype to produce inducible nitric oxide synthase (iNOS), which promotes the formation of an immunogenic TME and an immunogenic environment [43]. Conversely, higher radiation doses lead to pro-tumorigenic M2 phenotype TAM infiltration into the tumor [44]. Studies have indicated that a single radiation dose of 5-10 Gy results in mild vascular alterations within the TME. However, doses exceeding 10 Gy cause endothelial cell death, which leads to significant vascular damage and reduced blood flow. This compromised vasculature hinders the recruitment of effector T cells, resulting in decreased immune cell infiltration and potentially contributing to hypoxia within the TME [45]. High-dose daily fractionation (8 Gy × 2) has been reported to offer several advantages over low-dose daily fractionation (2 Gy × 10). High-dose daily fractionation preserves peripheral and tumor-infiltrating effector immune cells, down-regulates immune-suppressive cells, increases immune cell expression in the TME, and enhances tumor-specific immune responses [46]. Radiotherapy is promising for adjuvant immunotherapy. For example, Qu et al. [47] failed to achieve the desired therapeutic effect when treating rectal squamous cell carcinoma with high PD-L1 expression by chemotherapy and immunotherapy alone, but after adjuvant radiotherapy, the tumors achieved complete remission with a recurrence-free status within 12 months.
Light
Phototherapy is a tumor treatment modality that primarily involves two techniques (PDT and PTT). Compared to traditional cancer treatment options (radiotherapy and chemotherapy), phototherapy specifically targets desired cells or tissues, resulting in improved targeting and reduced side effects [48]. PDT is a non-invasive therapeutic approach that utilizes photosensitizers (PSs). These PS agents selectively accumulate in tumor tissues. When exposed to a specific wavelength of laser light, the PS is activated, leading to the generation of ROS by consuming molecular oxygen. This process causes DNA damage and ultimately leads to tumor cell death [49]. With respect to light dosimetry, the used impact rate in PDT is typically kept low (<200 mW/cm2) to prevent thermal damage to the tissue [50]. It is also important to note that the individual components of PDT are non-toxic and it is only when PS is irradiated with light that cytotoxic reactive oxygen species (ROS), such as mono-linear oxygen (1O2), superoxide anion (O2•−), and hydroxyl radical (•OH), are produced, which then cause cellular DNA damage [51].
Conventional PTT utilizes the heat generated by near-infrared materials (>50°C) to directly destroy tumor cells [52]. Compared to conventional treatments, PTT is less harmful to normal organs because PTT specifically localizes to tumor areas where PS accumulates and precisely applies laser irradiation. PTT is becoming a popular method for various diseases and cancers due to safety and precision [53]. However, the high temperatures of conventional PTT inevitably cause non-specific thermal damage to the surrounding healthy tissues. The necrosis induced by conventional PTT leads to severe local inflammation, further damage to healthy tissues, and even an increased risk of tumor metastasis [54]. Studies have also shown that conventional PTT suppresses host anti-tumor immunity by compromising immune antigens in the TME due to hyperthermia [55]. In contrast, mild PTT (mPTT [usually 42–45°C]) is a relatively low-temperature PTT method that offers advantages and features in terms of a modulating effect on the TME compared to conventional high-temperature PTT.
Vasculature alteration
PDT and PTT have dose-dependent effects on the vasculature. Early studies demonstrated that PDT causes multiple vascular effects, including altered vascular permeability, vasoconstriction/diastole, and vascular collapse [56]. In addition to direct damage to blood vessels, PDT also alters blood vessel permeability, which is crucial for systemic drug delivery. Studies have shown that when combined with medium doses of vascular-targeting agents, PDT significantly disrupts tumor perfusion and enhances drug delivery [57]. PDT has proven to be an effective treatment for a wide range of cancers, such as skin [58], head and neck [59], and superficial bladder cancers [60]. However, solid tumor hypoxia, which results from uncontrolled tumor growth and dysregulated angiogenesis, poses a challenge to PDT efficacy [61]. In addition, PDT leads to microvascular collapse, impeding O2 transport and exacerbating hypoxia in the TME, which further diminishes the effectiveness of PDT [62]. To address this issue, a commonly used approach is to design a variety of smart nanoplatforms based on the high expression of H2O2 in tumor cells. Under laser stimulation, nanomaterials, such as MnO2 [63], CaO2 [64], RuO2 [65], Fe3O4 [66], carbon dots [67], and biological catalase [68], react with H2O2 to produce O2, thereby alleviating hypoxia at the tumor site and improving the efficacy of PDT.
An early study [69] indicated that mild heat therapy enhances tumor blood flow and improves intravascular hemoglobin (Hb) oxygen saturation within the tumor. However, at higher temperatures, there is a transient increase in blood flow during the heating process, followed by the onset of vascular damage. The vascular damage leads to reduced tumor perfusion and oxygenation, decreased pH levels in the TME, and ultimately ischemia and cell death. Therefore, in addition to the development of nanoplatforms, mPTT also assists in improving the efficacy of PDT by increasing tissue oxygen saturation and ameliorating hypoxia in the TME.
In addition, the generation of ROS during PDT causes cellular DNA damage, whereas thermotherapy denature proteins involved in DNA repair [56] and thermotherapy has been shown to increase mitochondrial ROS levels [70], suggesting that PTT has a promising application in enhancing PDT.
Extracellular matrix remodeling
Solid tumors often exhibit increased ECM content, which contributes to elevated tissue stress and IFP. This finding, coupled with collapsed tumor blood vessels, can impede systemic drug delivery and compromise therapeutic efficacy [71]. In 1987 Barr et al. [72] conducted experimental studies in rats to determine the impact of PTT on the ECM. Barr et al. [72] used a 675-nm laser at 500 mW for 100 seconds on the rat colon, which resulted in an increase in temperature to 66 ± 7.5°C. Transmission electron microscopy revealed significant swelling and structural changes in submucosal collagen, indicating thermal damage and protein denaturation. In contrast, when the colon was treated with a hematoporphyrin derivative, PDT (100 mW power for 500 seconds), resulted in no ECM structural changes. This finding suggested that PDT preserves ECM structure (specifically, the submucosal collagen). Subsequent studies have also demonstrated that PTT with a near-infrared (NIR) laser or mild hyperthermia leads to collagen denaturation. Indeed, several studies have demonstrated that PTT or mild hyperthermia (43°C for 15 min) leads to collagen denaturation [73, 74]. Overall, these experimental findings indicate that with the assistance of some nanoparticles, PTT induces corresponding structural changes in the tumor ECM, including collagen reorganization. This facilitates more effective drug penetration into the tumor and contributes to an improved tumor treatment.
In 2023 Overchuk et al. [56] conducted a study on sub-therapeutic prostate-specific membrane antigen (PSMA)-targeted PDT (50 J/cm2) in subcutaneous prostate tumor xenografts. The findings revealed a 2-fold reduction in the total collagen density of the tumors compared to the previous study and electron microscopy showed a subendothelial region with reduced collagen coverage. Due to the precise therapeutic boundaries and non-thermal nature, PDT preserves ECM structures, which aids in healing and reducing scarring. Both PDT and PTT induce changes in the ECM of the tumor, which can be beneficial in the therapeutic regimen.
Immune response activation
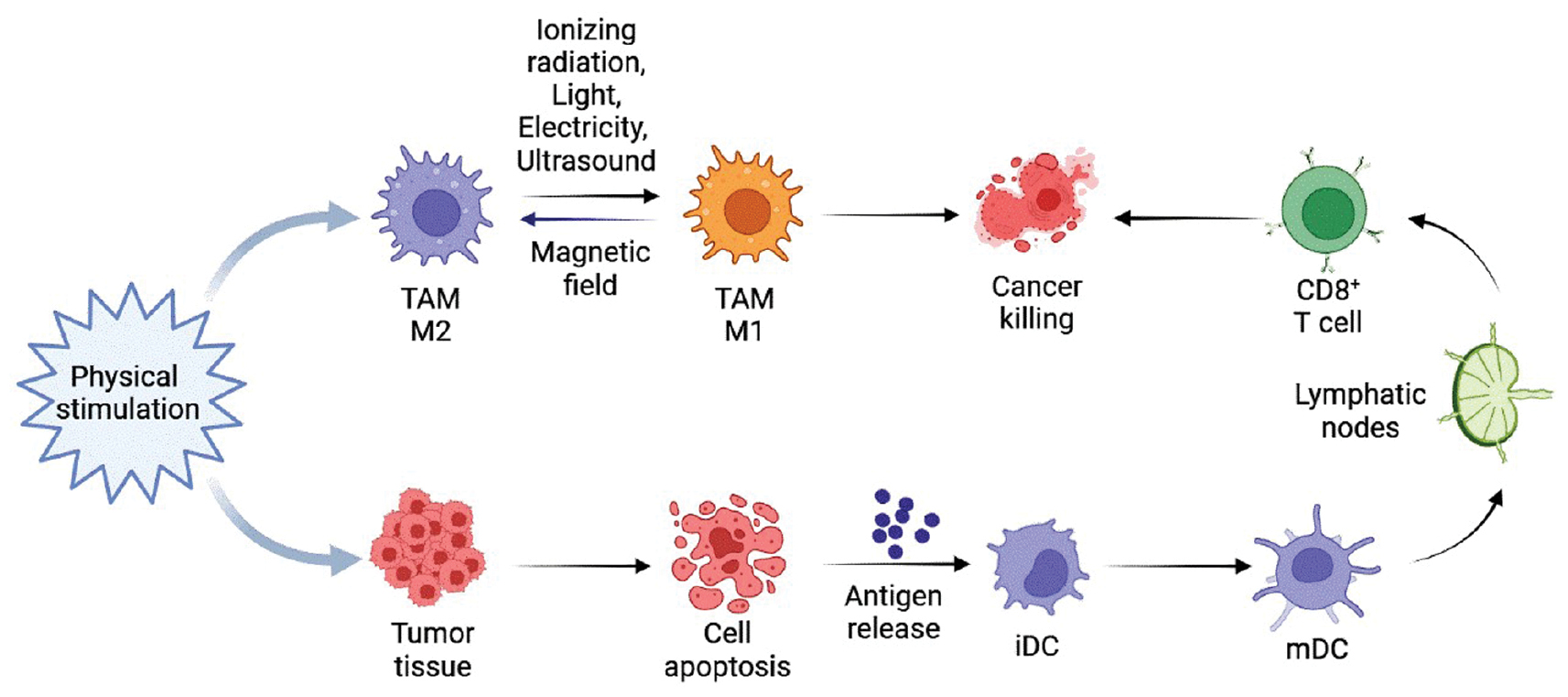
PDT and PTT elicit immune responses by releasing tumor-specific antigens (TSAs) and producing immune-modulating molecules, such as calreticulin (CRT), high-mobility group box 1 (HMGB1), ATP, and heat shock proteins (HSPs). These immune responses have a crucial role in targeting and eliminating tumor cells [75–77]. By inducing ICD, PDT and PTT lead to the release of inflammatory cytokines, such as IL-6, IL-1β, TNF-α, and chemokine C-X-C ligand 2 (CXCL2). This initial inflammation promotes the activation and recruitment of antigen-presenting cells (APCs), such as dendritic cells (DCs), which can enter local lymph nodes, take up and process TSAs, present TSAs to naive T cells, thereby activating long-term adaptive immunity [78]. PDT and PTT treatments induce ICD and reprogram TAMs, promoting innate immunity by upregulating M1 macrophages and downregulating M2 macrophages (Figure 3). This re-programming boosts anti-tumor immune responses and contributes to the therapeutic effects of PDT and PTT in cancer treatment [24]. However, unlike PDT, PTT may only induce ICD within a specific thermal window. For example, a study conducted by Sweeney et al. [79] demonstrated that PTT using Prussian blue nanoparticles (PBNPs) in neuroblastoma cell-induced ICD at the optimal thermal dose. The expression of ICD markers, such as the release of calreticulin, ATP, HMGB1, and CRT, is most evident on the cell surface when the cells are heated at temperatures ranging from 50–60°C for 10 min. Temperatures <50°C or >60°C were shown to be ineffective [75]. While some PDT regimens, particularly repeated PDT treatments, have shown the ability to activate adaptive immunity and induce effects in vitro alone [80], PDT and PTT typically require additional immune adjuvants or methods to enhance the immune response. Ghosh et al. [81] combined chemophototherapy and immunotherapy (a combination of PD-1 and anti-cytotoxic T-lymphocyte-associated protein 4 [CTLA-4] antibodies) to ablate medium-sized KPC pancreatic cancer tumors and induce memory immune responses. The results therein indicate that chemotherapy and immunotherapy alone cannot eliminate small KPC tumors. However, when used in combination, chemotherapy and immunotherapy can even clear medium-sized KPC tumors and protect against tumor re-attack. The enhanced synergy of these two treatments is facilitated by the penetration and accumulation of PDT-induced anti-PD-1 antibodies breaking through the physical barrier of the TME at the tumor site.
Figure 3 Diagram of the role of physical stimulation in the modulation of the tumor immune microenvironment. Except for magnetic fields, which polarize macrophages toward the M2 phenotype, other physical stimulations, such as ionizing radiation, light, electricity, and ultrasound, polarize macrophages toward the M1 phenotype. iCD: immature dendritic cell; mCD: mature dendritic cell.
Electricity
Electric fields can have various biological effects, including stimulating healing, causing direct tissue damage, or inducing cell death by disrupting cell mitosis. In the field of electric field applied oncology therapy, pulsed electric field (PEF)-based therapies include irreversible electroporation (IRE), gene electrotransfer (GET), electrochemistry (ECT), calcium electroporation (Ca-EP), and tumor-treating fields [TTF] [82]. Among these therapies, IRE is widely utilized for tumor ablation. IRE involves the application of short pulses of high-pressure electricity to create nanoscale perforations in cell membranes, subsequently inducing apoptosis [83]. In recent years, TTF has shown unique advantages in the treatment of glioblastomas, pleural mesotheliomas, and lung and pancreatic cancers [82]. Studies have indicated that the use of low-voltage, mid-frequency (100-500 kHz) electric fields, such as TTF, impede DNA repair, induce autophagy, promote anti-tumor immunity, inhibit tumor cell migration and invasion, and alter the permeability of cell membranes and the blood-brain barrier [84].
Vasculature alteration
It has been shown that PEFs inhibit angiogenesis in tumor tissues and suppress tumor angiogenesis, leading to a reduction in tumor growth [85]. In the study by B. Markelc et al, significant alterations in the morphology of endothelial cells were observed 1 h after application of an electric pulse. These cells became rounded and swollen, resulting in a constriction of the blood vessel lumen [86]. Other studies reported that ECT targets endothelial cells, triggering apoptosis and causing vascular destruction. Interestingly, electroporation exhibits greater efficacy in eliminating endothelial cells within small tumor vessels, while larger vessels appear to be more preserved [87]. Furthermore, electrical stimulation also breaks intracellular bioelectrical homeostasis, thereby promoting normalization of the tumor vasculature and facilitating drug delivery [88]. Li et al. [88] demonstrated that the combination of radio-stimulation and the chemotherapeutic agent, adriamycin, exhibits 1.8-fold higher anti-tumor efficacy compared to treatment with adriamycin alone.
Extracellular matrix remodeling
In 2022 Gouarderes et al. [89] demonstrated that PEFs induce remodeling of the ECM by activating MMPs and reducing collagen production. Regardless of the electrical stimulation protocol used in the experiments, the tissue collagen content was significantly reduced by 35%–50% after 1 week of electrical stimulation. PEFs promote collagen remodeling by transiently decreasing collagen production and increasing collagen degradation through sustained activation of MMPs by ROS. PEFs downregulate the expression of TGF-β, a major regulator of fibrosis, at the mRNA and protein levels. Furthermore, there was a substantial decrease in the gene expression of key enzymes involved in ECM cross-linking, such as lysyl oxidase (LOX) and transglutaminase.
Immune response activation
Electrical stimulation, like other physical stimulations, has the potential to directly eliminate tumor cells and elicit immune responses, making electrical stimulation a promising therapeutic approach for tumors [90]. Research has indicated that tumor cell death induced by electrical stimulation undergoes conversion from non-immunogenic to immunogenic, which is known as ICD. This process leads to the massive release of tumor antigens and damage-associated molecular patterns (DAMPs), attracting DCs to gather at the tumor site [91]. Furthermore, DCs are responsible for presenting tumor antigens to T cells, thus triggering an adaptive immune response [92]. Kong et al. [90] demonstrated that driving local charge release under ultrasound irradiation significantly enhances M1 polarization in macrophages. Additionally, electrical stimulation induces inflammation, prompts the release of pro-inflammatory cytokines and chemokines, and stimulates anti-tumor immunity. For example, Chen et al. [93] demonstrated that TTF generates pro-inflammatory cytokines and type I interferon.
Magnetic field
Magnetic fields are classified based on their characteristics and generation methods. With respect to characteristics, there are constant magnetic fields (CMFs) and dynamic magnetic fields (DMFs). Regarding the generation method, magnetic fields can be categorized as alternating magnetic fields (AMFs), geomagnetic fields (GMFs), pulsating magnetic fields (PuMFs), and pulsed magnetic fields [PMFs] [94]. Magnetothermal therapy (MHT) is a technique that utilizes the thermal effect of magnetic nanoparticles (MNPs) to treat tumors. In addition to the thermal effects, magnetic field exposure generates corresponding mechanical forces, including tension, compression, shear force, and torque. These forces significantly impact the cellular environment, including the plasma membrane and internal organelles (e.g., mitochondria, lysosomes, and nuclei). This impact results in noticeable morphologic changes and intracellular damage. Activation of apoptotic or non-apoptotic cellular signals subsequently induces tumor ablation [8].
Vasculature alteration
At the vascular level, sub-thermal therapy has been shown to increase permeability of the tumor vasculature and enhance blood flow through the tumor vasculature. Nanoparticle extravasation increases with rising temperatures from 40°C–42°C. However, temperatures >42°C lead to bleeding and stagnation of the tumor vasculature [95]. A static magnetic field (SMF) reduces blood flow and platelet adhesion in tumor microvessels, thereby inhibiting tumor angiogenesis in murine experiments [96]. DMFs have both generative and inhibitory effects on angiogenesis. The specific effects of DMFs on angiogenesis vary depending on various parameters, such as magnetic field strength and frequency. Hu et al. [97] conducted a study reporting a strong inhibitory effect of time-varying magnetic fields on tumor growth. The effect was more pronounced at weak magnetic fields (1-5 nT) compared to strong magnetic fields (2-5 mT). However, DMFs may promote angiogenesis in normal tissues and pulsed electromagnetic fields (PEMFs) with specific parameters have been shown to promote angiogenesis [98].
Extracellular matrix remodeling
Elevated temperatures have an impact on the ECM, and specifically on the structure of collagen [73, 99]. Magnetothermal therapy, similar to PTT, induces collagen denaturation and ECM remodeling. For example, when magnetic nanoparticles are positioned on top of collagen in the presence of AMF, the heat generated by the nanoparticles causes collagen to undergo phase change and melt [100]. Disruption of intracellular membranes has also been shown to effectively induce cell death [101]. Building upon this concept, Lopez et al. [23] found that nanoparticles specifically designed to target CAFs in pancreatic cancer are exposed to a low-frequency RMF, which resulted in interaction with lysosomal membranes. This interaction, facilitated by mechanical forces or activation of mechanosensitive ion channels on the lysosomal membranes, generated torque inside the lysosomes, effectively disrupting the lysosomal membranes and inducing death in CAFs.
Immune response activation
It has been shown that local heat therapy enhances anti-tumor immunity. Heat stress applied to tumor cells induces the release of HSPs that are recognized and activated by APCs [102]. This activation triggers the presentation of antigens to T cells, initiating adaptive immune responses [103]. In murine experiments, the use of magnetorheological fluid (MRF) or magnetotherapy to destroy primary tumors results in selective necrosis of malignant cells, while preserving tumor-infiltrating immune cells and inducing ICD. This in situ tumor injury activates DCs, recruiting DCs to the primary tumor site. The activated DCs then stimulate CD8+ T cells, leading to anti-tumor effects at the primary tumor site and distant sites [104]. It has also been shown that macrophages tend to be converted to a pro-tumor development M2 phenotype under high-gradient magnetic fields [105] [Figure 3].
Ultrasound
Ultrasound-related treatments have become widely used in the biomedical field due to safety, visualization capabilities, non-invasiveness, and relatively low cost of instruments [106]. Ultrasound waves are mechanical sound waves with frequencies higher than the human hearing range (16–20 kHz) that are capable of penetrating tissues up to a depth of approximately 10 cm [107]. Ultrasound waves enable precise localization of specific areas, selective destruction of pathologic tissues, and minimal damage to adjacent normal tissues and organs [108]. With respect to specific applications, therapeutic ultrasound can be categorized into non-thermal and thermal ultrasound energy, represented by SDT and high-intensity focused ultrasound (HIFU), respectively [109]. SDT activates the acoustic sensitizer through low-intensity ultrasound, leading to the production of ROS and subsequent destruction of tumor cells. As a non-invasive treatment for tumors, SDT shows promise as an anti-cancer therapy. The development and widespread utilization of nanomaterials has opened up opportunities for novel ultrasound sensitizers with tumor-targeting specificity. These sensitizers penetrate deep into the tumor, thereby improving the TME. HIFU, as a non-invasive procedure for cancer ablation, has also rapidly evolved in the treatment of solid tumors over the past few decades.
In addition, ultrasound induces a range of biological effects by activating acoustic sensitizers, including the generation of ROS [110], non-thermal effects, such as cavitation and mechanical effects, as well as thermal effects [111]. The thermal and mechanical effects of ultrasound have distinct applications in modulation of the TME.
Vasculature alteration
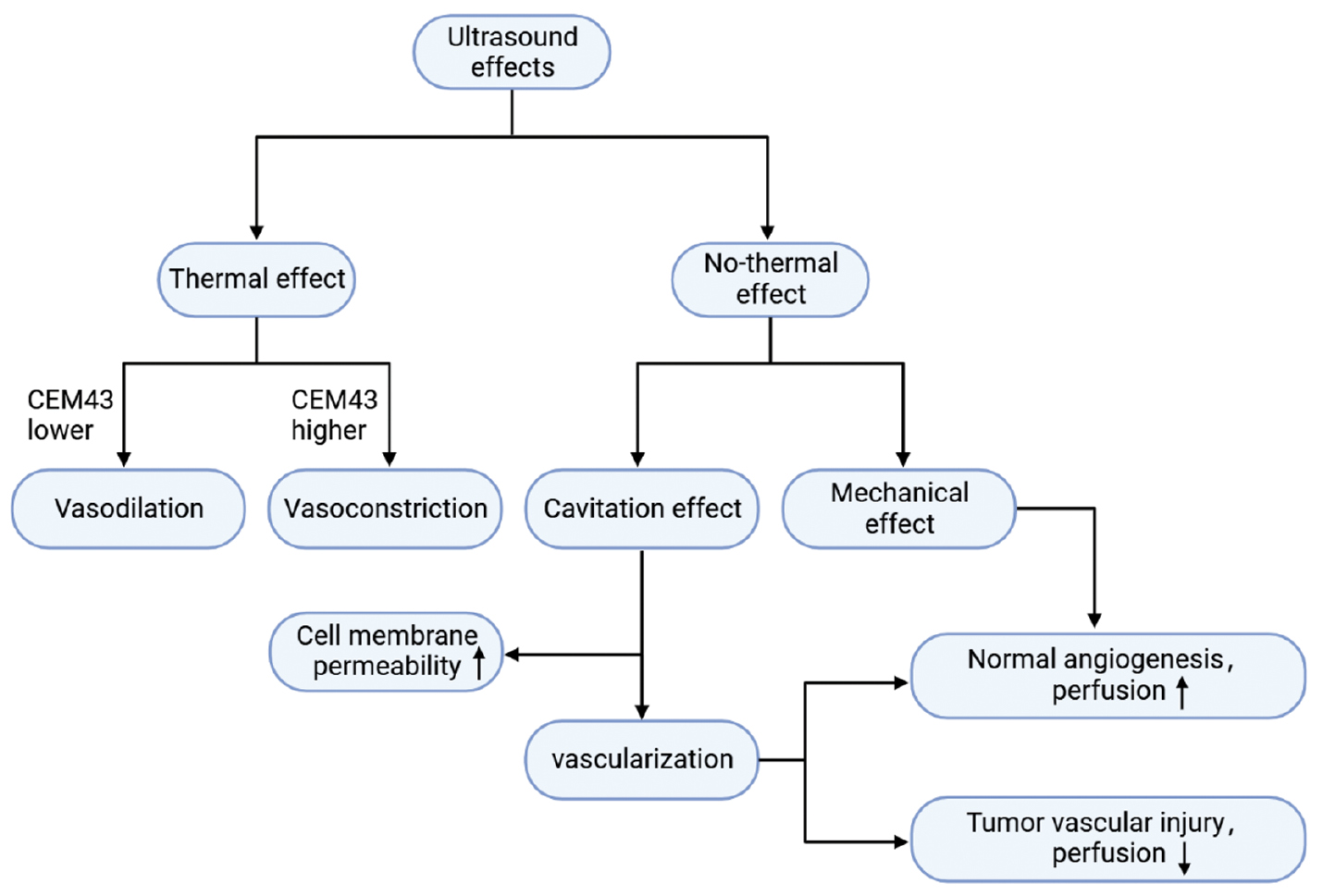
Hyperthermia induces several changes in the vasculature, with vasodilation being the most prominent. Such changes in the vasculature leads to increased blood flow, reduced hypoxia in the TME, improved acidity levels, and decreased interstitial pressure [112, 113]. Due to the direct reflection of the temperature receptor to activate the vascular smooth muscle, when the cumulative equivalent min at 43°C (CEM43) is low, the thermal effects of ultrasound result in vasodilation [114] and increase blood flow. Mild hyperthermia leads to a reduction in tumor IFP [115]. The effects of mild hyperthermia on blood vessels are reversible and do not cause tissue damage. Nevertheless, higher levels of CEM43 temporarily or permanently constrict blood vessels and cause congestion at the edges [106]. High-intensity ultrasound pulses also temporarily or permanently reduce blood vessel diameter. In addition to thermal effects, focused ultrasound (FU) transiently increases vascular permeability through mechanical effects [106] [Figure 4]. Price et al. [116] demonstrated that the cavitation effect can cause microvascular rupture, leading to the extravasation of red blood cells into the ECM. This finding indicates that ultrasound-induced cavitation disrupts endothelial cell membranes and enhances cell membrane permeability [117]. Moreover, other mechanisms have been proposed to explain the effect of cavitation on cell membrane permeability. For example, cavitation leads to the formation of intracellular ROS, which might contribute to increased permeability of cell membranes [118]. When microbubbles/nanobubbles (MBs/NBs). collapse, the localized transient warming to 4300–5000 K affects mobility of the phospholipid bilayer and enhances cellular permeability [119]. Increased endothelial cell permeability following ultrasound combined with MB treatment induces blood-brain barrier opening [120]. In addition to the effect on cell membrane permeability, the cavitation effect affects the vasculature and blood perfusion. Blood perfusion is reduced shortly after exposure to FU and MBs, and may be due to the increased fragility of newly formed blood vessels in tumors compared to healthy tissues. When exposed to cavitation MBs, these fragile tumor blood vessels sustain vascular injury and disruption of the capillary walls, leading to reduced perfusion [121]. Additionally, when combined with MBs, FU open up blood vessels and enhance perfusion. This effect may be mediated by the release of NO triggered by shear stress. The effect could also be related to increased mechano-transduction within endothelial cells due to mechanical interactions [122]. In conclusion, the impact on perfusion depends on various factors, such as the specific ultrasound treatment used, the type of tissue, and the vasculature structure.
Figure 4 Ultrasound effects on blood vessels.
Extracellular matrix remodeling
Tumors typically exhibit elevated pressure, which can be classified as solid pressure and IFP. Solid pressure refers to the pressure exerted by tumor cells, stromal cells, and ECM components as the density increases within the confined space of the host tissue. This solid pressure compresses pliable structures, like tumor blood and lymphatic vessels. Compression of lymphatic vessels reduces tumor drainage, leading to an increase in IFP [123]. Dysfunction of the lymphatic system and the dense ECM in tumors further exacerbates the increase in IFP. Exudate infiltrates from hyperpermeable vessels into the tumor interstitium, but due to the impaired lymphatic drainage and resistance posed by the dense ECM, the fluids are unable to adequately drain or penetrate the surrounding normal tissue [124]. Consequently, the tumor mesenchyme accumulates excess fluid that cannot be eliminated and the IFP gradually increases and eliminates the pressure gradient with the fluid, thereby limiting drug movement by convection. Reducing solid pressure and IFP to modulate the TME structure has been shown to improve drug delivery to the tumor mesenchyme [125].
Pulsed-HIFU remodeled the ECM in a murine A549 lung cancer experiment, resulting in increased vascular blood flow, decreased collagen content, and enhanced tissue permeability [126]. Because the size of MBs (2–3 μm in diameter) is limited by the vascular system, most MBs do not enter the ECM but how the size of MBs affects the tumor ECM is not clear. Xiao et al. [127] demonstrated a reduction in the IFP and an increase in drug penetration by a combination of 1 MPa and 10-min exposure time in ultrasound and MBs. To observe a reduction in the IFP, the presence of MBs is necessary because the ultrasound alone does not alter the IFP [128]. It is important to note that prolonged exposure to the ultrasound for >5 min destroys all MBs. Therefore, the observed decrease in the IFP reported by Xiao et al. [127] may have been primarily caused by hyperthermia rather than the direct effect of ultrasound on the IFP. Hyperthermia induced by ultrasound, which raises the body temperature to 42°C for 5 min, has been shown to reduce the IFP [115]. In the mentioned study [115], the authors also observed a reduction in the IFP after euthanasia in mice following ultrasound exposure. This finding suggests that the decrease in IFP was not solely a result of changes in blood flow but might also be associated with alterations in the ECM. Thus, the effects of ultrasound on the IFP may be attributed to a combination of factors, including changes in blood flow and potential modifications in the ECM.
Immune response activation
Ultrasound thermotherapy, ablation, tissue sectioning, and microbubble stabilization/inertial cavitation alter the TME, enhance immune activation, and inhibit tumor growth. Microbubble cavitation increases vascular permeability, which improves the delivery of immune cells, cytokines, antigens, and antibodies to the tumor. Vigorous microvesicle cavitation destroys tumor cells, effectively exposing tumor cells to a wide range of antigens, thereby promoting the maturation of APCs and subsequent adaptive immune cell activation [129]. In contrast, like other physical stimulations, ultrasound also induces ICD [130]. Ultrasound-induced ablation directly destroys tumor tissues, as in HIFU therapy, and the ablation releases tumor-associated antigens and a variety of biologically active molecules, which release endogenous DAMPs (e.g., HSP-60 and ATP) to activate APCs. Activated APCs initiate T cells to for antigen-specific cellular immune responses [103]. During ultrasound ablation therapy, the increase in temperature also enhances blood perfusion and promotes circulation and penetration of immune cells in the target area [129]. Mechanical HIFU, in addition to increasing immune cell infiltration, facilitates the conversion of macrophages to an immunostimulatory M1 phenotype [131]. FU alone has immunostimulatory potential. Increasing the intensity of focused ultrasound in glioblastomas within safe limits was shown to increase tumor-infiltrating lymphocytes and produce an immunostimulatory TME [132]. Many studies have been conducted to show that ultrasound effectively assists immunotherapy. For example, Hu et al. [133] showed that the therapeutic effect of anti-PD1 monotherapy alone was weak when used to treat tumors but combined with ultrasound combined with nanobubbles (USNBs) significantly enhanced the effect of anti-tumor immunotherapy.
Conclusion and future perspectives
Various physical stimulations have intersections and differences in modulation of the TME, which mainly includes influencing the vasculature, ECM, and immune responses. The TME changes induced by physical stimulation not only facilitate the improvement of drug or nanomaterial delivery in resistant conditions but also modifies the immunosuppressive microenvironment, paving the way for immunotherapy. In general, physical stimulation can be used to modulate the TME as an adjuvant therapy and a stand-alone treatment for tumors. Treatment alone has the following advantages: 1) non-invasive or minimally invasive; 2) significant local therapeutic effect superior to chemotherapy and reduced side effects; 3) a curative role for early-stage tumors, achieving tumor reduction for middle- and late-stage tumors; 4) accurate positioning and good targeting; and 5) use as an adjuvant or combined therapy. However, physical stimulation, as a kind of pure auxiliary stimulation or therapeutic means, only targets stimulation to the local tumor site and can do nothing to the metastatic or spreading tumors. Combination therapy has brought new prospects. With the deep exploration of the TME and the advances of modern nanotechnology, targeted tumor treatment strategies using nanomaterials as carriers coupled with various physical stimulations have garnered widespread attention [66]. For example, the combination of physical stimulation with chemotherapy and immunotherapy shows complementary advantages, significantly improving tumor treatment efficacy and reducing the formation of metastatic lesions [134]. Additionally, therapies utilizing biological carriers, such as bacteria, combined with physical stimulation have also demonstrated promising therapeutic effects [135]. In summary, this review has provided a reference for future research. As scientific inquiries delve further, combination therapy has become a focal point in current tumor treatment studies, creating favorable conditions for drug delivery and immunotherapy by modulating changes in the TME. Undoubtedly, combination therapy holds significant implications for future anti-tumor research. However, in the face of the complexity of the TME and the heterogeneity caused by individual differences, there is still a long way to go in exploring more precise and effective methods to modulate the TME and apply the findings in clinical tumor treatment. Despite the presence of numerous obstacles, we envision that the combination of physical stimuli-based cancer nanotherapy with chemotherapy drugs and immunotherapy will become an effective treatment approach in the near future.
Acknowledgement
We are grateful for the support of the National Natural Science Foundation of China (82272028, 81971621, and 82102087), the Key R&D Program of Hunan Province (2021SK2035), the Natural Science Foundation of Hunan Province (2022JJ30039 and 2022JJ40392), the Natural Science Foundation of Guangdong Province (2021A151501117), and the Project of Science and Technology Innovation of Hunan Province (2021SK51807).
Declaration of interests
The authors declare that they have no competing interests.
References
- Anderson NM, Simon MC. The tumor microenvironment. Curr Biol 2020;30(16):R921-5. [PMID: 32810447 DOI: 10.1016/j.cub.2020.06.081]
- Hang Y, Liu Y, Teng Z, Cao X, Zhu H. Mesoporous nanodrug delivery system: a powerful tool for a new paradigm of remodeling of the tumor microenvironment. J Nanobiotechnol 2023;21(1):101. [PMID: 36945005 DOI: 10.1186/s12951-023-01841-2]
- Martin JD, Miyazaki T, Cabral H. Remodeling tumor microenvironment with nanomedicines. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2021;13(6):e1730. [PMID: 34124849 DOI: 10.1002/wnan.1730]
- Zhang J, Xu Z, Li Y, Hu Y, Tang J, et al. Theranostic mesoporous platinum nanoplatform delivers halofuginone to remodel extracellular matrix of breast cancer without systematic toxicity. Bioeng Transl Med 2023;8(4):e10427. [PMID: 37476071 DOI: 10.1002/btm2.10427]
- Zhang T, Jia Y, Yu Y, Zhang B, Xu F, et al. Targeting the tumor biophysical microenvironment to reduce resistance to immunotherapy. Adv Drug Deliv Rev 2022;186:114319. [PMID: 35545136 DOI: 10.1016/j.addr.2022.114319]
- Philip PA, Lacy J, Portales F, Sobrero A, Pazo-Cid R, et al. Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): a multicentre, open-label phase 2 study. Lancet Gastroenterol Hepatol 2020;5(3):285-94. [PMID: 31953079 DOI: 10.1016/S2468-1253(19)30327-9]
- Nyrop KA, Deal AM, Shachar SS, Basch E, Reeve BB, et al. Patient-reported toxicities during chemotherapy regimens in current clinical practice for early breast cancer. Oncologist 2019;24(6):762-71. [PMID: 30552158 DOI: 10.1634/theoncologist.2018-0590]
- An J, Hong H, Won M, Rha H, Ding Q, et al. Mechanical stimuli-driven cancer therapeutics. Chem Soc Rev 2023;52(1):30-46. [PMID: 36511945 DOI: 10.1039/d2cs00546h]
- Thorat ND, Tofail SA, von Rechenberg B, Townley H, Brennan G, et al. Physically stimulated nanotheranostics for next generation cancer therapy: focus on magnetic and light stimulations. Appl Phys Rev 2019;6(4):041306. [DOI: 10.1063/1.5049467]
- Stapleton S, Dunne M, Milosevic M, Tran CW, Gold MJ, et al. Radiation and heat improve the delivery and efficacy of nanotherapeutics by modulating intratumoral fluid dynamics. ACS Nano 2018;12(8):7583-600. [PMID: 30004666 DOI: 10.1021/acsnano.7b06301]
- Yin S, Chen Z, Chen D, Yan D. Strategies targeting PD-L1 expression and associated opportunities for cancer combination therapy. Theranostics 2023;13(5):1520-44. [PMID: 37056572 DOI: 10.7150/thno.80091]
- Zhao Y, Shen M, Wu L, Yang H, Yao Y, et al. Stromal cells in the tumor microenvironment: accomplices of tumor progression? Cell Death Dis 2023;14(9):587. [PMID: 37666813 DOI: 10.1038/s41419-023-06110-6]
- Dai J, Su Y, Zhong S, Cong L, Liu B, et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther 2020;5(1):145. [PMID: 32759948 DOI: 10.1038/s41392-020-00261-0]
- Tang W, Yang Z, He L, Deng L, Fathi P, et al. A hybrid semiconducting organosilica-based O2 nanoeconomizer for on-demand synergistic photothermally boosted radiotherapy. Nat Commun 2021;12(1):523. [PMID: 33483518 DOI: 10.1038/s41467-020-20860-3]
- Wang M, Chang M, Li C, Chen Q, Hou Z, et al. Tumor-microenvironment-activated reactive oxygen species amplifier for enzymatic cascade cancer starvation/chemodynamic/immunotherapy. Adv Mater 2022;34(4):e2106010. [PMID: 34699627 DOI: 10.1002/adma.202106010]
- Chang X, Zhu M, Tang X, Yu X, Liu F, et al. Enhanced manipulation of tumor microenvironments by nanomotor for synergistic therapy of malignant tumor. Biomaterials 2022;290:121853. [PMID: 36272219 DOI: 10.1016/j.biomaterials.2022.121853]
- Li Y, Zhao P, Gong T, Wang H, Jiang X, et al. Redox dyshomeostasis strategy for hypoxic tumor therapy based on DNAzyme-loaded electrophilic ZIFs. Angew Chem Int Ed Engl 2020;59(50):22537-43. [PMID: 32856362 DOI: 10.1002/anie.202003653]
- Wang ZH, Peng WB, Zhang P, Yang XP, Zhou Q. Lactate in the tumour microenvironment: from immune modulation to therapy. EBioMedicine 2021;73:103627. [PMID: 34656878 DOI: 10.1016/j.ebiom.2021.103627]
- Liang Q, Zhou L, Li Y, Liu J, Liu Y. Nano drug delivery system reconstruct tumour vasculature for the tumour vascular normalisation. J Drug Target 2022;30(2):119-30. [PMID: 33960252 DOI: 10.1080/1061186X.2021.1927056]
- Zhao Y, Yu X, Li J. Manipulation of immune–vascular crosstalk: new strategies towards cancer treatment. Acta Pharm Sin B 2020;10(11):2018-36. [PMID: 33304777 DOI: 10.1016/j.apsb.2020.09.014]
- Öhlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med 2014;211(8):1503-23. [PMID: 25071162 DOI: 10.1084/jem.20140692]
- Yamazaki T, Young KH. Effects of radiation on tumor vasculature. Mol Carcinog 2022;61(2):165-72. [PMID: 34644811 DOI: 10.1002/mc.23360]
- Lopez S, Hallali N, Lalatonne Y, Hillion A, Antunes JC, et al. Magneto-mechanical destruction of cancer-associated fibroblasts using ultra-small iron oxide nanoparticles and low frequency rotating magnetic fields. Nanoscale Adv 2022;4(2):421-36. [PMID: 36132704 DOI: 10.1039/d1na00474c]
- Li D, Zhang M, Xu F, Chen Y, Chen B, et al. Biomimetic albumin-modified gold nanorods for photothermo-chemotherapy and macrophage polarization modulation. Acta Pharm Sin B 2018;8(1):74-84. [PMID: 29872624 DOI: 10.1016/j.apsb.2017.09.005]
- Baskar R, Dai J, Wenlong N, Yeo R, Yeoh KW. Biological response of cancer cells to radiation treatment. Front Mol Biosci 2014;1:24. [PMID: 25988165 DOI: 10.3389/fmolb.2014.00024]
- Peng J, Yin X, Yun W, Meng X, Huang Z. Radiotherapy-induced tumor physical microenvironment remodeling to overcome immunotherapy resistance. Cancer Lett 2023;559:216108. [PMID: 36863506 DOI: 10.1016/j.canlet.2023.216108]
- Krombach J, Hennel R, Brix N, Orth M, Schoetz U, et al. Priming anti-tumor immunity by radiotherapy: dying tumor cell-derived DAMPs trigger endothelial cell activation and recruitment of myeloid cells. Oncoimmunology 2019;8(1):e1523097. [PMID: 30546963 DOI: 10.1080/2162402X.2018.1523097]
- Marciscano AE, Haimovitz-Friedman A, Lee P, Tran PT, Tomé WA, et al. Immunomodulatory effects of stereotactic body radiation therapy: preclinical insights and clinical opportunities. In J Radiat Oncol Biol Phys 2021;110(1):35-52. [PMID: 30836168 DOI: 10.1016/j.ijrobp.2019.02.046]
- Baselet B, Sonveaux P, Baatout S, Aerts A. Pathological effects of ionizing radiation: endothelial activation and dysfunction. Cell Mol Life Sci 2019;76(4):699-728. [PMID: 30377700 DOI: 10.1007/s00018-018-2956-z]
- Hallahan DE, Virudachalam S. Accumulation of P-selectin in the lumen of irradiated blood vessels. Radiat Res 1999;152(1):6-13. [PMID: 10381836]
- Augsten M. Cancer-associated fibroblasts as another polarized cell type of the tumor microenvironment. Front Oncol 2014;4:62. [PMID: 24734219 DOI: 10.3389/fonc.2014.00062]
- Luo H, Tu G, Liu Z, Liu M. Cancer-associated fibroblasts: a multifaceted driver of breast cancer progression. Cancer Lett 2015;361(2):155-63. [PMID: 25700776 DOI: 10.1016/j.canlet.2015.02.018]
- Hellevik T, Pettersen I, Berg V, Winberg JO, Moe BT, et al. Cancer-associated fibroblasts from human NSCLC survive ablative doses of radiation but their invasive capacity is reduced. Radiat Oncol 2012;7:59. [PMID: 22500976 DOI: 10.1186/1748-717X-7-59]
- Kang HR, Moon JY, Ediriweera MK, Song YW, Cho M, et al. Dietary flavonoid myricetin inhibits invasion and migration of radioresistant lung cancer cells (A549-IR) by suppressing MMP-2 and MMP-9 expressions through inhibition of the FAK-ERK signaling pathway. Food Sci Nutr 2020;8(4):2059-67. [PMID: 32328272 DOI: 10.1002/fsn3.1495]
- Bai R, Ding T, Zhao J, Liu S, Zhang L, et al. The effect of PI3K inhibitor LY294002 and gemcitabine hydrochloride combined with ionizing radiation on the formation of vasculogenic mimicry of Panc-1 cells in vitro and in vivo. Neoplasma 2016;63(1):80-92. [PMID: 26639237 DOI: 10.4149/neo_2016_010]
- Guardamagna I, Lonati L, Savio M, Stivala LA, Ottolenghi A, et al. An integrated analysis of the response of colorectal adenocarcinoma caco-2 cells to X-ray exposure. Front Oncol 2021;11:688919. [PMID: 34150657 DOI: 10.3389/fonc.2021.688919]
- Marino S, Menna G, Di Bonaventura R, Lisi L, Mattogno P, et al. The extracellular matrix in glioblastomas: a glance at its structural modifications in shaping the tumoral microenvironment-a systematic review. Cancers 2023;15(6):1879. [PMID: 36980765 DOI: 10.3390/cancers15061879]
- Cheng JC, Chou CH, Kuo ML, Hsieh CY. Radiation-enhanced hepatocellular carcinoma cell invasion with MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction pathway. Oncogene 2006;25(53):7009-18. [PMID: 16732316 DOI: 10.1038/sj.onc.1209706]
- Gu Q, He Y, Ji J, Yao Y, Shen W, et al. Hypoxia-inducible factor 1α (HIF-1α) and reactive oxygen species (ROS) mediates radiation-induced invasiveness through the SDF-1α/CXCR4 pathway in non-small cell lung carcinoma cells. Oncotarget 2015;6(13):10893-907. [PMID: 25843954 DOI: 10.18632/oncotarget.3535]
- Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem 2003;88(4):660-72. [PMID: 12577300 DOI: 10.1002/jcb.10413]
- Fucikova J, Kepp O, Kasikova L, Petroni G, Yamazaki T, et al. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis 2020;11(11):1013. [PMID: 33243969 DOI: 10.1038/s41419-020-03221-2]
- Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 2018;25(3):486-541. [PMID: 29362479 DOI: 10.1038/s41418-017-0012-4]
- Klug F, Prakash H, Huber PE, Seibel T, Bender N, et al. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013;24(5):589-602. [PMID: 24209604 DOI: 10.1016/j.ccr.2013.09.014]
- Sheng Y, Zhang B, Xing B, Liu Z, Chang Y, et al. Cancer-associated fibroblasts exposed to high-dose ionizing radiation promote M2 polarization of macrophages, which induce radiosensitivity in cervical cancer. Cancers 2023;15(5):1620. [PMID: 36900416 DOI: 10.3390/cancers15051620]
- Park HJ, Griffin RJ, Hui S, Levitt SH, Song CW. Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat Res 2012;177(3):311-27. [PMID: 22229487 DOI: 10.1667/rr2773.1]
- Morisada M, Clavijo PE, Moore E, Sun L, Chamberlin M, et al. PD-1 blockade reverses adaptive immune resistance induced by high-dose hypofractionated but not low-dose daily fractionated radiation. Oncoimmunology 2018;7(3):e1395996. [PMID: 29399393 DOI: 10.1080/2162402X.2017.1395996]
- Qu F, Xiao L, Xiao Y, Gao C, Wang X, et al. Case Report: Intervention of radiotherapy improves the prognosis of rectal squamous cell carcinoma with high PD-L1 expression and enable patients to obtain NED status. Front Immunol 2023;14:1235697. [PMID: 37520582 DOI: 10.3389/fimmu.2023.1235697]
- Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, et al. Photodynamic therapy. J Natl Cancer Inst 1998;90(12):889-905. [PMID: 9637138 DOI: 10.1093/jnci/90.12.889]
- Lucky SS, Soo KC, Zhang Y. Nanoparticles in photodynamic therapy. Chem Rev 2015;115(4):1990-2042. [PMID: 25602130 DOI: 10.1021/cr5004198]
- Wilson BC, Weersink RA. The Yin and Yang of PDT and PTT. Photochem Photobiol 2020;96(2):219-31. [PMID: 31769516 DOI: 10.1111/php.13184]
- Chen J, Fan T, Xie Z, Zeng Q, Xue P, et al. Advances in nanomaterials for photodynamic therapy applications: status and challenges. Biomaterials 2020;237:119827. [PMID: 32036302 DOI: 10.1016/j.biomaterials.2020.119827]
- Gao P, Wang H, Cheng Y. Strategies for efficient photothermal therapy at mild temperatures: progresses and challenges. Chin Chem Lett 2022;33(2):575-86. [DOI: 10.1016/j.cclet.2021.08.023]
- Li J, Pu K. Semiconducting polymer nanomaterials as near-infrared photoactivatable protherapeutics for cancer. Acc Chem Res 2020;53(4):752-62. [PMID: 32027481 DOI: 10.1021/acs.accounts.9b00569]
- Orosz P, Echtenacher B, Falk W, Rüschoff J, Weber D, et al. Enhancement of experimental metastasis by tumor necrosis factor. J Exp Med 1993;177(5):1391-8. [PMID: 8478614 DOI: 10.1084/jem.177.5.1391]
- Gao J, Wang F, Wang S, Liu L, Liu K, et al. Hyperthermia-triggered on-demand biomimetic nanocarriers for synergetic photothermal and chemotherapy. Adv Sci (Weinh) 2020;7(11):1903642. [PMID: 32537410 DOI: 10.1002/advs.201903642]
- Overchuk M, Weersink RA, Wilson BC, Zheng G. Photodynamic and photothermal therapies: synergy opportunities for nanomedicine. ACS Nano 2023;17(9):7979-8003. [PMID: 37129253 DOI: 10.1021/acsnano.3c00891]
- He C, Agharkar P, Chen B. Intravital microscopic analysis of vascular perfusion and macromolecule extravasation after photodynamic vascular targeting therapy. Pharm Res 2008;25(8):1873-80. [PMID: 18446275 DOI: 10.1007/s11095-008-9604-5]
- Zeitouni NC, Bhatia N, Ceilley RI, Cohen JL, Del Rosso JQ, et al. Photodynamic therapy with 5-aminolevulinic acid 10% gel and red light for the treatment of actinic keratosis, nonmelanoma skin cancers, and acne: current evidence and best practices. J Clin Aesthet Dermatol 2021;14(10):E53-65. [PMID: 34976292]
- Domka W, Bartusik-Aebisher D, Mytych W, Dynarowicz K, Aebisher D. The use of photodynamic therapy for head, neck, and brain diseases. Int J Mol Sci 2023;24(14):11867. [PMID: 37511625 DOI: 10.3390/ijms241411867]
- Kubrak T, Karakuła M, Czop M, Kawczyk-Krupka A, Aebisher D. Advances in management of bladder cancer-the role of photodynamic therapy. Molecules 2022;27(3):731. [PMID: 35163996 DOI: 10.3390/molecules27030731]
- Zhang C, Zhao K, Bu W, Ni D, Liu Y, et al. Marriage of scintillator and semiconductor for synchronous radiotherapy and deep photodynamic therapy with diminished oxygen dependence. Angew Chem Int Ed Engl 2015;54(6):1770-4. [PMID: 25483028 DOI: 10.1002/anie.201408472]
- Yu Z, Zhou P, Pan W, Li N, Tang B. A biomimetic nanoreactor for synergistic chemiexcited photodynamic therapy and starvation therapy against tumor metastasis. Nat Commun 2018;9(1):5044. [PMID: 30487569 DOI: 10.1038/s41467-018-07197-8]
- Liang R, Liu L, He H, Chen Z, Han Z, et al. Oxygen-boosted immunogenic photodynamic therapy with gold nanocages@manganese dioxide to inhibit tumor growth and metastases. Biomaterials 2018;177:149-60. [PMID: 29890364 DOI: 10.1016/j.biomaterials.2018.05.051]
- Huang CC, Chia WT, Chung MF, Lin KJ, Hsiao CW, et al. An implantable depot that can generate oxygen in situ for overcoming hypoxia-induced resistance to anticancer drugs in chemotherapy. J Am Chem Soc 2016;138(16):5222-5. [PMID: 27075956 DOI: 10.1021/jacs.6b01784]
- Huang R, Ding Z, Jiang BP, Luo Z, Chen T, et al. Artificial metalloprotein nanoanalogues: in situ catalytic production of oxygen to enhance photoimmunotherapeutic inhibition of primary and abscopal tumor growth. Small 2020;16(46):e2004345. [PMID: 33089606 DOI: 10.1002/smll.202004345]
- Yang Y, Wang P, Shi R, Zhao Z, Xie A, et al. Design of the tumor microenvironment-multiresponsive nanoplatform for dual-targeting and photothermal imaging guided photothermal/photodynamic/chemodynamic cancer therapies with hypoxia improvement and GSH depletion. Chem Eng J 2022;441:136042. [DOI: 10.1016/j.cej.2022.136042]
- Jia Q, Ge J, Liu W, Zheng X, Chen S, et al. A magnetofluorescent carbon dot assembly as an acidic H2O2-driven oxygenerator to regulate tumor hypoxia for simultaneous bimodal imaging and enhanced photodynamic therapy. Adv Mater 2018;30(13):e1706090. [PMID: 29436031 DOI: 10.1002/adma.201706090]
- Wang H, Chao Y, Liu J, Zhu W, Wang G, et al. Photosensitizer-crosslinked in-situ polymerization on catalase for tumor hypoxia modulation & enhanced photodynamic therapy. Biomaterials 2018;181:310-7. [PMID: 30096565 DOI: 10.1016/j.biomaterials.2018.08.011]
- Horsman MR. Tissue physiology and the response to heat. Int J Hyperthermia 2006;22(3):197-203. [PMID: 16754339 DOI: 10.1080/02656730600689066]
- Kurokawa H, Ito H, Terasaki M, Matsui H. Hyperthermia enhances photodynamic therapy by regulation of HCP1 and ABCG2 expressions via high level ROS generation. Sci Rep 2019;9(1):1638. [PMID: 30733583 DOI: 10.1038/s41598-018-38460-z]
- Chauhan VP, Boucher Y, Ferrone CR, Roberge S, Martin JD, et al. Compression of pancreatic tumor blood vessels by hyaluronan is caused by solid stress and not interstitial fluid pressure. Cancer Cell 2014;26(1):14-5. [PMID: 25026209 DOI: 10.1016/j.ccr.2014.06.003]
- Barr H, Tralau CJ, MacRobert AJ, Krasner N, Boulos PB, et al. Photodynamic therapy in the normal rat colon with phthalocyanine sensitisation. Br J Cancer 1987;56(2):111-8. [PMID: 3663462 DOI: 10.1038/bjc.1987.166]
- Raeesi V, Chan WC. Improving nanoparticle diffusion through tumor collagen matrix by photo-thermal gold nanorods. Nanoscale 2016;8(25):12524-30. [PMID: 26822539 DOI: 10.1039/c5nr08463f]
- Marangon I, Silva AA, Guilbert T, Kolosnjaj-Tabi J, Marchiol C, et al. Tumor stiffening, a key determinant of tumor progression, is reversed by nanomaterial-induced photothermal therapy. Theranostics 2017;7(2):329-43. [PMID: 28042338 DOI: 10.7150/thno.17574]
- Anand S, Chan TA, Hasan T, Maytin EV. Current prospects for treatment of solid tumors via photodynamic, photothermal, or ionizing radiation therapies combined with immune checkpoint inhibition (A review). Pharmaceuticals (Basel) 2021;14(5):447. [PMID: 34068491 DOI: 10.3390/ph14050447]
- Yan G, Shi L, Zhang F, Luo M, Zhang G, et al. Transcriptomic analysis of mechanism of melanoma cell death induced by photothermal therapy. J Biophotonics 2021;14(8):e202100034. [PMID: 33729683 DOI: 10.1002/jbio.202100034]
- Nath S, Obaid G, Hasan T. The course of immune stimulation by photodynamic therapy: bridging fundamentals of photochemically induced immunogenic cell death to the enrichment of T-cell repertoire. Photochem Photobiol 2019;95(6):1288-305. [PMID: 31602649 DOI: 10.1111/php.13173]
- Garg AD, Nowis D, Golab J, Agostinis P. Photodynamic therapy: illuminating the road from cell death towards anti-tumour immunity. Apoptosis 2010;15(9):1050-71. [PMID: 20221698 DOI: 10.1007/s10495-010-0479-7]
- Sweeney EE, Cano-Mejia J, Fernandes R. Photothermal therapy generates a thermal window of immunogenic cell death in neuroblastoma. Small 2018;14(20):e1800678. [PMID: 29665282 DOI: 10.1002/smll.201800678]
- Lou J, Aragaki M, Bernards N, Chee T, Gregor A, et al. Repeated photodynamic therapy mediates the abscopal effect through multiple innate and adaptive immune responses with and without immune checkpoint therapy. Biomaterials 2023;292:121918. [PMID: 36442438 DOI: 10.1016/j.biomaterials.2022.121918]
- Ghosh S, He X, Huang WC, Lovell JF. Immune checkpoint blockade enhances chemophototherapy in a syngeneic pancreatic tumor model. APL Bioeng 2022;6(3):036105. [PMID: 36164594 DOI: 10.1063/5.0099811]
- Campana LG, Daud A, Lancellotti F, Arroyo JP, Davalos RV, et al. Pulsed electric fields in oncology: a snapshot of current clinical practices and research directions from the 4th world congress of electroporation. Cancers 2023;15(13):3340. [PMID: 37444450 DOI: 10.3390/cancers15133340]
- Collettini F, Enders J, Stephan C, Fischer T, Baur ADJ, et al. Image-guided irreversible electroporation of localized prostate cancer: functional and oncologic outcomes. Radiology 2019;292(1):250-7. [PMID: 31161973 DOI: 10.1148/radiol.2019181987]
- Moser JC, Salvador E, Deniz K, Swanson K, Tuszynski J, et al. The mechanisms of action of tumor treating fields. Cancer Res 2022;82(20):3650-8. [PMID: 35839284 DOI: 10.1158/0008-5472.CAN-22-0887]
- Nuccitelli R, Chen X, Pakhomov AG, Baldwin WH, Sheikh S, et al. A new pulsed electric field therapy for melanoma disrupts the tumor’s blood supply and causes complete remission without recurrence. Int J Cancer 2009;125(2):438-45. [PMID: 19408306 DOI: 10.1002/ijc.24345]
- Markelc B, Bellard E, Sersa G, Pelofy S, Teissie J, et al. In vivo molecular imaging and histological analysis of changes induced by electric pulses used for plasmid DNA electrotransfer to the skin: a study in a dorsal window chamber in mice. J Membr Biol 2012;245(9):545-54. [PMID: 22644389 DOI: 10.1007/s00232-012-9435-5]
- Zmuc J, Gasljevic G, Sersa G, Edhemovic I, Boc N, et al. Large liver blood vessels and bile ducts are not damaged by electrochemotherapy with bleomycin in pigs. Sci Rep 2019;9(1):3649. [PMID: 30842517 DOI: 10.1038/s41598-019-40395-y]
- Li C, Xiao C, Zhan L, Zhang Z, Xing J, et al. Wireless electrical stimulation at the nanoscale interface induces tumor vascular normalization. Bioact Mater 2022;18:399-408. [PMID: 35415302 DOI: 10.1016/j.bioactmat.2022.03.027]
- Gouarderes S, Ober C, Doumard L, Dandurand J, Vicendo P, et al. Pulsed electric fields induce extracellular matrix remodeling through matrix metalloproteinases activation and decreased collagen production. J Invest Dermatol 2022;142(5):1326-37.e9. [PMID: 34688615 DOI: 10.1016/j.jid.2021.09.025]
- Kong Y, Liu F, Ma B, Duan J, Yuan W, et al. Wireless localized electrical stimulation generated by an ultrasound-driven piezoelectric discharge regulates proinflammatory macrophage polarization. Adv Sci (Weinh) 2021;8(13):2100962. [PMID: 34258169 DOI: 10.1002/advs.202100962]
- Han JH, Shin HE, Lee J, Kang JM, Park JH, et al. Combination of metal-phenolic network-based immunoactive nanoparticles and bipolar irreversible electroporation for effective cancer immunotherapy. Small 2022;18(25):e2200316. [PMID: 35570584 DOI: 10.1002/smll.202200316]
- Burbach BJ, O’Flanagan SD, Shao Q, Young KM, Slaughter JR, et al. Irreversible electroporation augments checkpoint immunotherapy in prostate cancer and promotes tumor antigen-specific tissue-resident memory CD8+ T cells. Nat Commun 2021;12(1):3862. [PMID: 34162858 DOI: 10.1038/s41467-021-24132-6]
- Chen D, Le SB, Hutchinson TE, Calinescu AA, Sebastian M, et al. Tumor treating fields dually activate STING and AIM2 inflammasomes to induce adjuvant immunity in glioblastoma. J Clin Invest 2022;132(8):e149258. [PMID: 35199647 DOI: 10.1172/JCI149258]
- Zhang G, Liu X, Liu Y, Zhang S, Yu T, et al. The effect of magnetic fields on tumor occurrence and progression: recent advances. Prog Biophys Mol Biol 2023;179:38-50. [PMID: 37019340 DOI: 10.1016/j.pbiomolbio.2023.04.001]
- Kong G, Braun RD, Dewhirst MW. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res 2001;61(7):3027-32. [PMID: 11306483]
- Strieth S, Strelczyk D, Eichhorn ME, Dellian M, Luedemann S, et al. Static magnetic fields induce blood flow decrease and platelet adherence in tumor microvessels. Cancer Biol Ther 2008;7(6):814-9. [PMID: 18340115 DOI: 10.4161/cbt.7.6.5837]
- Hu JH, St-Pierre LS, Buckner CA, Lafrenie RM, Persinger MA. Growth of injected melanoma cells is suppressed by whole body exposure to specific spatial-temporal configurations of weak intensity magnetic fields. Int J Radiat Biol 2010;86(2):79-88. [PMID: 20148694 DOI: 10.3109/09553000903419932]
- Peng L, Fu C, Wang L, Zhang Q, Liang Z, et al. The effect of pulsed electromagnetic fields on angiogenesis. Bioelectromagnetics 2021;42(3):250-8. [PMID: 33675261 DOI: 10.1002/bem.22330]
- Liu X, Yan B, Li Y, Ma X, Jiao W, et al. Graphene oxide-grafted magnetic nanorings mediated magnetothermodynamic therapy favoring reactive oxygen species-related immune response for enhanced antitumor efficacy. ACS Nano 2020;14(2):1936-50. [PMID: 31961656 DOI: 10.1021/acsnano.9b08320]
- Kolosnjaj-Tabi J, Marangon I, Nicolas-Boluda A, Silva AKA, Gazeau F. Nanoparticle-based hyperthermia, a local treatment modulating the tumor extracellular matrix. Pharmacol Res 2017;126:123-37. [PMID: 28720518 DOI: 10.1016/j.phrs.2017.07.010]
- Contreras MF, Sougrat R, Zaher A, Ravasi T, Kosel J. Non-chemotoxic induction of cancer cell death using magnetic nanowires. Int J Nanomedicine 2015;10:2141-53. [PMID: 25834430 DOI: 10.2147/IJN.S77081]
- Todryk S, Melcher AA, Hardwick N, Linardakis E, Bateman A, et al. Heat shock protein 70 induced during tumor cell killing induces Th1 cytokines and targets immature dendritic cell precursors to enhance antigen uptake. J Immunol 1999;163(3):1398-408. [PMID: 10415040]
- van den Bijgaart RJ, Eikelenboom DC, Hoogenboom M, Fütterer JJ, den Brok MH, et al. Thermal and mechanical high-intensity focused ultrasound: perspectives on tumor ablation, immune effects and combination strategies. Cancer Immunol Immunother 2017;66(2):247-58. [PMID: 27585790 DOI: 10.1007/s00262-016-1891-9]
- Bouchlaka MN, Sckisel GD, Wilkins D, Maverakis E, Monjazeb AM, et al. Mechanical disruption of tumors by iron particles and magnetic field application results in increased anti-tumor immune responses. PLoS One 2012;7(10):e48049. [PMID: 23133545 DOI: 10.1371/journal.pone.0048049]
- Lei H, Pan Y, Wu R, Lv Y. Innate immune regulation under magnetic fields with possible mechanisms and therapeutic applications. Front Immunol 2020;11:582772. [PMID: 33193393 DOI: 10.3389/fimmu.2020.582772]
- Zhang N, Wang J, Foiret J, Dai Z, Ferrara KW. Synergies between therapeutic ultrasound, gene therapy and immunotherapy in cancer treatment. Adv Drug Deliv Rev 2021;178:113906. [PMID: 34333075 DOI: 10.1016/j.addr.2021.113906]
- Song X, Zhang Q, Chang M, Ding L, Huang H, et al. Nanomedicine-enabled sonomechanical, sonopiezoelectric, sonodynamic, and sonothermal therapy. Adv Mater 2023;35:e2212259. [PMID: 36812400 DOI: 10.1002/adma.202212259]
- Wang Z, Liu B, Sun Q, Feng L, He F, et al. Upconverted metal-organic framework janus architecture for near-infrared and ultrasound co-enhanced high performance tumor therapy. ACS Nano 2021;15(7):12342-57. [PMID: 34160201 DOI: 10.1021/acsnano.1c04280]
- Tachibana K. Emerging technologies in therapeutic ultrasound: thermal ablation to gene delivery. Hum Cell 2004;17(1):7-15. [PMID: 15369132 DOI: 10.1111/j.1749-0774.2004.tb00015.x]
- Li H, Shi W, Huang W, Yao EP, Han J, et al. Carbon quantum dots/TiO(x) electron transport layer boosts efficiency of planar heterojunction perovskite solar cells to 19. Nano Lett 2017;17(4):2328-35. [PMID: 28248512 DOI: 10.1021/acs.nanolett.6b05177]
- Zhou QL, Chen ZY, Wang YX, Yang F, Lin Y, et al. Ultrasound-mediated local drug and gene delivery using nanocarriers. Biomed Res Int 2014;2014:963891. [PMID: 25202710 DOI: 10.1155/2014/963891]
- Elming PB, Sørensen BS, Oei AL, Franken NAP, Crezee J, et al. Hyperthermia: the optimal treatment to overcome radiation resistant hypoxia. Cancers 2019;11(1):60. [PMID: 30634444 DOI: 10.3390/cancers11010060]
- Winslow TB, Eranki A, Ullas S, Singh AK, Repasky EA, et al. A pilot study of the effects of mild systemic heating on human head and neck tumour xenografts: analysis of tumour perfusion, interstitial fluid pressure, hypoxia and efficacy of radiation therapy. Int J Hyperthermia 2015;31(6):693-701. [PMID: 25986432 DOI: 10.3109/02656736.2015.1037800]
- Maruo A, Hamner CE, Rodrigues AJ, Higami T, Greenleaf JF, et al. Nitric oxide and prostacyclin in ultrasonic vasodilatation of the canine internal mammary artery. Ann Thorac Surg 2004;77(1):126-32. [PMID: 14726048 DOI: 10.1016/s0003-4975(03)01293-1]
- Watson KD, Lai CY, Qin S, Kruse DE, Lin YC, et al. Ultrasound increases nanoparticle delivery by reducing intratumoral pressure and increasing transport in epithelial and epithelial-mesenchymal transition tumors. Cancer Res 2012;72(6):1485-93. [PMID: 22282664 DOI: 10.1158/0008-5472.CAN-11-3232]
- Price RJ, Skyba DM, Kaul S, Skalak TC. Delivery of colloidal particles and red blood cells to tissue through microvessel ruptures created by targeted microbubble destruction with ultrasound. Circulation 1998;98(13):1264-7. [PMID: 9751673 DOI: 10.1161/01.cir.98.13.1264]
- Snipstad S, Sulheim E, de Lange Davies C, Moonen C, Storm G, et al. Sonopermeation to improve drug delivery to tumors: from fundamental understanding to clinical translation. Exp Opin Drug Deliv 2018;15(12):1249-61. [PMID: 30415585 DOI: 10.1080/17425247.2018.1547279]
- Lentacker I, De Cock I, Deckers R, De Smedt SC, Moonen CT. Understanding ultrasound induced sonoporation: definitions and underlying mechanisms. Adv Drug Deliv Rev 2014;72:49-64. [PMID: 24270006 DOI: 10.1016/j.addr.2013.11.008]
- Didenko YT, McNamara WB 3rd, Suslick KS. Effect of noble gases on sonoluminescence temperatures during multibubble cavitation. Phys Rev Lett 2000;84(4):777-80. [PMID: 11017370 DOI: 10.1103/PhysRevLett.84.777]
- Lelu S, Afadzi M, Berg S, Aslund AK, Torp SH, et al. Primary porcine brain endothelial cells as in vitro model to study effects of ultrasound and microbubbles on blood-brain barrier function. IEEE Trans Ultrason Ferroelectr Freq Control 2017;64(1):281-90. [PMID: 27529871 DOI: 10.1109/TUFFC.2016.2597004]
- Goertz DE, Karshafian R, Hynynen K, editors. Antivascular effects of pulsed low intensity ultrasound and microbubbles in mouse tumors. In 2008 IEEE Ultrasonics Symposium; 2008 Nov 2-5; Beijing, China. IEEE; 2008. [DOI: 10.1109/ULTSYM.2008.0161]
- Bertuglia S. Increase in capillary perfusion following low-intensity ultrasound and microbubbles during postischemic reperfusion. Crit Care Med 2005;33(9):2061-7. [PMID: 16148481 DOI: 10.1097/01.ccm.0000178356.90173.73]
- Stylianopoulos T, Martin JD, Snuderl M, Mpekris F, Jain SR, et al. Coevolution of solid stress and interstitial fluid pressure in tumors during progression: implications for vascular collapse. Cancer Res 2013;73(13):3833-41. [PMID: 23633490 DOI: 10.1158/0008-5472.CAN-12-4521]
- Stylianopoulos T, Munn LL, Jain RK. Reengineering the physical microenvironment of tumors to improve drug delivery and efficacy: from mathematical modeling to bench to bedside. Trends Cancer 2018;4(4):292-319. [PMID: 29606314 DOI: 10.1016/j.trecan.2018.02.005]
- Vlahovic G, Ponce AM, Rabbani Z, Salahuddin FK, Zgonjanin L, et al. Treatment with imatinib improves drug delivery and efficacy in NSCLC xenografts. Br J Cancer 2007;97(6):735-40. [PMID: 17712313 DOI: 10.1038/sj.bjc.6603941]
- Lee S, Han H, Koo H, Na JH, Yoon HY, et al. Extracellular matrix remodeling in vivo for enhancing tumor-targeting efficiency of nanoparticle drug carriers using the pulsed high intensity focused ultrasound. J Control Release 2017;263:68-78. [PMID: 28257990 DOI: 10.1016/j.jconrel.2017.02.035]
- Xiao N, Liu J, Liao L, Sun J, Jin W, et al. Ultrasound combined with microbubbles increase the delivery of doxorubicin by reducing the interstitial fluid pressure. Ultrasound Q 2019;35(2):103-9. [PMID: 30169494 DOI: 10.1097/RUQ.0000000000000381]
- Zhang Q, Jin H, Chen L, Chen Q, He Y, et al. Effect of ultrasound combined with microbubble therapy on interstitial fluid pressure and VX2 tumor structure in rabbit. Front Pharmacol 2019;10:716. [PMID: 31293427 DOI: 10.3389/fphar.2019.00716]
- Ho YJ, Li JP, Fan CH, Liu HL, Yeh CK. Ultrasound in tumor immunotherapy: current status and future developments. J Control Release 2020;323:12-23. [PMID: 32302759 DOI: 10.1016/j.jconrel.2020.04.023]
- Zhao H, Zhao B, Li L, Ding K, Xiao H, et al. Biomimetic decoy inhibits tumor growth and lung metastasis by reversing the drawbacks of sonodynamic therapy. Adv Healthc Mater 2020;9(1):e1901335. [PMID: 31762228 DOI: 10.1002/adhm.201901335]
- Abe S, Nagata H, Crosby EJ, Inoue Y, Kaneko K, et al. Combination of ultrasound-based mechanical disruption of tumor with immune checkpoint blockade modifies tumor microenvironment and augments systemic antitumor immunity. J Immunother Cancer 2022;10(1):e003717. [PMID: 35039461 DOI: 10.1136/jitc-2021-003717]
- Chen KT, Chai WY, Lin YJ, Lin CJ, Chen PY, et al. Neuronavigation-guided focused ultrasound for transcranial blood-brain barrier opening and immunostimulation in brain tumors. Sci Adv 2021;7(6):eabd0772. [PMID: 33547073 DOI: 10.1126/sciadv.abd0772]
- Hu J, He J, Wang Y, Zhao Y, Fang K, et al. Ultrasound combined with nanobubbles promotes systemic anticancer immunity and augments anti-PD1 efficacy. J Immunother Cancer 2022;10(3):e003408. [PMID: 35236741 DOI: 10.1136/jitc-2021-003408]
- Qiu Y, Wu Z, Chen Y, Liao J, Zhang Q, et al. Nano ultrasound contrast agent for synergistic chemo-photothermal therapy and enhanced immunotherapy against liver cancer and metastasis. Adv Sci (Weinh) 2023;10(21):e2300878. [PMID: 37162268 DOI: 10.1002/advs.202300878]
- Shi E, Shan T, Wang H, Mao L, Liang Y, et al. A bacterial nanomedicine combines photodynamic-immunotherapy and chemotherapy for enhanced treatment of oral squamous cell carcinoma. Small 2023:e2304014. [PMID: 37653616 DOI: 10.1002/smll.202304014]
- Wagner Ecker M, Schwager C, Wirkner U, Abdollahi A, Huber PE. MicroRNA expression after ionizing radiation in human endothelial cells. Radiat Oncol 2010;5:25. [PMID: 20346162 DOI: 10.1186/1748-717×5-25].