Recent Progress in the Green Synthesis, Characterization, and Applications of Selenium Nanoparticles
1Department of Chemistry, K.T.H.M. College, Nashik 422002, Maharashtra, India
2KVPS’s Institute of Pharmaceutical Education, Boradi, Shirpur, Maharashtra, India
3St. John Institute of Pharmacy and Research, Palghar, Maharashtra, India
*Correspondence to: Sajeda Samreen Sayyed Ibrahim, Department of Chemistry, K.T.H.M. College, Nashik 422002, Maharashtra, India. E-mail sajedasamreen424@gmail.com; Sharad S. Gaikwad, Assistant Professor, Department of Chemistry, K.T.H.M. College, Nashik, 422002, Maharashtra, India. E-mail gaikwad.sharad85@gmail.com
Received: August 1 2024; Revised: August 27 2024; Accepted: September 2 2024; Published Online: November 5 2024.
Cite this paper:
Ibrahim SSS, Ansari YN, Puri AV et al. Recent Progress in the Green Synthesis, Characterization, and Applications of Selenium Nanoparticles. BIO Integration 2024; 5: 1–15.
DOI: 10.15212/bioi-2024-0063. Available at: https://bio-integration.org/
Download citation
© 2024 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
Selenium nanoparticles (Se-NPs) have attracted researchers’ attention because of their unique attributes and potential for application in diverse areas, such as biological medicine, environmental remediation, and energy generation. This review summarizes recent progress in the green synthesis and characterization of Se-NPs. It elaborates on the fabrication of Se-NPs through chemical, biological, and physical techniques, including advantages and challenges. Moreover, techniques for evaluating the chemical and physical characteristics of NPs are described. The promising applications of Se-NPs are emphasized, including antioxidant, anticancer, and antimicrobial applications, and treatment of neurodegenerative diseases. Because of their exceptional properties and biocompatibility, Se-NPs are used in diverse industries. Recently, plant-extract synthesized Se-NPs have become increasingly used because of their benefits over chemically synthesized Se-NPs, including lower cost and greater environmental friendliness.
Keywords
Characterization, green synthesis, selenium, selenium nanoparticles.
Significance Statement
Recently synthesized Se-NPs from plant extracts are described, and their mechanisms, applications, advantages, toxicity, and other aspects are summarized. These Se-NPs are notable for their environmental sustainability, cost-effectiveness, and enhanced biocompatibility. Eco-friendly synthesis approaches eliminate hazardous chemicals, decrease production costs, and yield biocompatible nanoparticles with superior antioxidant and antimicrobial properties. Compared to chemically synthesized nanoparticles, the produced Se-NPs with the help of green synthesis are safer due to their reduced toxicity and are more suitable for a range of biomedical and environmental applications. This review advances green nanotechnology by highlighting innovative synthesis methods and various applications, and promoting sustainable research practices.
Introduction
Richard Feynman’s statement that “There’s plenty of room at the bottom” has opened new doors for the scientific community, leading to research interest in nanotechnology, which pertains to the properties of matter at the atomic scale [1]. “Nanotechnology,” a term originated by Norio Taniguchi, refers to the production of materials with one or more dimensions at the nanoscale. The goal of nanotechnology is to improve manufacturing processes while producing superior-quality products. Nanoparticles (NPs), nanocomposites, and nanowires are the structural and functional components of nanotechnology [2], the use of small materials and systems. Nanotechnology is expected to play a critical role in solving a variety of challenges, including advancements in healthcare, environmental sustainability, and improving industrial efficiency and has the potential to influence countries’ global economic standing [3]. Nanotechnology involves working with matter at the atomic and molecular scale, which is extremely small—usually between 1 and 100 nm [4]. Materials at the nanoscale have unique properties that make them suitable for commercial applications that benefit humanity, such as biological probes, diagnosis, catalysis, display devices, and optoelectronics [5].
In the field of nanotechnology, matter can be modified at the molecular and atomic scales, to produce materials with unique characteristics that can be applied to a range of challenges [6, 7]. Nanoscale materials have properties different from those of their larger counterparts, and can be commercialized [8, 9]. Synthesis of nano-materials, particularly metallic NPs, can be achieved through various methods, such as laser pyrolysis, supercritical fluid synthesis, spinning, the sol-gel method, mechanical milling, chemical vapor deposition, molecular condensation, chemical reduction, green synthesis, etching, sputtering, laser ablation, and electro-explosion [10]. The most economical and sustainable method among those discussed is the green synthesis of metallic NPs [11]. This method, compared with chemical methods, poses less risk of biological threats that might result in environmental toxicity. This method applies biological agents, such as plant parts and other microorganisms including bacteria and fungi, as reducing and stabilizing agents [12, 13].
Using living cells to produce NPs via biological pathways is a highly efficient and effective technique with greater mass yield than similar methods. Biochemicals and other components that can serve as stabilizing and reducing agents for the synthesis of green NPs are abundant in plants. This method is economical, safe, environmentally beneficial, and also more stable than other physical, chemical, and biological methods. The green synthesis of NPs can be categorized into three types: extracellular, intracellular, and phytochemical. The extraction of NPs from plant extracts is an economical process that achieves high yields, because of the abundance of phytochemical components that act as reducing and stabilizing agents in converting metal ions into metal NPs [14].
Selenium (Se) is an essential trace element in various physiological processes, such as metabolism and immune function [15]. Selenium NPs (Se-NPs) have unique physicochemical properties and biocompatibility, and consequently are useful in biomedicine, catalysis, and biotechnology and pharmaceutical sectors [16]. Recently, Se-NPs produced from plant extracts have become frequently used because of their advantages over chemically synthesized Se-NPs, including lower toxicity and higher sustainability [17]. Using plant extracts as reducing agents for Se-NPs is more cost-effective and environmentally friendly than conventional synthesis methods [18]. Active phytoconstituents in plant extracts act as capping agents and accelerate the conversion of selenite to fundamental selenium, thus yielding Se-NPs with diverse applications [19].
Selenium’s valuable properties render it useful in various scientific fields including medicine, biology, physics, and chemistry. Se-NPs are of special interest because they interact with a variety of proteins and have strong biological activity. Functional groups such as C–O, C–N, NH, and COO– found in proteins are responsible for this interaction. Additionally, Se-NPs demonstrate high adsorption capacity [20]. Many studies have successfully produced Se-NPs from extracts of plants, such as Terminalia arjuna [21], Vitis vinifera (raisin), Capsicum annum [22], and fenugreek seeds [23]. This discovery has offered a new path for the environmentally friendly synthesis of Se-NPs by using plant extracts; this path may be valuable in many industries. Consequently, the use of plant extracts as reducing and stabilizing agents in Se-NP production is currently a major topic of scientific research [24].
The use of plant extracts in synthesizing Se-NPs advantageously enables precise control over the size and shape of the particles. Additionally, this process is straightforward and can be replicated consistently, and therefore is suitable for large-scale industrial production [25]. The biogenic synthesis of Se-NPs by using plant extracts is a promising alternative to traditional methods that provides a sustainable and environmentally friendly option, while still preserving traditional knowledge, and has the potential to revolutionize multiple fields [26]. Because of their cost-effectiveness, sustainability, and eco-friendliness, plant extracts are increasingly used for the green synthesis of Se-NPs. This technique enables specific control over particle dimensions and therefore is highly suitable for diverse applications in biotechnology and medicine, such as biosensors, cancer therapy, antimicrobial agents, and targeted drug delivery [27–29].
Synthesis methods of Se-NPs
Top-down approach
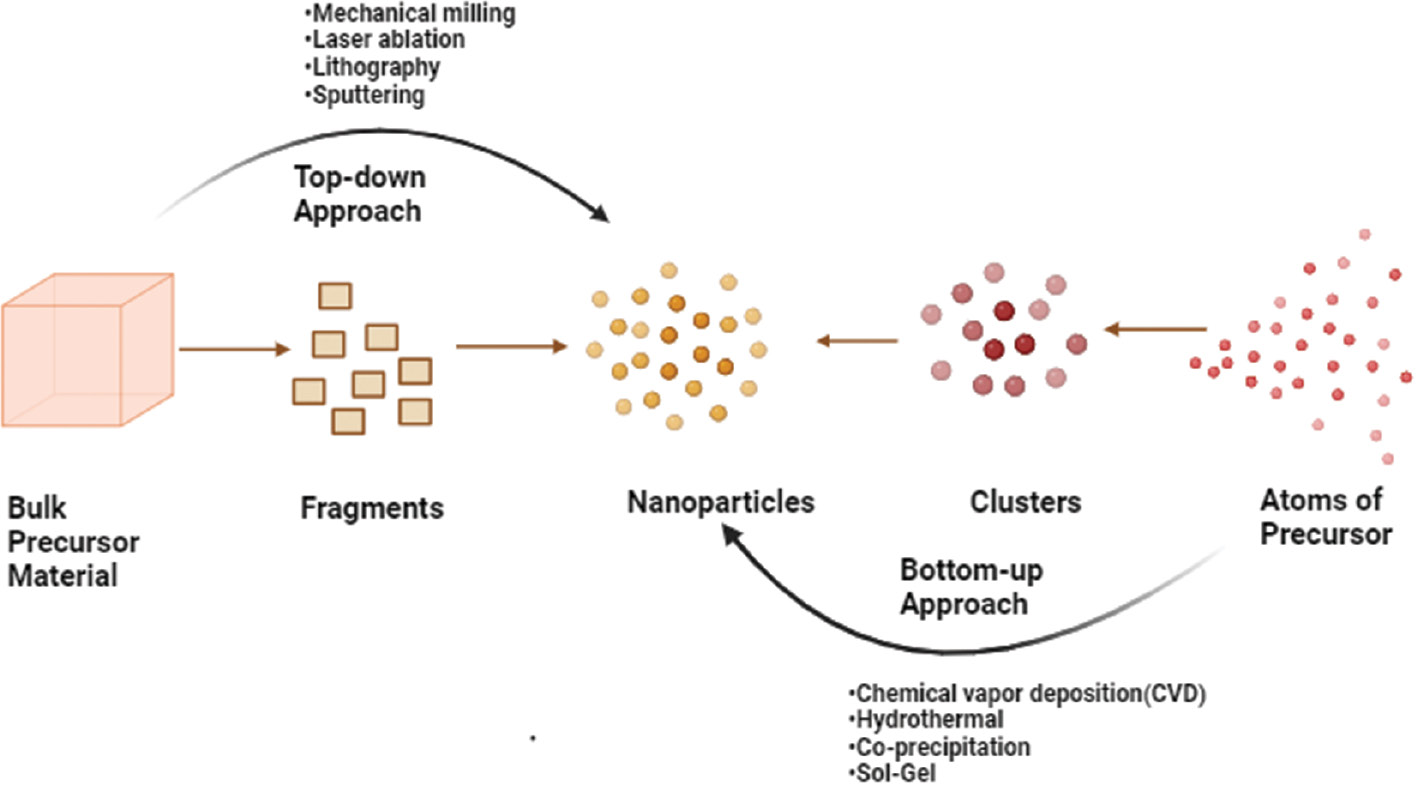
Selenium nanoparticles (Se-NPs) can be synthesized through biological methods utilizing the reducing and stabilizing properties of entities like plant extracts, microorganisms, and enzymes. Synthesis can be achieved through either top-down or bottom-up approaches, as shown in Figure 1. The first step in the top-down approach involves converting larger structures into nano-sized materials [30]. The top-down approach for the synthesis of nanoparticles (NPs) involves breaking down larger bulk materials into nanoscale particles. This method contrasts with the bottom-up approach, which builds NPs from atomic or molecular precursors. The top-down method has several drawbacks like the particles might not be uniform in size and shape, because of mechanical stress, vigorous shaking, and deformation during production. Although NPs can be produced on a larger scale, the top-down approach is not optimal in all cases [31]. When creating NPs, choosing the proper method is essential. The bottom-up approach tends to produce NPs with more distinct physical and chemical properties than the top-down approach. Therefore, the specific needs and objectives for NP synthesis must be carefully considered before selection of the most suitable method, to ensure that the desired results are achieved [32].
Figure 1 Illustration of the top-down and bottom-up approaches for the synthesis of nanoparticles.
Bottom-up approach
To synthesize NPs from molecules, certain materials are combined with agents that promote stability. Subsequently, the materials are subjected to specific conditions, such as heating, mixing, or chemical reactions [33]. NPs can be produced through a process called self-assembly, which enables control of the dimensions of the particles and addition of any necessary coatings or stabilizers. The production of metallic NPs starts with metal salts, which are broken into tiny atomic-sized particles. These particles then adhere and form NPs. This method is useful for producing NPs of the same size and shape, for applications such as drug delivery or catalysis. Self-assembly aids in control of the process and the production of uniform particles [34].
The bottom-up approach offers enhanced control over NP composition and surface characteristics. This technique also enables addition of specific coatings or functional groups to the NP surface, thus improving the particles’ stability and ability to interact with other materials. The bottom-up method is flexible and adaptable, thereby allowing for the production of NPs with various functionalities through the optimization of synthesis parameters, such as pH, temperature, and concentration, or use of different precursor materials [35]. In contrast, the top-down approach reduces larger structures, such as bulk materials or thin films, to nanoscale dimensions to form NPs. In creating NPs from molecules, specific stabilizing agents are combined with precursor materials and subjected to certain conditions, such as heating, mixing, or chemical reactions [36]. NP size and shape can be precisely controlled through a process called self-assembly, which includes adding necessary coating and stabilizing agents. Metallic NPs can be synthesized through a bottom-up synthesis method, in which metal salts are reduced to produce atomic-sized materials. These materials then undergo self-assembly through nucleation and growth, thus producing NPs with the desired dimensions. This method has many benefits, including the ability to create highly uniform NPs, thus aiding in applications that require particle uniformity, such as drug delivery systems or catalysis [37].
NPs can be made through either a bottom-up or top-down approach. The external appearance and composition of the particles can be more precisely controlled through the bottom-up method, which allows for specific coatings or functional groups to be added. Consequently, the particles are less prone to degradation and are better able to interact with other materials. The bottom-up approach can produce NPs with various functionalities through adjustment of synthesis parameters or use of different precursor materials. In contrast, the top-down approach reduces larger structures to nanoscale dimensions to synthesize NPs [38].
Biogenic synthesis of Se-NPs
Se-NPs can be produced by biological systems including fungi, bacteria, enzymes, and plant parts. Biogenic synthesis methods are increasingly used because of their eco-friendly and sustainable qualities. Unlike chemical approaches, which produce hazardous waste, biogenic synthesis converts soluble selenium ions, such as selenate or selenite, into NPs. This process uses biological agents such as plant extracts or microbial cells. Temperature, pH, concentration, and biological agent type all affect the process [39]. Biogenic synthesis is a cost-effective, environmentally friendly, highly stable, and biocompatible method for producing Se-NPs [40].
Plant-mediated Se-NPs synthesis
Se-NPs can be synthesized biologically using plant extracts, which facilitate the reduction of soluble selenium ions into nanoparticles. This green synthesis technique, known for its ease of use, low cost, and environmental friendliness, has captured substantial research attention [41]. Important plant parts, including leaves, seeds, flowers, stems, and roots, either dried or fresh, can be used in the synthesis process. Bioactive compounds that are phytochemicals, such as flavonoids, anthocyanins, or carotenoids, are extracted by boiling or sonication in water or organic solvents. To promote the reduction of selenium ions into NPs, selenium salts (precursors), such as selenite or selenate, are mixed with the plant extracts under optimal conditions, such as controlled pH and temperature [42]. Flavonoids and terpenoids are used in the synthesis to stabilize the metallic Se-NPs created by reducing selenium ions. The plant type, selenium ion concentration, and reaction time are several variables affecting the dimensions of Se-NPs [43]. The evaluation of metallic Se-NPs can be performed with methods including ultraviolet-visible (UV-vis) spectroscopy, scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), and Fourier transform infrared spectroscopy (FTIR) [44]. The synthesis of Se-NPs with plants offers several advantages, such as excellent biocompatibility; non-toxicity; and potential applications in healthcare, agriculture, and environmental remediation [45].
The method for synthesis of Se-NPs with selenious acid and plant extracts is as follows. The plant extract is prepared by mixing the plant material with water and allowing it to stand for a period of time. The extract is then filtered to remove any solid particles. The plant extract is mixed with selenious acid, which acts as a selenium-containing precursor. The mixture is stirred at room temperature for a specific time interval, typically 12–72 hours. The plant extract contains biomolecules that act as reducing agents and stabilizers for the Se-NPs. The reduction of selenious acid to Se-NPs occurs under the influence of these biomolecules. The Se-NPs are separated from the reaction mixture by centrifugation at high speed, and are subsequently washed thoroughly with water and solvent to remove any residual plant extract or other impurities [51, 52].
Bacteria-mediated Se-NP synthesis
Bacteria can be used to synthesize Se-NPs by converting soluble selenium ions into NPs with bacterial cells or cell-free extracts. This technique has attracted interest because of its simplicity, high yield, and potential for massive production. To increase the reduction of selenium ions into NPs, the synthesis process involves incubating bacterial cells or cell-free extracts with selenium salts, such as selenite or selenate, under controlled pH and temperature conditions. The resulting Se-NPs can be analyzed with various analytical methods, including UV-vis spectroscopy, TEM, and XRD. Bacillus subtilis, Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa are among the bacterial species demonstrated to be able to produce Se-NPs. The synthesis process involves bacterial cells secreting reducing agents, such as proteins, enzymes, or metabolites, which aid in the transformation of selenium ions into NPs. The dimensions of Se-NPs are influenced by variables such as the type of bacteria, concentration of selenium ions, and reaction time. The biological synthesis of Se-NPs with bacteria offers numerous advantages, including high stability, potential therapeutic and environmental applications, eco-friendliness, and possibilities for future use [79].
Fungi- mediated Se-NP synthesis
During the fabrication of Se-NPs with fungi, fungal cells or fungal extracts are used to transform soluble selenium ions into NPs. This technique is considered eco-friendly and sustainable, because of its affordability and high yield. For synthesis of Se-NPs, fungal extracts or cells are incubated with selenium salts under suitable pH, temperature, and incubation time conditions. The enzymes, polysaccharides, and proteins in the fungal extracts or cells act as reducing agents in the conversion of selenium ions to NPs. The generated Se-NPs can be analyzed with analytical methods such as UV-vis spectroscopy, XRD, and TEM. Fungi such as Candida glabrata, Aspergillus niger, and Penicillium species have been used to produce Se-NPs. The dimensions of Se-NPs are influenced by many factors, such as the type of fungus, selenium ion concentration, and reaction conditions. The advantages of producing Se-NPs biologically with fungi include high stability; low toxicity; and potential biomedicine, biotechnology, and environmental remediation applications. Se-NPs generated from fungi have been demonstrated to possess anticancer, antifungal, antibacterial, and antioxidant properties, and can be used as drug delivery systems [79, 80].
Characterization of Se-NPs
Ultraviolet-visible spectroscopy
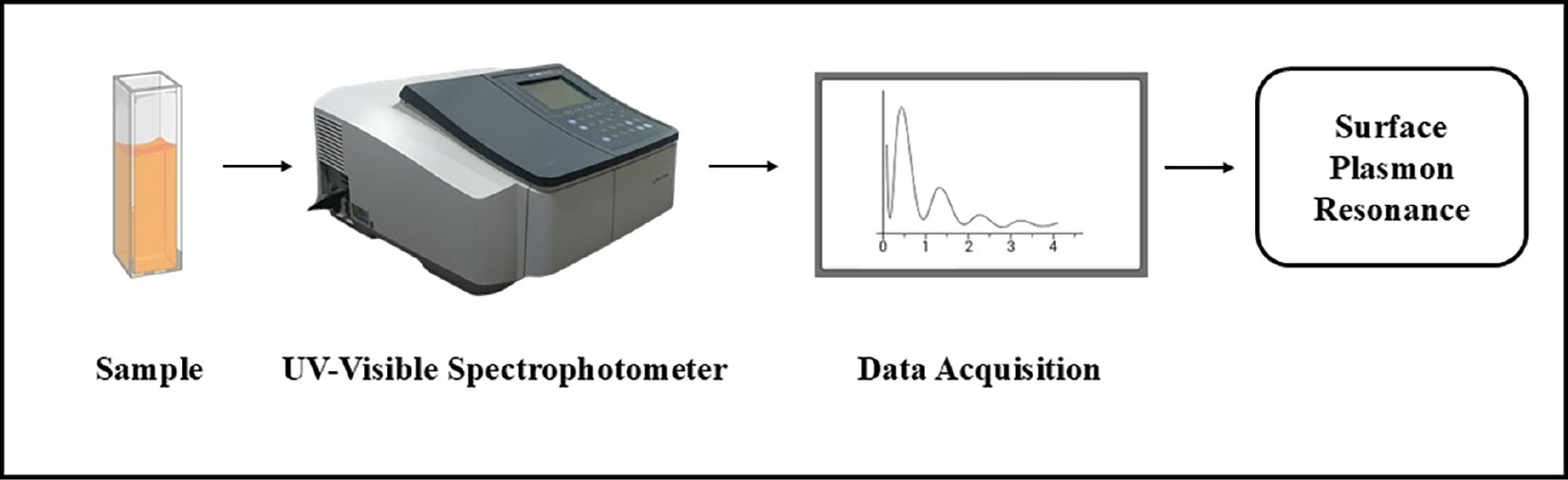
UV-vis spectroscopy is a tool used to estimate the optical properties of NPs. Figure 2 shows the UV-Vis absorption spectra of the synthesized nanoparticles, illustrating the surface plasmon resonance. This method provides valuable information regarding particle size, shape, and composition. NPs have quantized energy levels because of their small size [81]. The size of Se-NPs affects their electronic structure, and consequently how they absorb and scatter light. These effects are particularly noticeable in the UV-vis region. This method helps researchers examine how NPs interact with light, thus providing essential information for understanding NP size and quantum properties [82]. UV-vis spectroscopy studies have shown that Se-NPs have unique electrical structures that cause them to absorb light in a distinct manner. Surface plasmon resonance information is essential for understanding how light interacts with NPs. Researchers have confirmed the presence of Se-NPs according to peaks in the UV region (200–400 nm) [83], as depicted in Table 1.
Figure 2 Characterization of nanoparticles (NPs) by UV-Vis spectroscopy.
Table 1 Plant-Mediated Fabrication of Se-NPs.
| Sr. No | Plant Name | Plant Parts Used | UV (nm) | Average Size (nm) | Biological Activity | References |
|---|---|---|---|---|---|---|
| 1 | Allium sativum | Clove | 260 | 100 | Antimicrobial | [46] |
| 2 | Azadirachta indica | Leaves | 286 | 168 | Anthelmintic, antibacterial | [47] |
| 3 | Brassica oleracea | Florets | 370 | 25 | Antimicrobial | [48] |
| 4 | Carica papaya | Fruit | 364 | 101 | Antimicrobial | [49] |
| 5 | Cassia angustifolia | Seed | 286 | 00–00 | Antibacterial, antifungal | [50] |
| 6 | Cassia auriculata | Leaves | 252 | 50 | Anti-proliferative | [53] |
| 7 | Citrus lemon | Fruit juice | 400 | 90 | Antioxidant | [54] |
| 8 | Citrus paradise | Peel | 550 | 10 | Antibacterial | [20] |
| 9 | Citrus reticulata | Peel | 265 | 70 | Antimicrobial | [55] |
| 10 | Citrus sinensis | Peel | 250–300 | 20 | Antibacterial | [56] |
| 11 | Clausena dentata | Leaves | 420 | 80 | Larvicidal | [57] |
| 12 | Cleistocalyx operculate | Leaves | 302 | 200 | Antibacterial | [58] |
| 13 | Clitoria ternatea | Flower | 635 | 106 | Antibacterial | [59] |
| 14 | Diospyros montana | Bark | 289 | 150 | Antibacterial | [60] |
| 15 | Enicostema axillare | Leaves | 325 | 98 | Antibacterial | [61] |
| 16 | Hibiscus sabdariffa | Leaves | 320 | 50 | Antioxidant | [62] |
| 17 | Moringa oleifera | Leaves | 530 | 20 | Antioxidant | [63] |
| 18 | Moringa peregrina | Leaves | 279 | 150 | Antibacterial, anticancer | [64] |
| 19 | Nigella sativa | Seed oil | 530 | 75 | Larvicidal | [65] |
| 20 | Ocimum gratissimum | Leaves | 300 | 50 | Antimicrobial | [66] |
| 21 | Opuntia basilaris | Peel | 280 | 90 | Antibacterial | [67] |
| 22 | Portulaca oleracea | Leaves | 266 | 30 | Antimicrobial | [68] |
| 23 | Psidium guajava | Leaves | 381 | 20 | Antibacterial | [69] |
| 24 | Punica granatum | Peel extract | 330 | 145 | Antioxidant | [70] |
| 25 | Ribes nigrum | Fruit | 265 | 50 | Antioxidant | [71] |
| 26 | Solanum lycopersicum | Seed | 350 | 100 | Antimicrobial | [72] |
| 27 | Terminalia arjuna | Bark | 289 | 150 | Anticancer | [73] |
| 28 | Theobroma cacao | Seed | 276 | 50 | Antioxidant | [74] |
| 29 | Tinospora cordifolia | Stem | 285 | 200 | Antioxidant, anticancer | [75] |
| 30 | Trigonella foenum-graecum | Seed | 200–400 | 50–150 | Anticancer | [76] |
| 31 | Vitis vinifera | Fruits | 280 | 100 | Antioxidant | [77] |
| 32 | Withania somnifera | Root | 622 | 22 | Antioxidant | [78] |
Fourier transform infrared spectroscopy
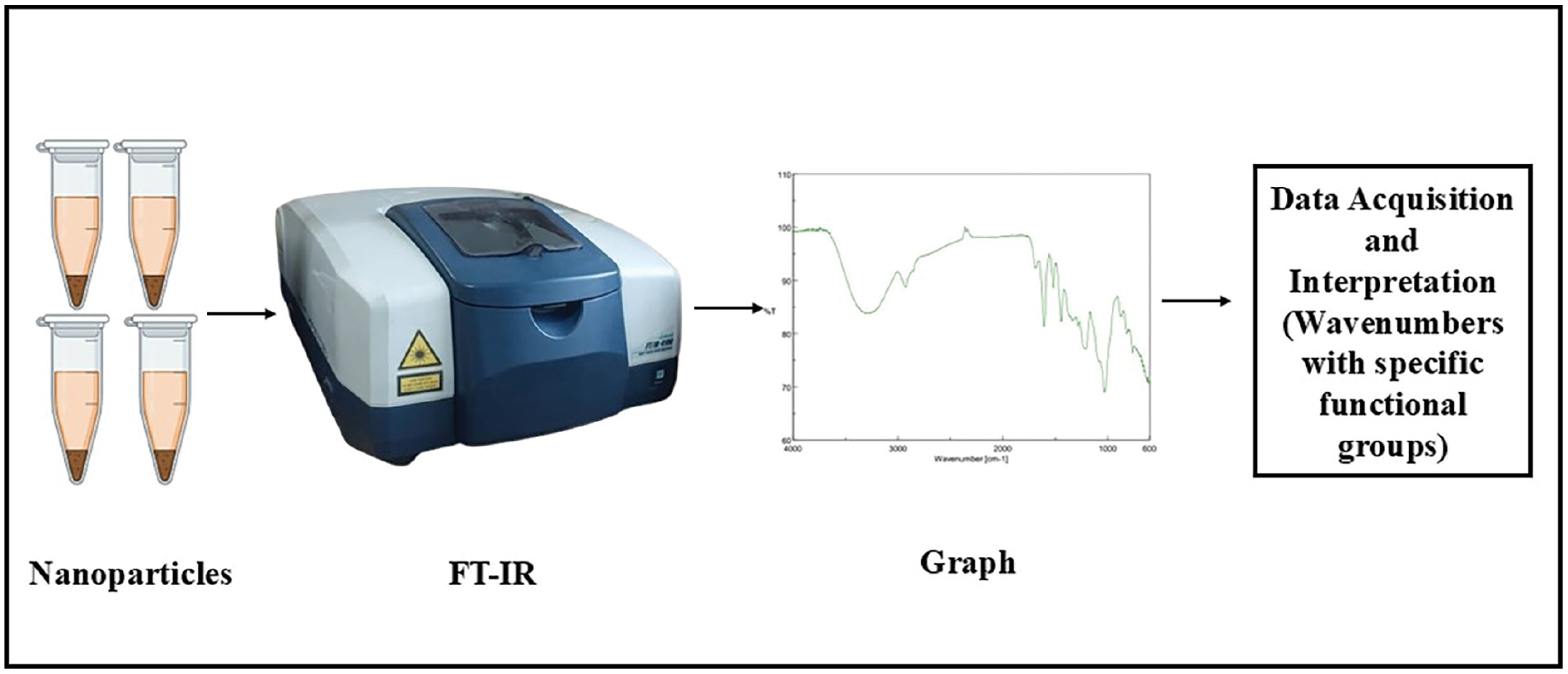
Fourier transform infrared spectroscopy (FT-IR) is a powerful technique for analyzing a material’s functional groups and chemical bonds, thereby providing information on Se-NP composition and surface properties. Figure 3 presents the procedure to get FTIR spectra of synthesized nanoparticles. FTIR analysis reveals details regarding the surface chemistry and organic capping agents [84]. This method can help confirm the synthesis process and understand NPs’ chemical environment. Plants rich in polyphenolic constituents are generally selected for synthesis. The active involvement of O–H, N–H, C=O, and C–O functional groups in the formation of Se-NPs can be validated by FTIR spectroscopy [85]. After the green synthesis of Se-NPs, the characteristic peaks observed in the FTIR spectrum are at 1375 cm−1 (indicating phenolic OH), 1030 cm−1 (corresponding to aromatic in-plane C–H bending), 1462 c−1 (representing asymmetric C-H bending in CH3 and CH2), and 1250 cm−1 (indicating secondary O-H) [86]. Between 3200 and 3500 cm−1, bonded O–H stretching can be confirmed. Analysis of the FTIR spectrum of Se-NPs synthesized from plant extracts, provides insights into the chemical composition, surface functionalization, and surface modifications of NPs.
Figure 3 Characterization of nanoparticles (NPs) by fourier transform infrared spectroscopy (FTIR).
Transmission electron microscopy
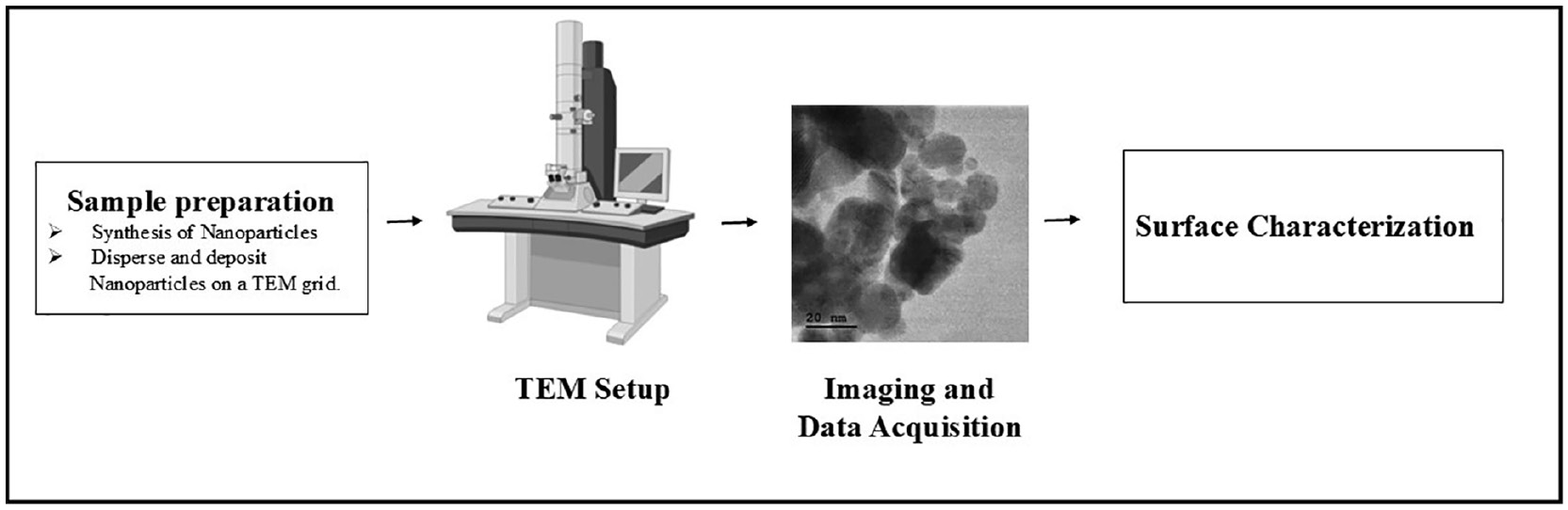
The fundamental morphology and dimensions of Se-NPs can be observed in high-resolution images produced by TEM. Figure 4 showcases TEM image of the synthesized nanoparticles, providing detailed insights into their morphology, size, and distribution at the nanoscale. This information is essential for interpreting NPs’ physical properties. NP size can be accurately measured with TEM. This aspect is important, because NPs’ size significantly influences their properties, including their optical, electronic, and catalytic behavior [87, 88]. TEM can reveal details regarding the internal structure of Se-NPs and also provides insights into the spatial distribution of Se-NPs within samples. This information is valuable for understanding how well NPs are dispersed or aggregated, both of which influence NPs’ activities and applications [88]. TEM can aid in identifying and visualizing any organic capping agents or ligands present on Se-NP surfaces. These coatings can potentially be incorporated during the synthesis process, and can affect NPs’ stability and interactions with other substances [89]. TEM is used to characterize NPs with dimensions below 10 nm. Energy-dispersive X-ray spectroscopy can be coupled with TEM to analyze the chemical composition of NPs, thereby confirming the presence of selenium and any other elements in the NPs. This method also aids in identifying any additional elements introduced during synthesis [90].
Figure 4 Characterization of nanoparticles (NPs) by transmission electron microscopy (TEM).
Scanning electron microscopy
SEM is a valuable technique for visualizing NP morphology and providing details on particle size, shape, and surface characteristics. This technique enables high-resolution examination of the surface topography and structure of NPs. SEM scans NP surfaces with a focused electron beam. Different signals are produced as the electrons interact with the sample, including secondary and backscattered electrons. These signals are identified and used to generate an image of the NP surface. Consequently, researchers can examine NPs’ structure, quantify their size and shape, and identify any surface defects. Alagesan et al. have revealed a distinct propensity of Se-NPs to aggregate—an observation substantiated by field-emission SEM images. These Se-NPs exhibit a spherical morphology, with diameters of 45–90 nm. During the synthesis of nanoparticles (NPs), particle aggregation becomes the dominant process, which masks the reduction of precursor atoms and the initial nucleation of these atoms [91].
Dynamic light scattering
Dynamic light scattering (DLS) is a prominent approach for characterizing NPs in solutions, and providing information regarding their size distribution and mobility. Figure 5 depicts the sample machine setup used for DLS analysis. The mechanism of characterizing Se-NPs with DLS is based on the principles of Brownian motion and the interaction of laser light with NPs in a solution [92]. This approach is widely used to characterize Se-NP size and distribution. This technique can determine the average size of NPs. The zeta potential of NPs can also be measured with DLS. The zeta potential measures NP surface charge and stability in a liquid medium, and is determined by analysis of the electrophoretic mobility of particles in an applied electric field [93]. Sani-e-Zahra et al. have used DLS analysis to calculate the average size of Se-NPs, thus demonstrating polydispersity in Se-NPs derived from tomato juice and seed extract sources. Two distinct model peaks were observed, at 989.5 nm and 151.7 nm, accompanied by a polydispersity index value of 0.432. The average size of the Se-NPs in the tomato juice extract was approximately 1020 nm [71].
Figure 5 Characterization of NPs by dynamic light scattering (DLS).
X-ray diffraction
XRD, based on Bragg’s law, illustrates X-ray diffraction by crystal planes. When X-rays collide with a crystalline sample, they interact with the lattice and are dispersed at different angles. The scattering angles can be used to calculate the interatomic distances within the crystal lattice. XRD can be used to determine the crystalline structure of Se-NPs. Exposing the NPs to X-rays provides diffraction patterns revealing information about the arrangement of atoms in the NPs. Consequently, the crystal structure can be identified, although the synthesis method and conditions can introduce variations. For example, selenium can exist in different crystalline forms, including hexagonal and amorphous forms, which can be distinguished by XRD. XRD data can be used to estimate the average Se-NP particle size through analysis of peak broadening in the XRD pattern, which is associated with the size of the crystalline domains in the NPs. Moreover, XRD can reveal the presence of impurities in the synthesized Se-NPs and can confirm Se-NPs’ chemical composition and stoichiometry [94]. Hashem et. al. have reported the XRD analysis of green synthesized Se-NPs and described the crystal and amorphous composition for precursor and synthesized Se-NPs, respectively [95].
Energy dispersive X-ray spectroscopy
Energy dispersive X-ray spectroscopy (EDX) provides useful information regarding NPs’ elemental compositions and chemical characteristics, through analysis of the energy distribution of X-rays emitted by a sample. Figure 6 illustrates the process of EDX in nanoparticle characterization. EDX provides quantitative data on the elemental composition, including the ratio of selenium to other elements present, which can aid in assessing NP purity. Examining the spatial distribution of elements within an NP and its elemental composition is helpful. This approach is based on the X-ray fluorescence principle. High-energy X-rays ionize and excite atoms in the sample, and characteristic X-ray spectra are emitted. Advanced EDX systems, such as X-ray photoelectron spectroscopy (XPS or ESCA), provide information regarding the chemical states of elements, thereby aiding in identifying chemical bonds and understanding NP surface chemistry. EDX spectroscopy can also be coupled with scanning electron microscopy to further enhance NP characterization [96]. Shahbaz et al. identified the solid absorption peaks of selenium ions at 1.35 keV, 11.20 keV, and 12.40 keV during the synthesis of selenium nanoparticles (Se-NPs) from plant extracts, using EDX spectra. According to EDX analysis, selenium coexists as peaks with other elements in elemental form [97].
Figure 6 Characterization of NPs by energy dispersive X-ray spectroscopy.
Zetasizer analysis
Particle size remains a key determinant of NP biodistribution, uptake, and clearance from the body. A Zetasizer instrument can be used to measure Se-NP particle size in terms of hydrodynamic diameter. The size distribution can also be characterized by the polydispersity index, which is expressed as mutually exclusive and opposite values: the lower the index, the higher the monodispersity. Zeta potential refers to NP surface charge and is used to determine the stability of an NP dispersion. Particles with a high zeta potential, whether positively or negatively charged, generate repulsive forces that prevent aggregation, thereby ensuring long-term stability in the solution. Zeta potential also affects the behaviors of NPs toward biological entities such as cell membranes and proteins. When Se-NPs are characterized with a Zetasizer, synthesis methods can be tailored to synthesize Se-NPs of known size, with surface charges suitable for a given application. This information is valuable for establishing proper Se-NP formulations for drug delivery, imaging, and therapeutic applications in which size and stability play important roles in effectiveness and safety [98].
Factors affecting the synthesis of Se-NPs
Sources of reducing and stabilizing agents
Plant extracts
The type and the part of the plant used (leaves, stems, or roots) affects the synthesis because of the varying concentrations of phytochemicals such as flavonoids, phenolic compounds, and terpenoids [99].
Microorganisms
Various strains of bacteria, fungi, and algae have enzymatic pathways that affect the reduction and stabilization of selenium ions [100].
Concentrations of precursor and reducing agents
Higher concentrations of selenium salts (e.g., sodium selenite or selenious acid) lead to the formation of larger NPs or higher yield, but can also increase the risk of aggregation [75, 101].
pH of the reaction medium
The pH of the synthesis medium affects the charge on the NPs and the ionization state of the reducing agents, and consequently influences the reduction rate, particle size, and stability. Neutral to slightly alkaline pH is favorable for Se-NP synthesis [102].
Temperature
Higher temperatures accelerate the reduction process, and affect the size distribution and crystallinity of Se-NPs [103]. Excessively high temperatures may lead to uncontrolled growth and aggregation.
Reaction time
The duration of the synthesis process influences the growth and stabilization of NPs [103]. Shorter reaction times may result in incomplete reduction, whereas longer times can lead to larger particles or aggregation. Finding an optimal reaction time is important for achieving desired NP characteristics.
Agitation and mixing
Proper mixing ensures uniform distribution of reducing agents and selenium precursors in the reaction medium, thus promoting homogeneous nucleation and growth of NPs [104]. Agitation speed can influence particle size and distribution.
Ionic strength and presence of additives
The ionic strength of the medium, influenced by the presence of salts or other additives, can affect the electrostatic interactions between particles, thereby influencing their stability and aggregation behavior. The choice of solvent can influence the solubility of the precursor and reducing agents, as well as the reduction kinetics. Aqueous solvents are frequently used in green synthesis, because of their eco-friendliness [24, 105].
Cytotoxicity of Se-NPs
In evaluating the suitability of green synthesized Se-NPs for biomedical purposes, investigating their cytotoxicity is crucial. The green synthesis method uses natural and environmentally friendly sources, such as plant extracts, to produce Se-NPs, which are presumed to be less harmful than chemically synthesized Se-NPs [106]. Several investigations have been conducted to determine the cytotoxicity of Se-NPs produced through green synthesis with various cell lines, including normal and cancer cells. The cytotoxicity of Se-NPs produced through green synthesis varies according to the concentration, size, surface charge, and duration of exposure. Although some studies have concluded that Se-NPs produced through green synthesis have minimal cytotoxicity at low doses, others have reported heightened cytotoxicity at higher concentrations. Notably, the cytotoxicity of Se-NPs may also be influenced by the cell type and biological environment in which they are used. Assays such as MTT, LDH, and Annexin V/propidium iodide can be used to evaluate the cytotoxicity of Se-NPs produced through green synthesis. These tests measure cell viability, membrane integrity, and apoptosis/necrosis after exposure to Se-NPs. Although green-synthesized Se-NPs are less hazardous than chemically synthesized Se-NPs, their cytotoxicity must be thoroughly investigated to ensure their safety and efficacy in various biological applications [107].
Future advances
Se-NPs have substantial potential in diverse applications, as illustrated in Figure 7. Se-NPs have a high surface-to-volume ratio, thus enhancing their activity and making them more effective than larger particles. Se-NPs have substantial potential in a range of biological applications, including medication delivery, cancer therapy, and antioxidants. Studies have demonstrated their anti-cancer, antioxidant, antimicrobial, and anti-biofilm properties. The application of nano-Se medications has shown promising results in treating Huntington’s disease. Se-NPs have notable semiconducting, photoelectric, and X-ray-sensing properties; are used in photocells, photocopying, photometers, and xerography; and are also important in renewable energy devices. Se-NPs are valuable in environmental applications because of their mercury-capturing properties.
Figure 7 Applications of green synthesized Se-NPs.
Se-NPs in anticancer applications
Cancer is a major research focus, because it is the most destructive disease in the 21st century. Current challenges include problems of drug-induced toxicity and resistance. Various treatment methods are being tested to combat cancer. With the help of nanotechnology, personalized medicine has become more effective, by enabling better targeting while decreasing toxicity. Inorganic NPs, such as Se-NPs, have been successfully used to induce cytotoxicity in cancer cells. Se-NPs have the potential to decrease drug resistance and limit chemotherapeutic drug toxicity. Se-NPs derived from the probiotic bacterial strain Lactobacillus casei ATCC393 have been biogenically synthesized and demonstrated to suppress colon cancer cell proliferation, both in vitro and in vivo. At a treatment dose of 15 g/mL, Caspases 3/7 and 9 are activated by Se-NPs, thereby limiting the development of Caco-2 colon cancer cells. Moreover, Se-NPs have been found to activate intrinsic apoptotic pathway-associated apoptotic processes in CT26 and HT29 colon cancer cells [108]. The immunomodulatory effects of Se-NPs as an immunoadjuvant have been examined by Yazdi, et al., to develop a preventive tumor-associated antigen-based vaccine effective against breast tumors in mice [109]. For the prevention of cervical cancer, Se-NPs have been synthesized through green chemistry methods, and altered with a hydrophilic biocompatible polymer such as chitosan to incorporate anticancer drugs such as paclitaxel. Se-NPs act primarily by inducing apoptosis through caspase activation and mitochondrial dysfunction, thus generating reactive oxygen species (ROS), and leading to oxidative stress and DNA damage, inhibited cell proliferation via cell cycle arrest, and downregulation of proliferative signaling pathways [110].
Se-NPs in antimicrobial applications
Antimicrobials, including antibiotics, antivirals, antifungals, and antiparasitics, are currently essential in medical practice. Despite warnings regarding the adverse effects of antibiotic resistance, which was discovered in penicillin-resistant bacteria, antibiotics continue to be overused. Since the discovery of Staphylococcus in 1940, with extensive use of antimicrobials in food, medicine, and agriculture, multidrug-resistant microorganisms have proliferated and have become more difficult to eradicate with potent antibiotics. The growth of antimicrobial resistance has necessitated development of alternative antimicrobials. Gold, silver, copper, titanium dioxide, and zinc oxide NPs are among those currently being researched. Many studies have shown excellent efficacy of Se-NPs as broad-spectrum antibacterial agents against bacteria, viruses, fungi, and parasites. According to Sans-Serramitjana et al., the application of Se-NPs against oral pathogenic microorganisms such as C. albicans, E. faecalis, P. gingivalis, and S. mutans appears to be promising for in vitro reduction of planktonic and sessile microbial populations. Se-NPs exhibit antimicrobial activity by generating ROS that damage microbial cell walls and DNA, disrupting cell membranes to cause leakage, interacting with sulfur-containing proteins and consequently inhibiting microbial functions, and preventing biofilm formation. These mechanisms collectively enhance the effectiveness against various bacteria [111].
NPs in antifungal applications
Se-NPs have antifungal properties and have found use in various biological applications. These NPs primarily interfere with essential fungal enzymes and proteins, thus disrupting metabolic processes. Lazcano-Ramirez. et al. have conducted assays with Se-NPs at serial dilutions from 0 to 1.7 mg/mL, and have reported their antifungal activity against the commercially important plant pathogenic fungi Fusarium oxysporum and Colletotrichum gloeosporioides. Both Se-NPs showed antifungal activity against the plant pathogens at 0.25 mg/mL doses. Nile et al. have functionalized biogenic Se-NPs synthesized with the help of Paenibacillus terreus with nystatin (Se-NP@PVP nystatin nanoconjugates) and used them to inhibit Candida albicans growth, morphogenesis, and biofilm formation. Although Se-NPs produced during biological processes are inert, nanoconjugates have demonstrated antifungal activity against C. albicans by preventing growth, morphogenesis, and biofilm formation [112].
Se-NPs in antidiabetic applications
Diabetes is a common metabolic disorder that affects many people and can greatly reduce their quality of life. According to the World Health Organization, diabetes is expected to affect 366 million people by 2030, and is associated with 1.5 million annual fatalities worldwide. Several factors contribute to the development of diabetes, including poor eating habits, stress, inactivity, obesity, inflammation, heredity, and age. However, various methods are available to manage diabetes and its associated complications, including dietary changes; engaging in physical activity; and closely monitoring blood pressure, glucose levels, and cholesterol. The protein hormone insulin is typically administered through subcutaneous injections to regulate blood glucose levels in people with diabetes. However, frequent insulin injections can cause discomfort, localized infection, fatty deposition, hypertrophy, and trypanosomiasis. Se-NPs have been used in studies to address diabetes, because of their strong ability to regulate blood glucose levels. Se-NPs elicit antidiabetic effects by enhancing insulin sensitivity, decreasing oxidative stress and inflammation, regulating glucose metabolism, and protecting pancreatic beta cells, thereby improving glucose control and mitigating diabetes complications. Gutierrez et al. have administered Se-NPs derived from luteolin (Lu) and diosmin (DIO) to mice with streptozotocin-induced diabetes, to treat hyperlipidemia, hyperglycemia, and hepato-renal dysfunction. This treatment resulted in enhanced serum biochemical parameters, better glycemic control, and decreased lipotoxicity while maintaining β-cell function. The findings suggest that synthesized NPs can effectively manage diabetic diseases and have high potential for ameliorating the disorders associated with diabetes mellitus [113].
Toxicity assessment
Se-NPs have greater effects on organisms than inorganic selenium forms. In addition, each individual’s need for antioxidant defense determines how selenium affects health status. Selenium becomes toxic when present in excess. Selenium toxicity in general and Se-NP toxicity have been assumed to be related: both selenium and Se-NPs have pro-oxidative properties that increase ROS concentrations. The bioaccumulation phenomenon may amplify this effect in various tissues, among which the liver is most susceptible [114]. However, in the toxicological examination of Se-NPs, only the function of the antioxidant system; body weight; and bioaccumulation in the liver, kidneys, and heart have received substantial attention. The ways in which Se-NPs interact with the gastrointestinal tract, immunological system, muscles, and other indirect targets of selenium are poorly understood [115]. Se-NPs are less harmful than selenium in most tests. Sublethal doses of 20 nm Se-NPs at 0.05, 0.5, or 4 mg Se/kg body weight (BW)/d were not found to lead to differences in brain neurotransmitters or hematological markers between control and sodium selenite-treated groups (0.5 mg Se/kg BW/d) during a 28-day trial [116]. Se-NPs did not show more efficient bioaccumulation in blood and tissues after dietary administration of 10 mg Se/kg BW. Plasma, liver, and kidney GPx activity did not differ between Se-methionine and Se-NP treatment. Moreover, Se-NPs led to less immediate liver injury and less toxicity than Se-Met. A decrease in the dietary selenium stockpile and an increase in the lethal dosage in Se-NPs fed mice demonstrated the efficacy of Se-NPs in avoiding selenium toxicity. The hypothesized mechanism involves the cell’s unique selenium uptake and phase 2 response. Despite the varying toxicological effects of Se-NPs, biologically or ecologically fabricated and altered NPs have been reported to enhance animal health, with diminished toxicity [117]. Specific doses of Se are believed to be harmful. Therefore, the toxicity of Se nanomaterials is believed to depend on both the size/shape and dosage of Se-NPs. Many studies have shown that biogenic Se-NPs are less harmful than sodium selenite in animals. Bano et al. have researched the toxicological effects of Se-NPs in animals and concluded that low concentrations of Se-NPs can be considered safe [118].
Conclusion
Se-NPs can be fabricated through physical, chemical, and biological methods. The green synthesis approach is gaining attention for its economical and eco-friendly advantages. Using natural sources such as extracts of plants and microorganisms as reducing and stabilizing agents in Se-NP synthesis is a promising technique. The green synthesis of Se-NPs has substantial potential in various fields, such as medicine, agriculture, and environmental remediation. Moreover, Se-NPs have potential in cancer therapy, wound healing, and drug delivery systems in medicine. In agriculture, Se-NPs have been found to improve plant growth and resilience to environmental challenges and diseases. In environmental remediation, Se-NPs have been demonstrated to remove contaminants from wastewater and soil. The future of Se-NPs will entail developing new green synthesis procedures and optimizing existing methods to increase Se-NP yield and stability. Combining Se-NPs with other nanomaterials and traditional medicines is expected to create more effective and targeted treatments for various ailments. The green synthesis of Se-NPs has excellent potential for diverse applications, and further study in this field will be critical for producing safe and effective nanomaterials that substantially benefit society. The phytoconstituents that cap selenium nanoparticles (Se-NPs) enhance their therapeutic effectiveness in a dose-dependent manner, opening up new possibilities for use in the food, pharmaceutical, and biomedical industries. The biosynthesis of plant-based NPs is a relatively simple process that is easily scalable for large-scale production. This Review provided a comprehensive overview of the current status and future prospects of this emerging field. Future research on Se-NPs should focus on optimizing green synthesis methods for sustainability and scalability, advancing characterization techniques, and exploring the potential of Se-NPs in targeted drug delivery systems. In-depth mechanistic studies are needed to understand their biological activity, and comprehensive toxicity and environmental impact assessments will be essential to ensure safety. Efforts should also be directed toward clinical translation, including preclinical studies and regulatory framework development. By summarizing recent advances in synthesis methods, characterization techniques, and potential applications, this Review provides insights into the unique properties and promising therapeutic potential of Se-NPs. Exciting new avenues may enable the design and development of robust biogenic Se-NPs that can be produced, stored, and marketed globally without risk.
Conflict of interest
The authors declare that there are no conflicts of interest.
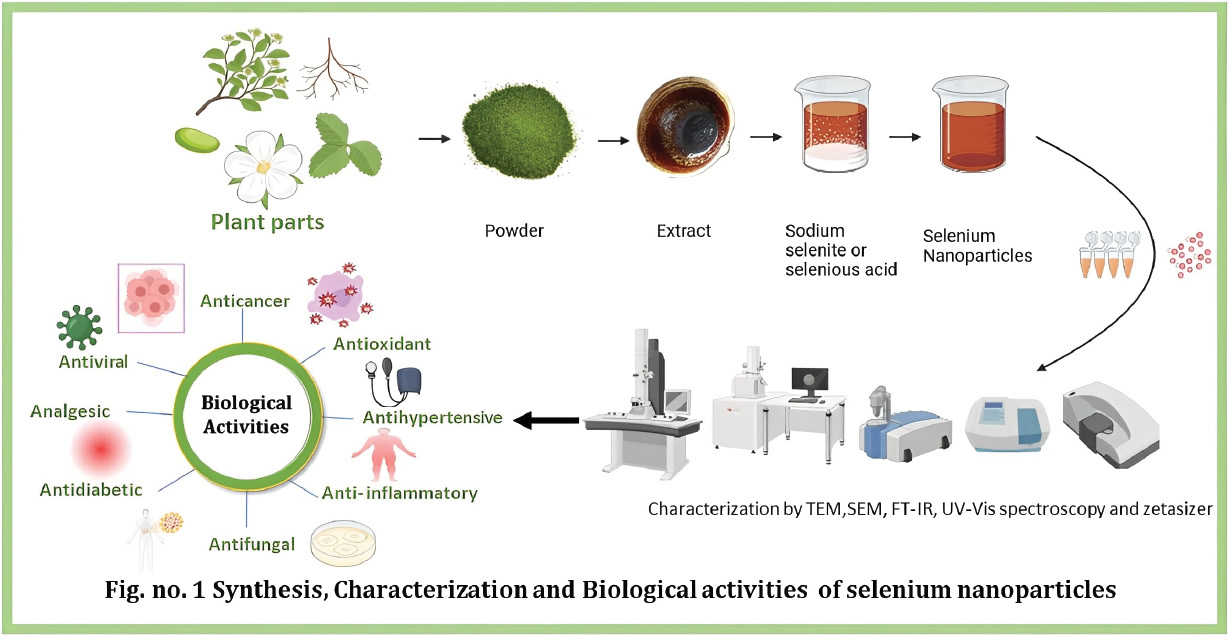
Graphical abstract
The graphical abstract summarizes the eco-friendly synthesis of Se-NPs by mainly using plant extracts followed by characterization thoroughly highlighting their biological activity. The green route followed to synthesize the nanoparticles is depicted in the synthesis chart, and characterizations have been done using UV-Vis spectroscopy, FTIR, Zetasizer, SEM, and TEM for further evidence of particle formation, stability, morphology, and size distribution as well. The recently synthesized nanoparticles’ biological activities, derived from a range of plant sources, are summarized, highlighting their pharmacological potential and diverse therapeutic applications as reported in this review paper.
References
- Kuhn R, Bryant IM, Jensch R, Böllmann J. Applications of environmental nanotechnologies in remediation, wastewater treatment, drinking water treatment, and agriculture. Appl Nano 2024;3(1):54-90. [DOI: 10.3390/applnano3010005]
- Szczyglewska P, Feliczak-Guzik A, Nowak I. Nanotechnology–general aspects: a chemical reduction approach to the synthesis of nanoparticles. Molecules 2023;28(13):4932. [DOI: 10.3390/molecules28134932]
- Bayda S, Adeel M, Tuccinardi T, Cordani M, Rizzolio F. The history of nanoscience and nanotechnology: from chemical-physical applications to nanomedicine. Molecules 2019;25(1):112. [PMID: 31892180 DOI: 10.3390/molecules25010112]
- Adya AK, Canetta E. Chapter 14 – nanotechnology and its applications to animal biotechnology. In: Verma AS, Singh A, editors. Animal biotechnology. San Diego: Academic Press; 2014. pp. 247-63.
- Ansari JA, Malik JA, Ahmed S, Manzoor M, Ahemad N, et al. Recent advances in the therapeutic applications of selenium nanoparticles. Mol Biol Rep 2024;51(1);688. [PMID: 38796570 DOI: 10.1007/s11033-024-09598-z]
- Chanda N, Khan Y, Kaur S, Mekapothula S, Kulkarni RR, et al. An effective strategy for the synthesis of biocompatible gold nanoparticles using cinnamon phytochemicals for phantom CT imaging and photoacoustic detection of cancerous cells. Pharm Res 2011;28(2):279-91. [PMID: 20872051 DOI: 10.1007/s11095-010-0276-6]
- Surwade SP, Fadhl S, Alhassan J, Luxton T, Peloquin D, et al. Augmented antibacterial activity of ampicillin with silver nanoparticles against methicillin-resistant Staphylococcus aureus (MRSA). J Antibiot (Tokyo) 2019;72(1):50-3. [PMID: 30361634 DOI: 10.1038/s41429-018-0111-6]
- Akter S, Sikder T, Rahman M, Ullah AKMA, Hossain KFB, et al. A systematic review on silver nanoparticles-induced cytotoxicity: physicochemical properties and perspectives. J Adv Res 2018;9:1-16. [DOI: 10.1016/j.jare.2017.10.008]
- He H, Wang L, Xue Y, Cai R, Guo P, et al. In situ green synthesis and characterization of sericin-silver nanoparticle composite with effective antibacterial activity and good biocompatibility. Mater Sci Eng C 2017;80:509-16. [PMID: 28866194 DOI: 10.1016/j.msec.2017.06.015]
- Kumari S, Raturi S, Kulshrestha S, Chauhan K, Dhingra S, et al. A comprehensive review on various techniques used for synthesizing nanoparticles. J Mater Res Technol 2023;27:1739-63. [DOI: 10.1016/j.jmrt.2023.09.291]
- Huston M, DeBella M, DiBella M, Gupta A. Green synthesis of nanomaterials. Nanomaterials 2021;11(8):2130. [DOI: 10.3390/nano11082130]
- Dikshit PK, Kumar J, Das AK, Sadhu S, Sharma S, et al. Green synthesis of metallic nanoparticles: applications and limitations. Catalysts 2021;11(8):902. [DOI: 10.3390/catal11080902]
- Sharma NK, Vishwakarma J, Rai S, Alomar TS, AlMasoud N, et al. Green route synthesis and characterization techniques of silver nanoparticles and their biological adeptness. ACS Omega 2022;7(31):27004-20. [PMID: 35967040 DOI: 10.1021/acsomega.2c01400]
- Mustapha T, Misni N, Ithnin NR, Daskum AM, Unyah NZ. A review on plants and microorganisms mediated synthesis of silver nanoparticles, role of plants metabolites and applications. Int J Environ Res Public Health 2022;19(2):674. [PMID: 35055505 DOI: 10.3390/ijerph19020674]
- Genchi G, Lauria G, Catalano A, Sinicropi MS, Carocci A. Biological activity of selenium and its impact on human health. Int J Mol Sci 2023;24(3):2633. [PMID: 36768955 DOI: 10.3390/ijms24032633]
- Bisht N, Phalswal P, Khanna PK. Selenium nanoparticles: a review on synthesis and biomedical applications. Mater Adv 2022;3(3):1415-31. [DOI: 10.1039/D1MA00639H]
- Adeyemi JO, Oriola AO, Onwudiwe DC, Oyedeji AO. Plant extracts mediated metal-based nanoparticles: synthesis and biological applications. Biomolecules 2022;12(5):627. [PMID: 35625555 DOI: 10.3390/biom12050627]
- Ikram M, Javed B, Raja NI, Mashwani ZU. Biomedical potential of plant-based selenium nanoparticles: a comprehensive review on therapeutic and mechanistic aspects. Int J Nanomedicine 2021;16:249-68. [PMID: 33469285 DOI: 10.2147/ijn.S295053]
- Husen A, Siddiqi KS. Plants and microbes assisted selenium nanoparticles: characterization and application. J Nanobiotechnology 2014;12(1):28. [PMID: 25128031 DOI: 10.1186/s12951-014-0028-6]
- Alvi GB, Iqbal MS, Ghaith MMS, Haseeb A, Ahmed B, et al. Biogenic selenium nanoparticles (SeNPs) from citrus fruit have anti-bacterial activities. Sci Rep 2021;11(1):4811. [DOI: 10.1038/s41598-021-84099-8]
- Prasad KS, Selvaraj K. Biogenic synthesis of selenium nanoparticles and their effect on As(III)-induced toxicity on human lymphocytes. Biol Trace Elem Res 2014;157(3):275-83. [PMID: 24469678 DOI: 10.1007/s12011-014-9891-0]
- Shah V, Medina-Cruz D, Vernet-Crua A, Truong LB, Sotelo E, et al. Pepper-mediated green synthesis of selenium and tellurium nanoparticles with antibacterial and anticancer potential. J Funct Biomater 2023;14(1):24. [PMID: 36662072 DOI: 10.3390/jfb14010024]
- Husseini HH, Zainulabdeen JA. The effect of selenium nanoparticles with fenugreek extract on oxidative stress related to polycystic ovary syndrome. Eurasian Chem Commun 2023;5(4):371-81. [DOI: 10.22034/ecc.2023.369594.1551]
- Pyrzynska K, Sentkowska A. Biosynthesis of selenium nanoparticles using plant extracts. J Nanostruct Chem 2022;12(4):467-80. [DOI: 10.1007/s40097-021-00435-4]
- Ngcongco K, Krishna SB, Pillay K. Biogenic metallic nanoparticles as enzyme mimicking agents. Front Chem 2023;11:1107619. [PMID: 36959878 DOI: 10.3389/fchem.2023.1107619]
- Ao B, Du Q, Liu D, Shi X, Tu J, et al. A review on synthesis and antibacterial potential of bio-selenium nanoparticles in the food industry. Front Microbiol 2023;14:1229838. [PMID: 37520346 DOI: 10.3389/fmicb.2023.1229838]
- Yang T, Lee S-Y, Park K-C, Park S-H, Chung J, Lee S. The effects of selenium on bone health: from element to therapeutics. Molecules 2022;27(2):392. [PMID: 35056706 DOI: 10.3390/molecules27020392]
- Torres-Ortiz D, Hernández-Vázquez D, López-Campos M, Fernández F, Becerra-Becerra E, Esparza R, et al. Green synthesis and antiproliferative activity of gold nanoparticles of a controlled size and shape obtained using shock wave extracts from Amphipterygium adstringens. Bioengineering 2023;10(4):437. [PMID: 37106624 DOI: 10.3390/bioengineering10040437]
- Maiyo F, Singh M. Selenium nanoparticles: potential in cancer gene and drug delivery. Nanomedicine 2017;12(9):1075-89. [PMID: 28440710 DOI: 10.2217/nnm-2017-0024]
- Mohajerani E, Burnett L, Smith JV, Kurmus H, Milas J, et al. Nanoparticles in construction materials and other applications, and implications of nanoparticle use. Materials 2019;12(19):3052. [PMID: 31547011 DOI: 10.3390/ma12193052]
- Seleiman MF, Almutairi KF, Alotaibi M, Shami A, Alhammad BA, et al. Nano-fertilization as an emerging fertilization technique: why can modern agriculture benefit from its use? Plants 2021;10(1):2. [DOI: 10.3390/plants10010002]
- Spoială A, Ilie C-I, Crăciun LN, Ficai D, Ficai A, et al. Magnetite-silica core/shell nanostructures: from surface functionalization towards biomedical applications—a review. Appl Sci 2021;11(22):11075. [DOI: 10.3390/app112211075]
- Lipińska W, Grochowska K, Siuzdak K. Enzyme immobilization on gold nanoparticles for electrochemical glucose biosensors. Nanomaterials 2021;11(5):1156. [DOI: 10.3390/nano11051156]
- Maksimyak PP, Zenkova CY, Tkachuk VM. Carbon nanoparticles. Production, properties, perspectives of use. Phys Chem Solid State 2020;21(1):13-8. [DOI: 10.15330/pcss.21.1.13-18]
- Escudero A, Carrillo-Carrión C, Romero-Ben E, Franco A, Rosales-Barrios C, et al. Molecular bottom-up approaches for the synthesis of inorganic and hybrid nanostructures. Inorganics 2021;9(7):58. [DOI: 10.3390/inorganics9070058]
- Krishna PG, Chandra Mishra P, Naika MM, Gadewar M, Ananthaswamy PP, et al. Photocatalytic activity induced by metal nanoparticles synthesized by sustainable approaches: a comprehensive review. Front Chem 2022;10:917831. [PMID: 36118313 DOI: 10.3389/fchem.2022.917831]
- Shoeibi S, Mozdziak P, Golkar-Narenji A. Biogenesis of selenium nanoparticles using green chemistry. Top Curr Chem 2017;375(6):88. [PMID: 29124492 DOI: 10.1007/s41061-017-0176-x]
- Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem 2019;12(7):908-31. [DOI: 10.1016/j.arabjc.2017.05.011]
- Patra JK, Baek K-H. Green nanobiotechnology: factors affecting synthesis and characterization techniques. J Nanomater 2014;2014:417305. [DOI: 10.1155/2014/417305]
- Puri A, Patil S, Mulik M. Biomedical applications of biogenic phytonanoparticles: a review. Int J Botany Stud 2021;6(3):534-40.
- Singh J, Dutta T, Kim K-H, Rawat M, Samddar P, et al. ‘Green’ synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J Nanobiotechnol 2018;16(1):84. [PMID: 30373622 DOI: 10.1186/s12951-018-0408-4]
- Vahdati M, Tohidi Moghadam T. Synthesis and characterization of selenium nanoparticles-lysozyme nanohybrid system with synergistic antibacterial properties. Sci Rep 2020;10(1):510. [PMID: 31949299 DOI: 10.1038/s41598-019-57333-7]
- Singh H, Saini N, Shukla R, Desimone MF, Pandya S, et al. Revisiting the green synthesis of nanoparticles: uncovering influences of plant extracts as reducing agents for enhanced synthesis efficiency and its biomedical applications. Int J Nanomedicine 2023;18:4727-50. [PMID: 37621852 DOI: 10.2147/ijn.S419369]
- Tabibi M, Aghaei S, Amoozegar MA, Nazari R, Zolfaghari MR. Characterization of green synthesized selenium nanoparticles (SeNPs) in two different indigenous halophilic bacteria. BMC Chem 2023;17(1):115. [PMID: 37716996 DOI: 10.1186/s13065-023-01034-w]
- Zhang T, Qi M, Wu Q, Xiang P, Tang D, et al. Recent research progress on the synthesis and biological effects of selenium nanoparticles. Front Nutr 2023;10:1183487. [PMID: 37260518 DOI: 10.3389/fnut.2023.1183487]
- Anu K, Singaravelu G, Murugan K, Benelli G. Green-synthesis of selenium nanoparticles using garlic cloves (Allium sativum): biophysical characterization and cytotoxicity on vero cells. J Clust Sci 2017;28(1):551-63. [DOI: 10.1007/s10876-016-1123-7]
- Mulla NA, Otari SV, Bohara RA, Yadav HM, Pawar SH. Rapid and size-controlled biosynthesis of cytocompatible selenium nanoparticles by Azadirachta indica leaves extract for antibacterial activity. Mater Lett 2020;264:127353. [DOI: 10.1016/j.matlet.2020.127353]
- Dhanraj G, Rajeshkumar S. Anticariogenic effect of selenium nanoparticles synthesized using Brassica oleracea. J Nanomater 2021;2021:8115585. [DOI: 10.1155/2021/8115585]
- Vundela SR, Kalagatur NK, Nagaraj A, Kadirvelu K, Chandranayaka S, et al. Multi-biofunctional properties of phytofabricated selenium nanoparticles from Carica papaya fruit extract: antioxidant, antimicrobial, antimycotoxin, anticancer, and biocompatibility. Front Microbiol 2022;12:769891. [DOI: 10.3389/fmicb.2021.769891]
- Antony R, Yadav P, Kannaiyan P. Enhanced antimicrobial and cytotoxicity on cancer cell using bio-originated selenium nanoparticles. Asian J Chem 2020;32:543-9. [DOI: 10.14233/ajchem.2020.22420]
- Anu K, Devanesan S, Prasanth R, AlSalhi MS, Ajithkumar S, et al. Biogenesis of selenium nanoparticles and their anti-leukemia activity. J King Saud Univ Sci 2020;32(4):2520-6. [DOI: 10.1016/j.jksus.2020.04.018]
- Wen S, Hui Y, Chuang W. Biosynthesis and antioxidation of nano-selenium using lemon juice as a reducing agent. Green Process Synth 2021;10(1):178-88. [DOI: 10.1515/gps-2021-0018]
- Sasidharan S, Sowmiya R, Balakrishnaraja R. Biosynthesis of selenium nanoparticles using Citrus reticulata peel extract. World J Pharm Res 2015;4:1322-30.
- Dang-Bao T, Ho TG, Do BL, Phung Anh N, Phan TD, et al. Green orange peel-mediated bioinspired synthesis of nanoselenium and its antibacterial activity against methicillin-resistant Staphylococcus aureus. ACS Omega 2022;7(40):36037-46. [PMID: 36249379 DOI: 10.1021/acsomega.2c05469]
- Sowndarya P, Ramkumar G, Shivakumar MS. Green synthesis of selenium nanoparticles conjugated Clausena dentata plant leaf extract and their insecticidal potential against mosquito vectors. Artif Cells Nanomed Biotechnol 2017;45(8):1490-5. [PMID: 27832715 DOI: 10.1080/21691401.2016.1252383]
- Vu TT, Nguyen PTM, Pham NH, Le TH, Nguyen TH, et al. Green synthesis of selenium nanoparticles using Cleistocalyx operculatus leaf extract and their acute oral toxicity study. J Compos Sci 2022;6(10):307. [DOI: 10.3390/jcs6100307]
- Deb Barma M, Doraikanan S. Synthesis, characterization and antimicrobial activity of selenium nanoparticles with Clitoria ternatea on oral pathogens. Int J Health Sci 2022;6(S1):2529-38. [DOI: 10.53730/ijhs.v6nS1.5316]
- Puri A, Patil S. Biogenic synthesis of selenium nanoparticles using Diospyros montana bark extract: characterization, antioxidant, antibacterial, and antiproliferative activity. Biosci Biotech Res Asia 2022;19(2):423-41. [DOI: 10.13005/bbra/2997]
- Perumal S, Gopal Samy MV, Subramanian D. Selenium nanoparticle synthesis from endangered medicinal herb (Enicostema axillare). Bioprocess Biosyst Eng 2021;44(9):1853-63. [PMID: 33855637 DOI: 10.1007/s00449-021-02565-z]
- Fan D, Li L, Li Z, Zhang Y, Ma X, et al. Biosynthesis of selenium nanoparticles and their protective, antioxidative effects in streptozotocin induced diabetic rats. Sci Technol Adv Mater 2020;21(1):505-14. [PMID: 32939175 DOI: 10.1080/14686996.2020.1788907]
- Abu-Zeid EH, Fattah DMA, Arisha AH, Ismail TA, Alsadek DM, et al. Protective prospects of eco-friendly synthesized selenium nanoparticles using Moringa oleifera or Moringa oleifera leaf extract against melamine induced nephrotoxicity in male rats. Ecotoxicol Environ Saf 2021;221:112424. [DOI: 10.1016/j.ecoenv.2021.112424]
- Al-Qaraleh SY, Al-Zereini WA, Oran SA. Phyto-decoration of selenium nanoparticles using Moringa peregrina: chemical characterization and bioactivity evaluation. Biointerface Res Appl Chem 2022;13:1-15.
- Farag SM, El-Sayed AA, Abdel-Haleem DR. Larvicidal efficacy of Nigella sativa seeds oil and its nanoparticles against Culex pipiens and Musca domestica. J Egypt Soc Parasitol 2020;50(1):215-20. [DOI: 10.21608/jesp.2020.88840]
- Ogunleye GE, Oyinlola KA, Akintade O, Fashogbon R, Adesina T. Green synthesis, characterization and antimicrobial potential of selenium nanoparticles from Ocimum gratissimum. Turk J Agric Food Sci Technol 2022;10:2903-12. [DOI: 10.24925/turjaf.v10isp2.2903-2912.5615]
- Hashem AH, Selim TA, Alruhaili MH, Selim S, Alkhalifah DHM, et al. Unveiling antimicrobial and insecticidal activities of biosynthesized selenium nanoparticles using prickly pear peel waste. J Funct Biomater 2022;13(3):112. [PMID: 35997450 DOI: 10.3390/jfb13030112].
- Fouda A, Al-Otaibi WA, Saber T, AlMotwaa SM, Alshallash KS, et al. Antimicrobial, antiviral, and in-vitro cytotoxicity and mosquitocidal activities of Portulaca oleracea-based green synthesis of selenium nanoparticles. J Funct Biomater 2022;13(3):157. [PMID: 36135592 DOI: 10.3390/jfb13030157]
- Alam H, Khatoon N, Raza M, Ghosh PC, Sardar M. Synthesis and characterization of nano selenium using plant biomolecules and their potential applications. BioNanoScience 2019;9(1):96-104. [DOI: 10.1007/s12668-018-0569-5]
- Hashem AH, Saied E, Ali OM, Selim S, Al Jaouni SK, et al. Pomegranate peel extract stabilized selenium nanoparticles synthesis: promising antimicrobial potential, antioxidant activity, biocompatibility, and hemocompatibility. Appl Biochem Biotechnol 2023;195(10):5753-76. [PMID: 36705842 DOI: 10.1007/s12010-023-04326-y]
- Zhao M, Wu Y, Zhang F, Zheng S, Wang L, et al. Preparation of Ribes nigrum L. polysaccharides-stabilized selenium nanoparticles for enhancement of the anti-glycation and α-glucosidase inhibitory activities. Int J Biol Macromol 2023;253:127122. [PMID: 37776928 DOI: 10.1016/j.ijbiomac.2023.127122]
- Sani Z, Iqbal MS, Abbas K, Qadir MI. Synthesis, characterization and evaluation of biological properties of selenium nanoparticles from Solanum lycopersicum. Arab J Chem 2022;15(7):103901. [DOI: 10.1016/j.arabjc.2022.103901]
- Puri A, Mohite P, Patil S, Chidrawar VR, Ushir YV, et al. Facile green synthesis and characterization of Terminalia arjuna bark phenolic–selenium nanogel: a biocompatible and green nano-biomaterial for multifaceted biological applications. Front Chem 2023;11:1273360. [DOI: 10.3389/fchem.2023.1273360]
- Mellinas C, Jiménez A, Garrigós MD. Microwave-assisted green synthesis and antioxidant activity of selenium nanoparticles using Theobroma cacao L. bean shell extract. Molecules 2019;24(22):4048. [PMID: 31717413 DOI: 10.3390/molecules24224048]
- Abhijeet P, Swati P. Tinospora cordifolia stem extract-mediated green synthesis of selenium nanoparticles and its biological applications. Pharmacognosy Res 2022;14(3):289-96. [DOI: 10.5530/pres.14.3.42]
- Ramamurthy CH, Sampath KS, Arunkumar P, Kumar MS, Sujatha V, et al. Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioprocess Biosyst Eng 2013;36(8):1131-9. [PMID: 23446776 DOI: 10.1007/s00449-012-0867-1]
- Sharma G, Sharma AR, Bhavesh R, Park J, Ganbold B, et al. Biomolecule-mediated synthesis of selenium nanoparticles using dried Vitis vinifera (raisin) extract. Molecules 2014;19(3):2761-70. [PMID: 24583881 DOI: 10.3390/molecules19032761]
- Mohammed Ali IA, AL-Ahmed HI, Ben Ahmed A. Evaluation of green synthesis (Withania somnifera) of selenium nanoparticles to reduce sperm DNA fragmentation in diabetic mice induced with streptozotocin. Appl Sci 2023;13(2):728. [DOI: 10.3390/app13020728]
- Xu C, Guo Y, Qiao L, Ma L, Cheng Y, et al. Biogenic synthesis of novel functionalized selenium nanoparticles by Lactobacillus casei ATCC 393 and its protective effects on intestinal barrier dysfunction caused by enterotoxigenic Escherichia coli K88. Front Microbiol 2018;9:1129. [PMID: 29967593 DOI: 10.3389/fmicb.2018.01129]
- Zhang H, Zhou H, Bai J, Li Y, Yang J, et al. Biosynthesis of selenium nanoparticles mediated by fungus Mariannaea sp. HJ and their characterization. Colloids Surf A Physicochem Eng Asp 2019;571:9-16. [DOI: 10.1016/j.colsurfa.2019.02.070]
- Nassar ARA, Eid AM, Atta HM, El Naghy WS, Fouda A. Exploring the antimicrobial, antioxidant, anticancer, biocompatibility, and larvicidal activities of selenium nanoparticles fabricated by endophytic fungal strain Penicillium verhagenii. Sci Rep 2023;13(1):9054. [PMID: 37270596 DOI: 10.1038/s41598-023-35360-9]
- Puri A, Mohite P, Maitra S, Subramaniyan V, Kumarasamy V, et al. From nature to nanotechnology: the interplay of traditional medicine, green chemistry, and biogenic metallic phytonanoparticles in modern healthcare innovation and sustainability. Biomed Pharmacother 2024;170:116083. [PMID: 38163395 DOI: 10.1016/j.biopha.2023.116083]
- Mourdikoudis S, Pallares RM, Thanh NTK. Characterization techniques for nanoparticles: comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018;10(27):12871-934. [DOI: 10.1039/C8NR02278J]
- Kumar A, Shah SR, Jayeoye TJ, Kumar A, Parihar A, et al. Biogenic metallic nanoparticles: biomedical, analytical, food preservation, and applications in other consumable products. Front Nanotechnol 2023;5:1175149. [DOI: 10.3389/fnano.2023.1175149]
- Joudeh N, Linke D. Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists. J Nanobiotechnol 2022;20(1):262. [PMID: 35672712 DOI: 10.1186/s12951-022-01477-8]
- Kamnev AA, Dyatlova YA, Kenzhegulov OA, Vladimirova AA, Mamchenkova PV, et al. Fourier Transform Infrared (FTIR) spectroscopic analyses of microbiological samples and biogenic selenium nanoparticles of microbial origin: sample preparation effects. Molecules 2021;26(4):1146. [PMID: 33669948 DOI: 10.3390/molecules26041146]
- Sidhu AK, Verma N, Kaushal P. Role of biogenic capping agents in the synthesis of metallic nanoparticles and evaluation of their therapeutic potential. Front Nanotechnol 2022;3:801620. [DOI: 10.3389/fnano.2021.801620]
- Catauro M, Papale F, Bollino F, Piccolella S, Marciano S, et al. Silica/quercetin sol–gel hybrids as antioxidant dental implant materials. Sci Technol Adv Mater 2015;16(3):035001. [PMID: 27877802 DOI: 10.1088/1468-6996/16/3/035001]
- Su D. Advanced electron microscopy characterization of nanomaterials for catalysis. Green Energy Environ 2017;2(2):70-83. [DOI: 10.1016/j.gee.2017.02.001]
- Yang C, Wang C, Khan Z, Duan S, Li Z, et al. Algal polysaccharides–selenium nanoparticles regulate the uptake and distribution of selenium in rice plants. Front Plant Sci 2023;14:1135080. [PMID: 36968401 DOI: 10.3389/fpls.2023.1135080]
- Javed R, Zia M, Naz S, Aisida SO, Ain NU, et al. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: recent trends and future prospects. J Nanobiotechnol 2020;18(1):172. [DOI: 10.1186/s12951-020-00704-4]
- Kasinathan K. Green synthesis for advanced materials of Graphene Oxide (GO) with ZnO for enhanced photocatalytic activity at room temperature. In: Makhlouf ASH, Scharnweber D, editors. Handbook of nanoceramic and nanocomposite coatings and materials. Butterworth-Heinemann; 2015. pp. 115-27.
- Alagesan V, Venugopal S. Green synthesis of selenium nanoparticle using leaves extract of Withania somnifera and its biological applications and photocatalytic activities. BioNanoScience 2019;9(1):105-16. [DOI: 10.1007/s12668-018-0566-8]
- Carvalho PM, Felício MR, Santos NC, Gonçalves S, Domingues MM. Application of light scattering techniques to nanoparticle characterization and development. Front Chem 2018;6:237. [PMID: 29988578 DOI: 10.3389/fchem.2018.00237]
- Shahabadi N, Zendehcheshm S, Khademi F. Selenium nanoparticles: synthesis, in-vitro cytotoxicity, antioxidant activity and interaction studies with ct-DNA and HSA, HHb and Cyt c serum proteins. Biotechnol Rep 2021;30:e00615. [DOI: 10.1016/j.btre.2021.e00615]
- Cruz LY, Wang D, Liu J. Biosynthesis of selenium nanoparticles, characterization and X-ray induced radiotherapy for the treatment of lung cancer with interstitial lung disease. J Photochem Photobiol B Biol 2019;191:123-7. [PMID: 30616036 DOI: 10.1016/j.jphotobiol.2018.12.008]
- Amin MA, Ismail MA, Badawy AA, Awad MA, Hamza MF, et al. The potency of fungal-fabricated selenium nanoparticles to improve the growth performance of Helianthus annuus L. and control of cutworm agrotis ipsilon. Catalysts 2021;11(12):1551. [DOI: 10.3390/catal11121551]
- Rades S, Hodoroaba V-D, Salge T, Wirth T, Lobera MP, et al. High-resolution imaging with SEM/T-SEM, EDX and SAM as a combined methodical approach for morphological and elemental analyses of single engineered nanoparticles. RSC Adv 2014;4(91):49577-87. [DOI: 10.1039/C4RA05092D]
- Shahbaz M, Akram A, Mehak A. Haq EU, Fatima N, et al. Evaluation of selenium nanoparticles in inducing disease resistance against spot blotch disease and promoting growth in wheat under biotic stress. Plants 2023;12(4):761. [DOI: 10.3390/plants12040761]
- Ullah A, Yin X, Wang F, Xu B, Mirani ZA, et al. Biosynthesis of selenium nanoparticles (via Bacillus subtilis BSN313), and their isolation, characterization, and bioactivities. Molecules 2021;26(18):5559. [PMID: 34577029 DOI: 10.3390/molecules26185559]
- Goufo P, Singh RK, Cortez I. A reference list of phenolic compounds (including stilbenes) in grapevine (Vitis vinifera L.) roots, woods, canes, stems, and leaves. Antioxidants 2020;9(5):398. [PMID: 32397203 DOI: 10.3390/antiox9050398]
- Singh NA, Narang J, Garg D, Jain V, Payasi D, et al. Nanoparticles synthesis via microorganisms and their prospective applications in agriculture. Plant Nano Biol 2023;5:100047. [DOI: 10.1016/j.plana.2023.100047]
- Kondaparthi P, Deore M, Naqvi S, Flora SJ. Dose-dependent hepatic toxicity and oxidative stress on exposure to nano and bulk selenium in mice. Environ Sci Pollut Res 2021;28(38):53034-44. [PMID: 34023997 DOI: 10.1007/s11356-021-14400-9]
- Tuyen NNK, Huy VK, Duy NH, An H, Nam NTH, et al. Green synthesis of selenium nanorods using Muntigia calabura leaf extract: effect of pH on characterization and bioactivities. Waste Biomass Valori 2024;15(4):1987-98. [DOI: 10.1007/s12649-023-02269-3]
- Lade BD, Shanware AS. Phytonanofabrication: methodology and factors affecting biosynthesis of nanoparticles. In: Shabatina T, Bochenkov V, editors. Smart nanosystems for biomedicine, optoelectronics and catalysis. London, England: IntechOpen; 2020.
- Zambonino MC, Quizhpe EM, Mouheb L, Rahman A, Agathos SN, et al. Biogenic selenium nanoparticles in biomedical sciences: properties, current trends, novel opportunities and emerging challenges in theranostic nanomedicine. Nanomaterials (Basel) 2023;13(3):424. [PMID: 36770385 DOI: 10.3390/nano13030424]
- Sampath S, Sunderam V, Manjusha M, Dlamini Z, Lawrance AV. Selenium nanoparticles: a comprehensive examination of synthesis techniques and their diverse applications in medical research and toxicology studies. Molecules 2024;29(4):801. [PMID: 38398553 DOI: 10.3390/molecules29040801]
- Puri A, Mohite P, Ansari Y, Mukerjee N, Alharbi HM, et al. Plant-derived selenium nanoparticles: investigating unique morphologies, enhancing therapeutic uses, and leading the way in tailored medical treatments. Mater Adv 2024;5(9):3602-28. [DOI: 10.1039/d3ma01126g]
- Long Q, Cui LK, He SB, Sun J, Chen QZ, et al. Preparation, characteristics and cytotoxicity of green synthesized selenium nanoparticles using Paenibacillus motobuensis LY5201 isolated from the local specialty food of longevity area. Sci Rep 2023;13(1):53. [PMID: 36593245 DOI: 10.1038/s41598-022-26396-4]
- Nikam PB, Salunkhe JD, Minkina T, Rajput VD, Kim BS, et al. A review on green synthesis and recent applications of red nano selenium. Results Chem 2022;4:100581. [DOI: 10.1016/j.rechem.2022.100581]
- Spyridopoulou K, Aindelis G, Pappa A, Chlichlia K. Anticancer activity of biogenic selenium nanoparticles: apoptotic and immunogenic cell death markers in colon cancer cells. Cancers 2021;13(21):5335. [PMID: 34771499 DOI: 10.3390/cancers13215335]
- Yazdi MH, Varastehmoradi B, Faghfuri E, Mavandadnejad F, Mahdavi M, et al. Adjuvant effect of biogenic selenium nanoparticles improves the immune responses and survival of mice receiving 4T1 cell antigens as vaccine in breast cancer murine model. J Nanosci Nanotechnol 2015;15(12):10165-72. [PMID: 26682463 DOI: 10.1166/jnn.2015.11692]
- Menon S, Jayakodi S, Yadav KK, Somu P, Isaq M, et al. Preparation of paclitaxel-encapsulated bio-functionalized selenium nanoparticles and evaluation of their efficacy against cervical cancer. Molecules 2022;27(21):7290. [PMID: 36364115 DOI: 10.3390/molecules27217290]
- Sans-Serramitjana E, Obreque M, Muñoz F, Zaror C, Mora ML, et al. Antimicrobial activity of selenium nanoparticles (SeNPs) against potentially pathogenic oral microorganisms: a scoping review. Pharmaceutics 2023;15(9):2253. [PMID: 37765222 DOI: 10.3390/pharmaceutics15092253]
- Nile SH, Thombre D, Shelar A, Gosavi K, Sangshetti J. Antifungal properties of biogenic selenium nanoparticles functionalized with nystatin for the inhibition of Candida albicans Biofilm formation. Molecules 2023;28(4):1836. [PMID: 36838823 DOI: 10.3390/molecules28041836]
- Gutiérrez RMP, Gómez JT, Urby RB, Soto JGC, Parra HR. Evaluation of diabetes effects of selenium nanoparticles synthesized from a mixture of luteolin and diosmin on streptozotocin-induced type 2 diabetes in mice. Molecules 2022;27(17):5642. [PMID: 36080407 DOI: 10.3390/molecules27175642]
- Fernandes AP, Gandin V. Selenium compounds as therapeutic agents in cancer. Biochim Biophys Acta Gen Subj 2015;1850(8):1642-60. [PMID: 25459512 DOI: 10.1016/j.bbagen.2014.10.008]
- Tinggi U. Selenium: its role as antioxidant in human health. Environ Health Prev Med 2008;13(2):102-8. [PMID: 19568888 DOI: 10.1007/s12199-007-0019-4]
- Hadrup N, Loeschner K, Mandrup K, Ravn-Haren G, Frandsen HL, et al. Subacute oral toxicity investigation of selenium nanoparticles and selenite in rats. Drug Chem Toxicol 2019;42(1):76-83. [PMID: 30032689 DOI: 10.1080/01480545.2018.1491589]
- Bano I, Skalickova S, Arbab S, Urbankova L, Horky P. Toxicological effects of nanoselenium in animals. J Anim Sci Biotechnol 2022;13(1):72. [PMID: 35710460 DOI: 10.1186/s40104-022-00722-2]