The Physiological Repercussions of Thermo-Oxidized Sesame Oil
1Amity Institute of Pharmacy, Amity University, Lucknow Campus, Noida, Uttar Pradesh 226010, India
2Department of Botany and Microbiology, Gurukul Kangri (Deemed to be University), Haridwar, Uttrakhand 249404, India
3Babu Sunder Singh College of Pharmacy, Nigoha, Uttar Pradesh 227309, India
*Correspondence to: Dr. Mohammad Yasir, Assistant professor-Grade III, Department of Pharmacognosy, Amity Institute of Pharmacy, Amity University, Lucknow Campus, Noida, Uttar Pradesh 226010, India. Mobile: +91-9179417284. E-mail: yasirmohammad6@gmail.com, myasir@lko.amity.edu
Received: July 2 2024; Revised: September 3 2024; Accepted: September 13 2024; Published Online: November 14 2024
Cite this paper:
Pratap R, Yasir M, Dubey RC et al. The Physiological Repercussions of Thermo-Oxidized Sesame Oil. BIO Integration 2024; 5: 1–11.
DOI: 10.15212/bioi-2024-0045. Available at: https://bio-integration.org/
Download citation
© 2024 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
Cooking oil is an important source of essential nutrients for human health. Prolonged heating of vegetable oil can lead to the emergence of health conditions. This study examined the physicochemical characteristics of sesame seed oil after heating at 180°C for 4 or 8 hours. We assessed the acid value, iodine value, peroxide value, p-anisidine value, refractive index, and saponification value; conducted gas chromatography; and performed preclinical testing on Wistar rats. The acid value, specific gravity, refractive index, p-anisidine value, saponification value, peroxide value, and iodine value in the present investigation have been calculated to fall within the following range: 0.42–2.11 milligrams of potassium hydroxide (KOH) per gram (mg KOH/g oil), 0.92–0.8912, 1.473–1.4746, 0.67–15.32 p-Anisidine Value (AnV), 188–197.25 milligrams of potassium hydroxide (KOH) or sodium hydroxide (NaOH) per gram of fat or oil (mg KOH/g oil), 3.56–12.56 milliequivalents (meq) of active oxygen per kilogram of oil (meq/kg), and 108–81 grams of iodine per 100 grams of oil (gm/100g oil). Gas chromatography detected alterations in the quantities of mono-unsaturated fatty acids and poly-unsaturated fatty acids, as well as the production of trans fats, which have negative effects on health. Animal studies indicated changes in lipid profiles, liver profiles, and amounts of antioxidant enzymes (glutathione, superoxide dismutase [SOD], catalase, and thiobarbituric acid reactive substances [TBARS]) in tissue homogenates. The findings indicate that consuming thermo-oxidized oil can have detrimental effects. These findings highlight the need for a thorough comprehension of thermo-oxidized oil utilisation to adequately tackle public health issues.
Keywords
Catalytic enzymes, edible oils, gas chromatography, iodine value, MUFA.
Introduction
Sesame seed oil (SSO) is derived from sesame seeds for culinary purposes. SSO is rich in mono- and poly-unsaturated fatty acids (MUFAs and PUFAs, respectively), and the antioxidants sesamol and sesamin, and is used to promote health in traditional medicines and skincare products [1]. SSO is frequently used in Asian, Middle Eastern, and Mediterranean cuisines. SSO, extracted from sesame plant seeds (Sesamum indicum), has been an important ingredient in numerous international cuisines for centuries. SSO is rich in MUFAs and PUFAs, which are essential for maintaining good health [2]. Sesame seed sauce is a widely used flavour enhancer in stir-fries, marinades, salads, sauces, and dips. Its elevated smoke point makes it optimal for frying and sautéing. Untoasted SSO, with its mild taste and light color, is preferred for cooking and frying [3]. Beyond its culinary applications, SSO confers heart benefits, owing to its unsaturated fat and antioxidant content. SSO is produced from sesame seeds through either pressing or solvent extraction methods [4].
SSO, valued for its cooking versatility and health benefits, exhibits varying physicochemical properties according to the extraction methods, processing conditions, and sesame seed type. SSO color can vary from pale yellow to golden or amber, depending on its purity and processing methods [5]. A spectrophotometer or standard color charts can be used to determine color measurements. The density of oil refers to its mass per unit volume at a given temperature. The refractive index, measuring the extent of light bending in oil, can be determined with a refractometer to determine oil purity and composition. The presence of free fatty acids (FFAs) in oil indicates hydrolytic rancidity, as measured by the acid value [6]. The peroxide value indicates the extent of lipid oxidation by quantifying the presence of peroxides. The milliequivalents of active oxygen per kilogram of indicate oil’s quality and freshness. The saponification value measures the average molecular weight of fatty acids in oil, through determination of the amount of alkali necessary for saponification. Physicochemical parameters are frequently used to assess SSO quality [7].
In warm, well-drained areas, sesame plants yield sesame seed. These plants grow best in warm, tropical, and subtropical regions. Effective cultivation requires proper irrigation, weed control, and pest management for healthy plant growth and copious seed production. Seeds are sown either at the onset of the rainy season or when the soil temperature reaches approximately 68°F (20°C). Removing the outer coat of a sesame seed reveals its white or black edible kernel [8]. Through cold-pressing or expeller-pressing, SSO is extracted before being filtered for impurities. When heated and exposed to oxygen, such as during cooking, frying, and other heat treatments, SSO undergoes thermo-oxidation. At high temperatures, SSO undergoes hydrolysis, thus leading to the release of FFAs due to oxidation [9]. These process may potentially negatively affect an oil’s taste, aroma, and nutritional properties. At high temperatures, SSO’s unsaturated fatty acids can form harmful compounds including free radicals and lipid peroxides through polymerization. Thermo-oxidation causes SSO to darken, develop a polymerized layer, and acquire off-flavors because of the formation of volatile compounds, including aldehydes, ketones, and hydrocarbons. SSO should be used below its smoke point, and prolonged heating and oxygen exposure should be avoided to minimize thermo-oxidation. Storing SSO in a cool, dark place before its expiration date optimizes its quality [10]. Various edible vegetable oils are used by populations worldwide, and their thermal oxidative effects have been demonstrated to be harmful in several scientific studies. The safety of reusing oil in food preparation remains questionable, partly because of the limited human studies reported and the contradictory findings reported in animals. The present work was aimed at evaluating the toxic effects of thermo-oxidized SSO in various body organs in rats.
Material and methods
Materials
SSO was purchased from Patanjali Ayurved Ltd., Sonipat, Haryana. Only analytical grade chemicals were used.
Method for preparation of oil samples
SSO samples in 1 L amounts were heated to 180°C for 4 or 8 hours in conical flasks placed on a heating plate with a thermostat. Subsequently, the oil was cooled to ambient temperature and kept in amber-colored bottles in a refrigerator at 4°C [11].
Physicochemical characterization of normal and thermo-oxidized SSO samples
Estimation of acid value and percentage free fatty acids
The acid value was determined via the titration method. In a 250 mL conical flask, 2 grams oil and 50 mL neutralized ethyl alcohol were added; 0.1 M potassium hydroxide (KOH) was used to titrate the heated solution, and phenolphthalein served as an indicator [12, 13].
The acid value was calculated with Eq. (1), and FFAs were calculated as indicated in Eq. (2)
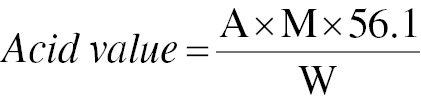
where A is the amount in mL of 0.1 M KOH consumed per sample; M is the molarity of KOH; and W is the weight in g of the oil sample.
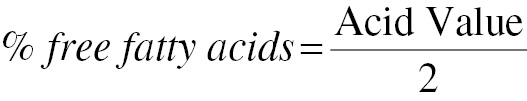
Specific gravity of the oil samples
The specific gravity was measured with a dry pycnometer. Specific gravity was determined by comparison of the density of the oil to that of water. A pycnometer was filled with distilled water, which was weighed with an electronic balance. Oil weight was also measured. The pycnometer was ensured to have no air leakage. The specific gravity value was determined with Eq. (3) [14].
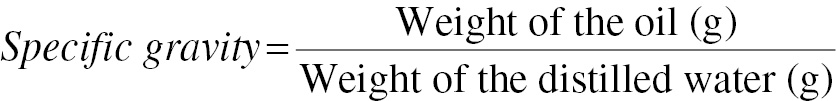
Smoke point of the oil
The point at which, under certain circumstances, an oil or fat starts to visibly emit a continuous plume of blue smoke is considered as smoke point. The smoke point was determined with the cleveland open cup (COC) method, as described in American Oil Chemists’ Society (AOCS) Official Method Cc 9a-48. In the COC, the sample was added to the fill line, a thermometer was placed in the vertical center, and a beam light was positioned across the center before heating. The cup’s temperature was raised to 50°C initially, and increased at 5°C per minute thereafter. A thin, continuous blue smoke stream indicated that the sample had reached its smoke point [15].
Determination of refractive index
According to the AOCS Fourth Edition (1994) procedure, 1–2 minutes should be allowed for the oil sample to seep into the prism before measurement of the refractive index. The light should be adjusted for a clear reading to determine the refractive index or butyro refractometer number. The refractive index of oils rises with the elongation of fatty acid chains and the degree of unsaturation. The refractive index, the ratio of the vacuum wavelength to the wavelength in the medium, varies with temperature and wavelength.
The calibration of the device was conducted with either distilled water with a refractive index of 1.333 at 20.0°C or a glass prism with a known refractive index, and optical contact was ensured with a bromonaphthalene drop. The refractometer temperature was set to 15°C for dry and clean prisms [16].
p-anisidine value
The p-anisidine value was determined with the AOCS [1992] method. Thermo-oxidized oil (1.0±0.5 g) was transferred into a 25 mL volumetric flask, which was filled with iso-octane to determine the p-anisidine value of Cd 18–90. The optical density was determined at 350 nm with isooctane as the blank in a Shimadzu UV-1800 spectrophotometer. After 5 mL oil and 1 mL reagent were added to a 10 mL graduated test tube, the mixture was incubated for 10 minutes before the optical density was measured [17]. p-anisidine was calculated with Eq. (4)
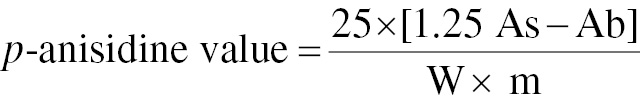
where As is the absorbance of the fat solution after reaction with the p-anisidine reagent; Ab is the absorbance of the fat solution; and m is the mass in g of the test sample.
Determination of saponification value
A 1 g amount of oil requires a specified amount of KOH for saponification, which can be calculated according to the International Union of Pure and Applied Chemistry (IUPAC) 7th edition [1987] standard method with 0.5 N HCl, alcoholic KOH solution, and phenolphthalein indicator. We reacted 2.5 g oil with 25 mL (0.5 N) ethanolic KOH for 1 hour under reflux. The result was highlighted by the disappearance of pink colour [18]. The saponification values of the oil samples were calculated with Eq. (5)
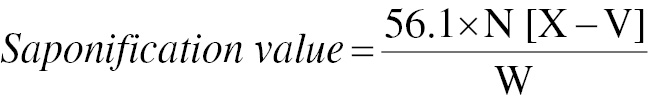
where N is the normality of hydrochloric acid (HCI); X is the volume of HCI used in the blank in mL; V is the volume of HCI used in the sample in mL; and W is the weight of the sample in g.
Peroxide value
Peroxide value was determined according to previously described method [19]. Briefly, A saturated potassium iodide solution of 0.1 mL was added to 5 g of oil, and the mixture was agitated for one minute. Then, 30 mL of filtered water was added. The solution was titrated with 0.01 M sodium thiosulphate solution, using a 1% starch solution as an indicator.
The PV was calculated with the equation
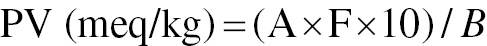
where A is the amount of 0.01 M sodium thiosulfate solution used for the titration; B is the mass of the oil sample (g); F is the factor of 0.01 M sodium thiosulfate solution used.
Iodine value
The iodine value was determined with the IUPAC standard method from 1987. The unsaturation degree of 100 grams of oil or fat is indicated by the absorbed iodine value. Iodine monochloride solution converts double bonds in fats and oils into halogenated compounds. Iodine reacts with unsaturated fatty acids’ double bonds. Dissolving 0.2 g sample in 20 mL carbon tetrachloride resulted in the release of free iodine after addition of 25 mL Wij’s reagent, as determined by titration of the excess iodine with sodium thiosulfate and use of starch as indicator. Solutions of 10% potassium iodide, 0.1 N sodium thiosulfate, and starch were added for the analysis. The flask was stoppered and shaken gently in the dark with iodine, and 15 mL potassium iodide solution was added. Titration with standardized sodium thiosulfate was performed until the color disappeared. A blank sample was analyzed under the same conditions. Iodine value was calculated with Eq. (7)
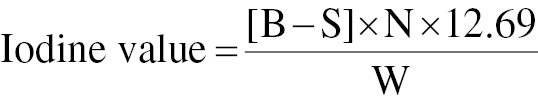
where B is the volume of 0.1 N Na2S2O3 required to titrate the blank in mL; S is the volume of 0.1 N Na2S2O3 required to titrate the sample in mL; N is the normality of Na2S2O3; and W is the weight of the sample in g [19].
Gas chromatography-flame ionization detection analysis of oil samples
Oil (50–100 mg), anhydrous sodium sulfate (200 mg), 5.0 mL sulfuric acid (2% sulfuric acid in methanol), sodium chloride (200 mg), and n-hexane (2.0 mL) were combined in a test tube, incubated for 5 minutes, and heated at 80°C for 15 minutes. Extraction was performed with petroleum ether or diethyl ether under alkaline or acidic conditions. The mixture was spun in a centrifuge at 5000 RPM for 10 minutes. The mixture was stirred for approximately 2 minutes. The hexane fraction was filtered through a 0.22 μm syringe filter, extracted with a micropipette, and transferred to a glass vial for gas chromatography analysis [20].
Instrument conditions for gas chromatography-flame ionization detection analysis
The gas chromatography-flame ionization detection (GC-FID) setup was optimized with an HP-88 column (100 m × 0.25 mm ID × 0.20 μm), 1.0 mL injection volume, 20:1 split ratio, 200°C inlet temperature, 280°C detector temperature, maximum temperature of 260°C, air flow rate of 400 mL/min, hydrogen gas flow of 40 mL/min, and makeup gas flow of 25 mL/min; 70.667 minutes was required to complete the run. The peaks in the sample chromatogram matching the fatty acid methyl esters standard were integrated after chromatography [20].
In vivo study of toxicological effects of thermally oxidized SSO
Albino Wistar rats 8–10 weeks old, weighing 100–120 g, were acquired from the animal house at the Sri Satya Sai College of Pharmacy, SSSUTMS, Sehore, Madhya Pradesh, India. The animals were housed in iron-topped cages containing wood chips under controlled environmental conditions (22–26°C temperature, 40–70% humidity, 12 h light/12 h dark cycle) with access to a pellet diet and water ad libitum.
The study consisted of four groups, each with six animals. For 30 days, the animals received standard feed supplemented with various oil types. The control group was administered standard oil, whereas groups II–IV were subjected to oil heated to 180°C for durations of 4 and 8 hours, respectively. Blood samples were collected before anesthesia and subjected to biochemical analyses including SGOT, SGPT, total cholesterol, triglyceride, HDL, low-density lipoprotein (LDL), glucose, hematological parameters (Hb, red blood cell [RBC] count, white blood cell [WBC] count, and platelet count), antioxidant enzyme levels, and serum separation without anticoagulant.
Measurement of antioxidant enzyme levels in organ tissues
Superoxide dismutase
The level of superoxide dismutase (SOD) in tissue supernatants was determined. The spectrophotometric inhibition rate of pyrogallol auto-oxidation was determined at 420 nm with a Spectramax Plus spectrophotometer after addition of enzyme extract. One unit of enzyme caused 50% inhibition of pyrogallol auto-oxidation. Enzyme activity was quantified in U/mg protein [21].
Catalase
A spectrophotometer was used to measure catalase activity anc2 the 1-minute absorbance decrease at 240 nm. One unit of enzyme was equal to 1 μmol H2O2 consumed per minute. Protein content was quantified in parts per million [21].
Glutathione peroxidase
After addition of 0.01 mL supernatant, 2 mL phosphate buffer (pH 7.4), 0.5 mL 5,5′-dithiobisnitro benzoic acid, and 0.4 mL double-distilled water to equal volumes of homogenate and 10% trichloroacetic acid, the mixture was centrifuged to obtain the supernatant. Within 15 minutes of vortexing, the absorbance was read at 412 nm [22].
Thiobarbituric acid reactive substances
Test tubes containing 0.1 mL homogenate were heated with 1 mL each of 10% trichloroacetic acid and 0.67% thiobarbituric acid in a boiling water bath for 20 minutes. The test tubes were centrifuged in a bath of crushed ice for 10 minutes at 6000 rpm. At 540 nm, the absorbance of the supernatant was determined [22].
Statistical analysis
The data for antioxidant enzyme levels, blood biochemistry, and hematological parameters were statistically analyzed with one-way analysis of variance in GraphPad Prism 10 and presented as graphs. Mean values and standard deviations are reported. The averages of three estimates for each parameter are reported for both the sample and the treatment group. A P value < 0.05 was considered significant.
Results
Physicochemical parameters of normal and thermo-oxidized SSO samples
The physicochemical parameters of both normal and thermally oxidised SSO samples have been evaluated as follows: The acid values for normal, 4 h, and 8 h thermally oxidised SSO samples were recorded as 0.42±0.023, 0.65±0.02, and 2.11±0.14 mgKOH/g oil, respectively. The % FFA values were measured at 0.21±0.010, 0.32±0.02, and 1.05±0.15 %. Specific gravities were determined to be 0.92±0.002, 0.9112±0.002, and 0.8912±0.002. Smoke points were noted as 220±1.5, 205±1.2, and 185±2.11. Refractive indices were found to be 1.473±0.002, 1.4741±0.002, and 1.4746±0.003. The p-anisidine values were recorded at 0.67±0.090, 3.87±0.13, and 15.32±0.12. Saponification values were measured as 188±0.41, 190±0.48, and 197.25±0.49. Peroxide values were noted to be 3.56±0.072, 7.67±0.03, and 12.56±2.08. Lastly, iodine values were recorded as 108±0.02, 85.5±0.29, and 81±0.14 (Table 1).
Table 1 Physicochemical Properties of Normal and Thermo-Oxidized SSO Samples
| Oil Sample | Acid Value | Free Fatty Acids (%) | Specific Gravity | Smoke Point | Refractive Index | p-Anisidine Value | Saponification Value | Peroxide Value | Iodine Value |
|---|---|---|---|---|---|---|---|---|---|
| SSO | 0.42±0.023 | 0.21±0.010 | 0.92±0.002 | 220±1.5 | 1.473±0.002 | 0.67±0.090 | 188±0.41 | 3.56±0.072 | 108±0.02 |
| SSO4 | 0.65±0.02 | 0.32±0.02 | 0.9112±0.002 | 205±1.2 | 1.4741±0.002 | 3.87±0.13 | 190±0.48 | 7.67±0.03 | 85.5±0.29 |
| SSO8 | 2.11±0.14 | 1.05±0.15 | 0.8912±0.002 | 185±2.11 | 1.4746±0.003 | 15.32±0.12 | 197.25±0.49 | 12.56±2.08 | 81±0.14 |
Gas chromatography-flame ionization detection analysis
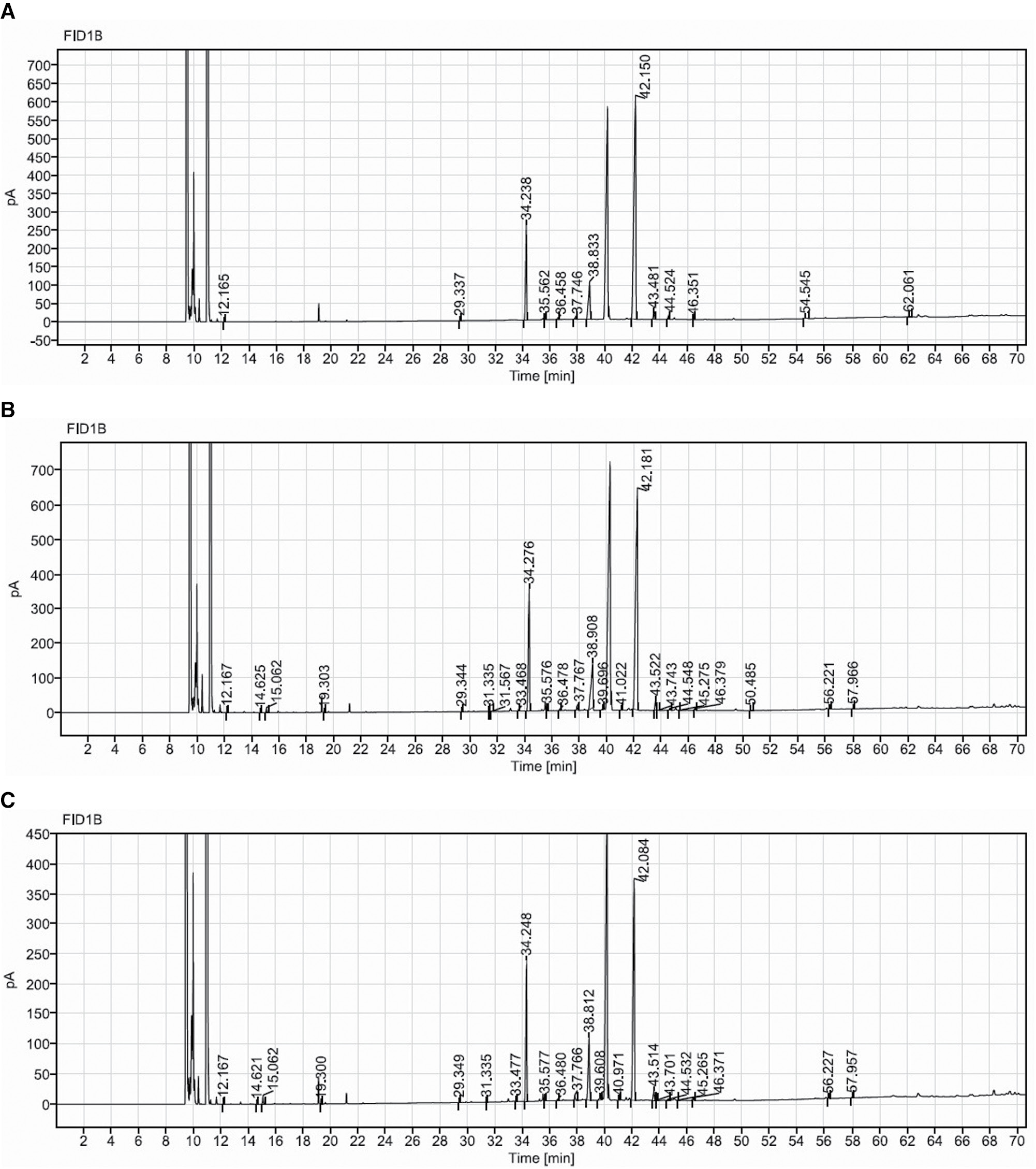
The GC-FID analysis of normal SSO is shown in Figure 1. A total of 13, 24, and 22 fatty acids in SSO, SSO4, and SSO8 were identified as saturated fatty acids (28.44%), MUFAs (0.32%), and PUFAs (71.24%), respectively. The heating process altered both saturated and trans fatty acids (Table 2). These fatty acids formed during thermo-oxidation and comprised caproic acid methyl ester (C6:0), caprylic acid methyl ester (C8:0), capric acid methyl ester (C10:0), methyl myristoleate (C14:1), pentadecanoic acid methyl ester (C15:0), cis-10-pentadecenoic acid methyl ester (C15:1), lignoceric acid methyl ester (C24:0), and nervonic acid methyl ester (C24:1n9) in SSO4; SSO8 contained one more fatty acid form, linolelaidic acid methyl ester (C18:2n6t).
Figure 1 GC-FID analysis of sesame seed oil (SSO) samples: (A) Normal SSO, (B) SSO thermally oxidized for 4 hours, (C) SSO thermally oxidized for 8 hours.
Table 2 Percentages of Saturated, Monounsaturated, and Polyunsaturated Fatty Acids in SSO After Thermo-Oxidation
| Oil Sample | Saturated Fatty Acid (SFA) (%) | MUFA (%) | PUFA (%) | Trans Fat (%) |
|---|---|---|---|---|
| SSO | 28.44 | 0.32 | 71.24 | 0.00 |
| SSO4 | 36.15 | 1.07 | 62.66 | 0.13 |
| SSO8 | 43.53 | 1.40 | 54.32 | 0.76 |
Biochemical analysis
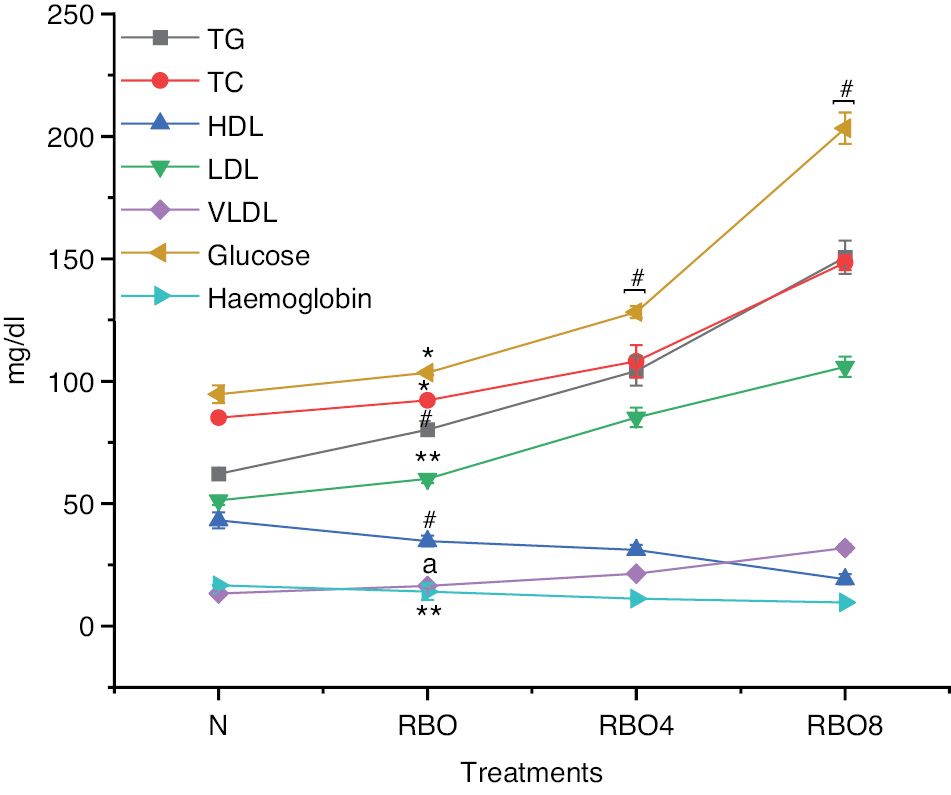
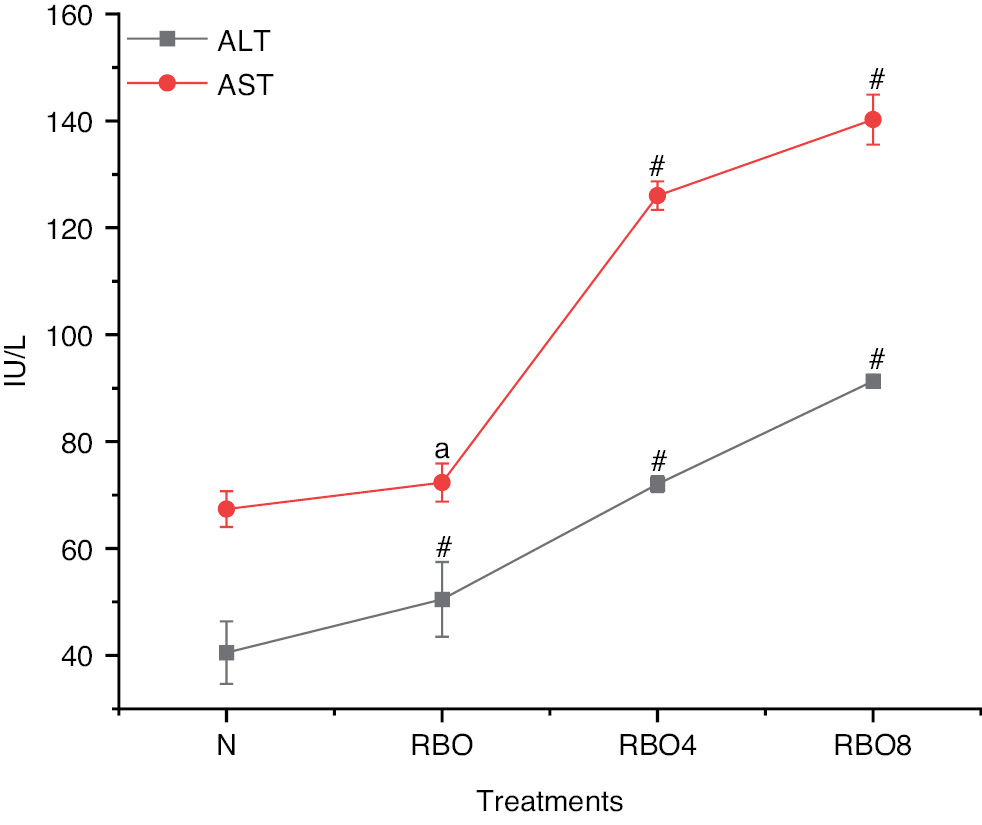
The biochemical analysis of lipid levels in blood revealed that the total cholesterol value for group IV SSO8 was significantly higher (161.27±3.48) than those in group III SSO4 (116.93±3.95) and group II SSO (93.94±2.01). However, compared with the normal control group I, the total cholesterol value (85.13±1.69) in group IV SSO8 was only slightly higher but was still greater than that in group II SSO (Figure 2). Triglycerides in group IV SSO8 were more substantial (156.03±6.19 mg/dL) in comparison to group III (113.25±6.02 mg/dL) and group II (75.19±3.52 mg/dL). However, when compared to group II, 75.19±3.52 mg/dL was found, which is somewhat higher when compared to normal control group I SSO (Figure 2). The HDL levels in group IV SSO8 were measured at 17.81±2.08, which is lower than the levels observed in group III at 23.61±2.08 and group II at 34.76±4.05. In contrast, the normal control group I exhibited HDL levels of 43.17±3.28, indicating a significant decrease in HDL levels when compared to group II to group IV (Figure 2). The LDL level in group IV SSO8 was significantly raised (104.23±2.08) compared to group III (82.58±6.95) and group II (57.67±3.15). However, in comparison to the normal control group, LDL levels increased from group II to group IV (Figure 2). Very low density lipoprotein (VLDL) levels in group IV SSO8 were measured at 32.79±1.57, which is higher than the levels observed in group III at 22.85±2.07 and group II at 17.69±2.01. In comparison, the normal control group I exhibited a VLDL level of 13.29±0.91 (Figure 2). The creatinine level in group IV SSO8 was greater (0.94±0.21) than those in group III SSO4 (0.66±0.02) and group II (0.44±0.01), but was significantly greater than that in the normal group (Figure 2). The liver function test for alanine transaminase (ALT) in group IV SSO8 was measured at 95.28±3.70, which is higher than the values recorded for group III at 74.07±2.08 and group II at 58.43±2.63. In comparison, the normal control group I exhibited a value of 40.53±5.84 (Figure 3). The aspartate aminotransferase (AST) value in group IV SSO8 was measured at 145.12±3.68, which is significantly greater than the values recorded in group III at 129.67±0.99 and group II at 75.94±3.82. In comparison, the normal control group I exhibited a value of 67.36±3.35 (Figure 3) (Table S1).
Figure 2 Biochemical analysis of normal fresh oil and thermo-oxidized sesame seed oil (SSO) treated groups under various conditions. Oil samples were prepared by thermal oxidation at 180°C for 4 h or 8 h. Animals were divided into normal, SSO, SSO4, and SSO8 groups. The rats in the control group that were provided with regular water exhibited typical weight gain patterns. Other groups were exposed to treated oil in the feed for 30 days. Subsequently, blood was collected, and biochemical and hematological parameters were tested. Biochemical parameters (triglycerides, total cholesterol, HDL, LDL, VLDL, creatinine, glucose, and hemoglobin) were analyzed with an autoanalyzer. The results are expressed as mean ± SD (n=6). #P<0.001, * P<0.05, ** P<0.01, as compared with the normal group. anon-significant values.
Figure 3 Liver enzymes in groups treated with normal fresh oil or thermo-oxidized sesame seed oil (SSO) under various conditions. The experimental conditions were as described in Figure 2. Oil samples were prepared by thermal oxidation at 180°C for 4 h or 8 h. Liver enzymes including ALT and AST were analyzed with an auto analyzer. The results are expressed as mean ± SD (n=6). #P <0.001, anon-significant values, as compared with the normal groups.
Effect on hematological parameters
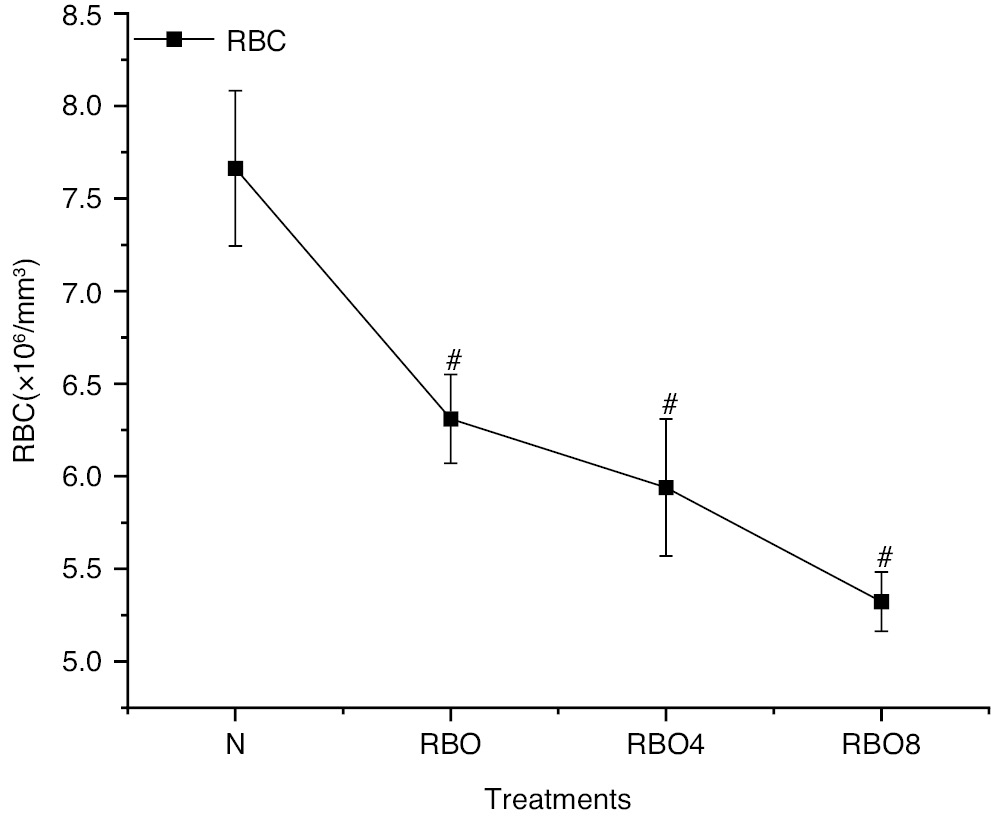
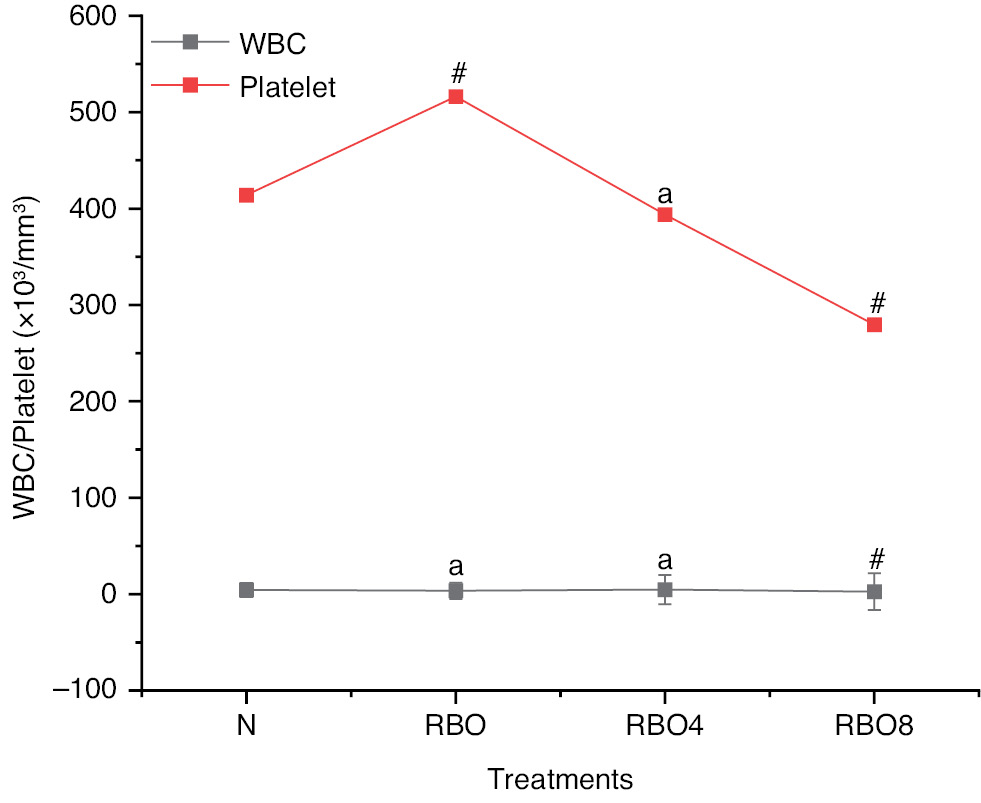
Hematological values such as hemoglobin in Wistar rats in group IV SSO8 were lower (10.59±0.36) than those in group III SSO4 (12.92±0.58) and group II SSO (15.77±0.37) (Figure 2). The RBC count, WBC count, and platelet count exhibited no significant differences (Figures 4 and 5). However, the blood glucose level for group IV SSO8 was elevated (186.78±5.51 mg/dL) compared to group III (134.85±4.09 mg/dL) and group II (105.78±2.40 mg/dL), indicating an increase relative to the normal level of 94.71±3.62 mg/dL (Figure 2) (Table S2).
Figure 4 RBC counts in normal fresh oil and thermo-oxidized ses ame seed oil (SSO) treated groups under various conditions. The experimental conditions were as described in Figure 2. Oil samples were prepared by thermal oxidation at 180°C for 4 h or 8 h. Blood was collected after the treatment, and RBC counts were analyzed with an autoanalyzer. The results are expressed as mean ± SD (n=6). #P<0.001 as compared with the normal groups.
Figure 5 WBC and platelet counts in groups treated with normal fresh oil or thermo-oxidized sesame seed oil (SSO) under various conditions. The experimental conditions were as described in Figure 2. Oil samples were prepared by thermal oxidation at 180°C for 4 h or 8 h. Blood was collected after the treatment, and WBC and platelet counts were analyzed with an autoanalyzer. The results are expressed as mean ± SD (n=6). #P <0.001, anon-significant values, as compared with the normal groups.
Antioxidant enzyme results in the liver and heart
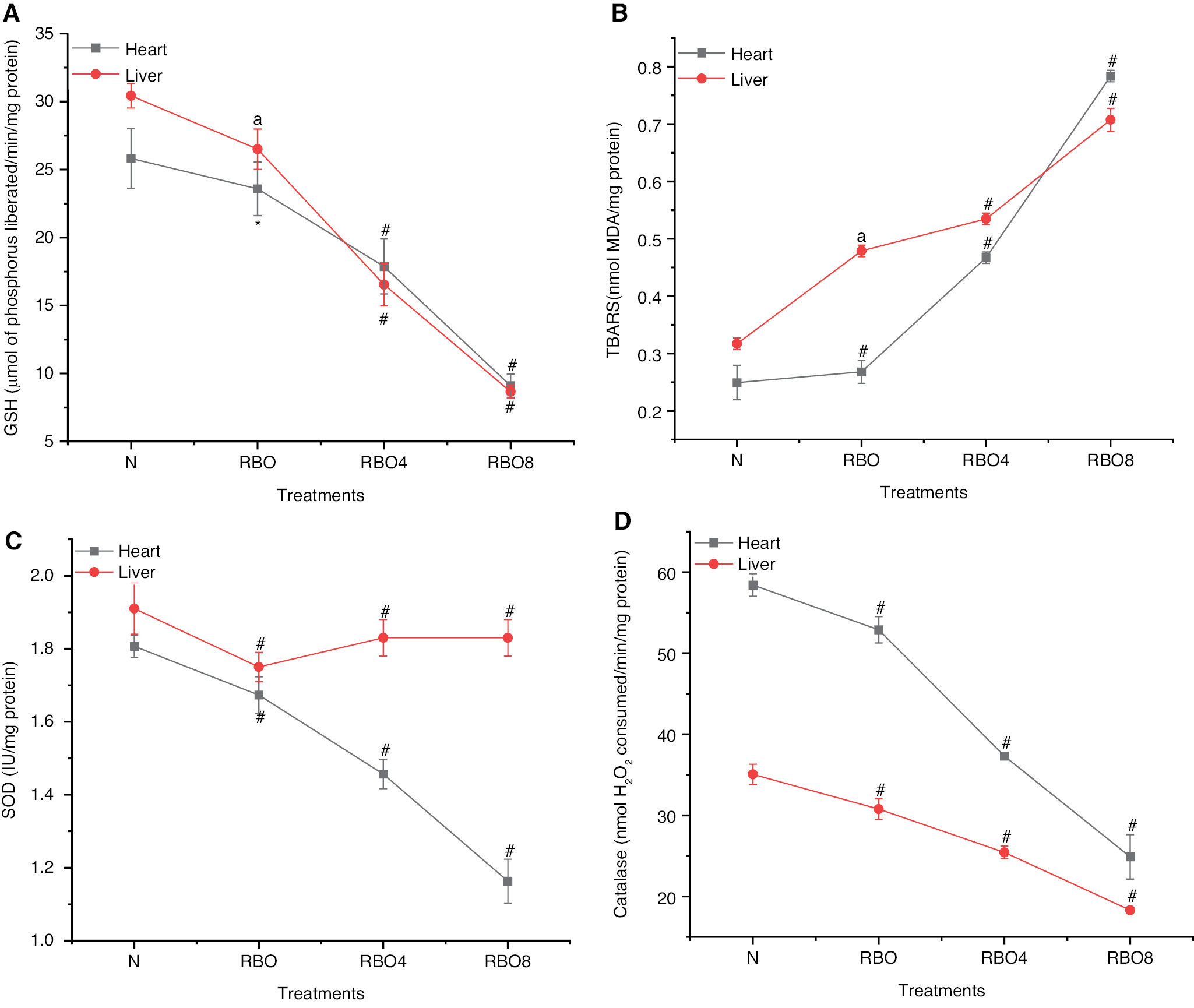
In the SSO8 and SSO4 groups, the heart mean glutathione (GSH) levels were significantly lower (P<0.001) than those in the normal control group. In the SSO group, GSH levels did not differ from those in the normal control group (Figure 6A). In the SSO8 and SSO4 groups, the mean thiobarbituric acid reactive substance (TBARS) levels were significantly (P<0.001) higher than that in the normal control group (Figure 6B). In the SSO8 and SSO4 groups, the mean SOD levels were significantly (P<0.001) lower than those in the control group (Figure 6C). In the SSO8 and SSO4 groups, catalase levels were significantly (P<0.001) lower than those in the normal control group (Figure 6D).
Figure 6 (A) GSH levels in heart and liver tissue. We examined GSH (glutathione) levels in heart and liver tissues from rats treated with normal fresh oil or thermo-oxidized sesame seed oil (SSO) under various conditions. GSH levels decreased, thus indicating oxidative damage in the tissue. (B) TBARS levels in heart and liver tissue. Similarly, we assessed thiobarbituric acid reactive substance (TBARS) levels in the same tissue samples. TBARS levels increased, thus further confirming oxidative damage due to SSO treatment. (C) SOD levels in heart and liver tissue: We also measured superoxide dismutase (SOD) levels in the heart and liver tissue of rats exposed to SSO. Interestingly, SOD levels decreased, in agreement with oxidative damage. (D) Catalase levels in heart and liver tissue. Finally, we evaluated catalase levels in the same tissue samples. Catalase levels decreased, thus reinforcing the presence of oxidative damage caused by SSO treatment. The results are expressed as mean ± SD (n=6). #P<0.001, *P<0.05 and anon-significant values, as compared with the normal groups.
In comparison to the GSH levels in the liver in the normal control group, the mean GSH levels were significantly (P<0.001) lower in both the SSO8 and SSO4 groups. The SSO group had significantly lower GSH levels (P<0.05) than the normal control group (Figure 6A). The SSO8 and SSO4 groups showed significantly (P<0.001) greater mean TBARS levels than the control group (Figure 6B). In the SSO8 and SSO4 groups, the mean SOD levels were significantly (P<0.001) lower than that in the normal control group (Figure 6C). In the SSO8 and SSO4 groups, the mean catalase levels were significantly lower (P<0.001) than those in the normal control group (Figure 6D) (Table S3).
Histopathological effects in the liver and heart
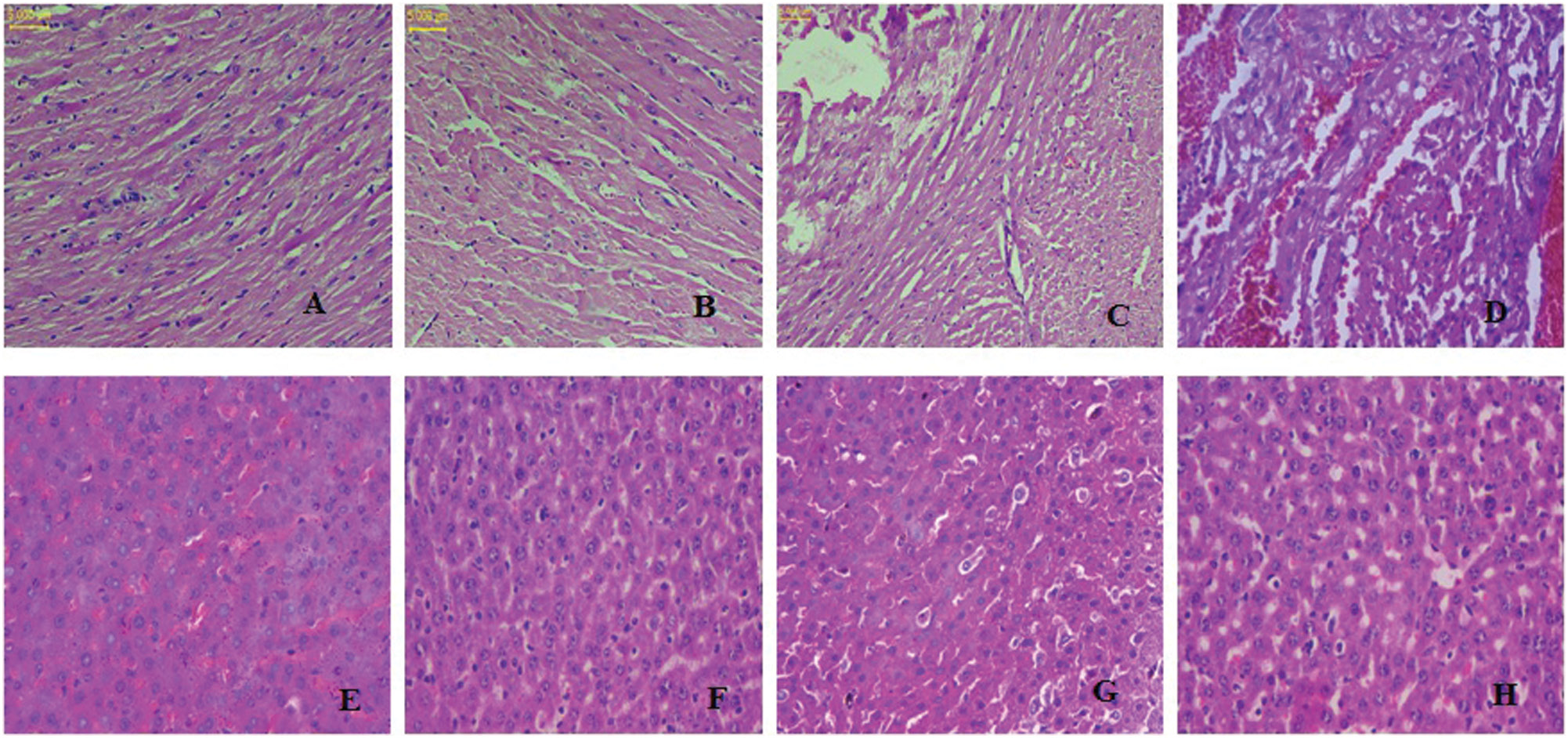
The heart tissue in rats exposed to thermally heated SSO for 8 hours showed minimal damage and fewer lesions containing fatty particles than the normal, undamaged rat heart tissue. After 4 hours of thermal heating of SSO, the presence of some fatty particles was observed in the myocardial cells in the treated group. The normal-SSO-treated group exhibited a regular architecture with no pathological alterations (Figure 7A–D). After 8 hours of thermal heating of SSO, we observed fewer fatty particles in rat hepatocytes and larger lipid vacuoles than were present in normal controls. After 4 hours of thermal heating of SSO, the presence of some fatty particles in hepatocytes was observed. The liver morphology of the normal-SSO-treated group appeared normal (Figure 7E–H).
Figure 7 Histopathological evaluation of normal and thermo-oxidized SSO treatment in a) cardiac (A–D) and b) liver tissues (E–H) from albino Wistar rats.
Discussion
Daily meals are nutritionally enriched by edible oils. In food preparation, thermally oxidized oil is an economical choice. Prolonged heating of vegetable oil can lead to the emergence of harmful health conditions due to lipid oxidation, thus negating the nutritional benefits [23]. SSO undergoes thermo-oxidation when exposed to high temperatures and air. Heating oil in the presence of oxygen alters its taste, smell, nutrition, and potential health effects [10]. The nutritional properties of SSO can be altered by thermo-oxidation. Peroxides, aldehydes, and polymerized compounds, are oxidation byproducts that can modify an oil’s fatty acid composition and decrease its nutritional value. The naturally occurring antioxidants in SSO, such as sesamol and sesamin, may degrade during thermo-oxidation, thus decreasing the health benefits [24]. During thermo-oxidation, the generation of trans fats, free radicals, and lipid peroxides leads to increased inflammation, oxidative stress, and higher risks of chronic diseases, such as cardiovascular disorders and cancer [25]. The physicochemical properties of thermally oxidized SSO adversely affected rat health in our study. Heating of SSO at 180±2°C resulted in changes in the peroxide value, FFA level, acid value, p-anisidine, saponification value, refractive index, and iodine value, possibly because of oil degradation. Thermally oxidized oil undergoes significant physicochemical changes [18].
GC-FID analysis indicated the formation of MUFA methyl myristoleate (C14:1), cis-10-pentadecenoic acid methyl ester (C15:1), palmitoleic acid methyl ester (C16:1), cis-10-heptadecenoic acid methyl ester (C17:1), cis-11-eicosenoic acid methyl ester (C20:1), and the trans fats trans-9-octadecenoic methyl ester and linolelaidic acid methyl ester in SSO4 and SSO8 . Lipid peroxidation in SSO was indicated due to formation of trans fats.
In the SSO4 and SSO8 groups, rat blood samples showed significantly higher levels of glucose, total cholesterol, triglycerides, LDL, VLDL, AST, and ALT than observed in the SSO and control groups. These harmful health issues must be addressed. No prior studies have investigated the effects of thermally oxidized SSO on blood biochemistry in rats. Hageman et al. have reported altered hematological levels in rat blood cells after consumption of heated vegetable oil, similarly to this study [26]. Animal blood cell proportions have been found to be unaffected by the ingestion of heated maize and the physicochemical characteristics of sesame oils; moreover, hyperlipidemia impairs the antioxidant defense system. Obesity lowers the antioxidant capacity of tissues and plasma [27]. SSO8 has been reported to decrease levels of GSH in rats. Enzymatic antioxidants such as SOD and catalase eliminate reactive oxygen species and free radicals. Thermally oxidized RBO decreases antioxidant enzyme activities in the liver and heart, thereby resulting in damage. Histopathological studies have indicated vacuolated swelling of the cytoplasm in hepatocytes from rats treated with SSO8 and SSO4. Heart wall and myocardial cells show damaged linings of cells and the presence of fat globules, thereby indicating organ malfunction, which may be fatal for individuals.
Conclusion
Overconsumption of heated vegetable oils may harm essential organs. Heating oil for extended periods generates intricate oxidation compounds, thus transforming oil’s chemical and physical nature. This study indicated that heating oil negatively affects the liver and heart. Heated oils increase the risk of cardiovascular disease through elevating blood pressure, altering lipid levels, and inducing vascular injury [28]. Economic considerations have overshadowed health concerns in the use of thermo-oxidized oil in the food industry and society. Limiting the use of oxidized oil in homes and businesses requires essential regulations, initiatives, and knowledge [29]. Multiple animal studies have not yet definitively established the impact of heated oil on human health. Studies are needed to examine the long-term health effects of heating oil and to promote this knowledge among the public. Evaluating oil’s physicochemical properties is essential for ensuring oil quality and preventing the utilization of hazardous oil. Oil’s chemical and physical properties undergo substantial changes with heating. This study underscores the importance of informed dietary decisions and highlights the need for further investigation of the complex links between diet and overall health. The findings in this manuscript underscore the significance of precise dietary analysis in enhancing public health outcomes. This study underscores the importance of informed dietary choices and advocates for further investigation of the intricate relationship between diet and health.
Acknowledgments
We thank the Amity Institute of Pharmacy, Amity University, Lucknow Campus, and Gurukul Kangri (Deemed to be University), Haridwar, Uttarakhand, India, for providing the necessary laboratory facilities for the study.
Future perspectives
Numerous animal studies have yet to conclusively determine the effects of heated oil on human health. Thermo-oxidation processes form oxidation products associated with inflammation, oxidative stress, and heightened risk of chronic diseases including cardiovascular disease. Future research may be aimed at finding safer alternatives or optimizing production processes to decrease the generation of harmful compounds. Thermal oxidation of SSO may lead to adverse health effects, according to research. The intake of products containing thermo-oxidized oil leads to oxidative stress, thereby increasing the likelihood of chronic diseases, such as cardiovascular disease. Future research should focus on finding safer alternatives or improving manufacturing processes to minimize production of harmful compounds.
Ethical approval
Institutional Animal Ethics Committee (IAEC), College of Pharmacy, Sri Satya Sai University of Technology and Medical Sciences, opposite oil fed plant, Pachama, NH-18, Sehore, Madhya Pradesh: IAEC approval number 1587/PO/Re/S/11/CPCSEA.
Funding
No funding was received for the publication of this research.
Conflicts of interest
The authors certify that there are no conflicts of interest with any financial organization regarding the material discussed in the article.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
R.P. and M.Y. made substantial contributions to the conception and design of the manuscript, acquisition, analysis, and interpretation of the data. A.K.S. and R.C.D. participated in drafting and critical revision of the manuscript. All authors read and approved the final version of the manuscript. All authors contributed equally to the manuscript, and read and approved the final version of the manuscript.
Supplementary materials
Supplementary Material can be downloaded from https://bio-integration.org/wp-content/uploads/2024/11/bioi20240045_Supplemental.zip.
References
- Jayaraj P, Narasimhulu CA, Rajagopalan S, Parthasarathy S, Desikan R. Sesamol: a powerful functional food ingredient from sesame oil for cardioprotection. Food Funct 2020;11(2):1198-210. [PMID: 32037412 DOI: 10.1039/c9fo01873e]
- Kumari K, Mudgal VD, Viswasrao G, Srivastava H. Studies on the effect of ohmic heating on oil recovery and quality of sesame seeds. J Food Sci Technol 2016; 53:2009-16. [PMID: 27413228 DOI: 10.1007/s13197-016-2176-1]
- Raeisi Dehkordi H, Amiri M, Zimorovat A, Moghtaderi F, Zarei S, et al. Canola oil compared with sesame and sesame canola oil on glycaemic control and liver function in patients with type 2 diabetes: a three way randomized triple blind cross over trial. Diabetes Metab Res Rev 2021;37(5):e3399. [PMID: 32860716 DOI: 10.1002/dmrr.3399]
- Ye L, Xiao F, Xie J, Feng L, Tang Z, et al. Synergistic renoprotective effects of sesame oil and erythropoietin on ischemic kidney injury after renal transplantation. AMB Express 2020;10:1-7. [PMID: 31912323 DOI: 10.1186/s13568-019-0934-y]
- Devarajan S, Singh R, Chatterjee B, Zhang B, Ali A. A blend of sesame oil and rice bran oil lowers blood pressure and improves the lipid profile in mild-to-moderate hypertensive patients. J Clin Lipidol 2016;10(2):339-49. [PMID: 27055965 DOI: 10.1016/j.jacl.2015.12.011]
- Kapoor S, Parmar SS, Yadav M, Chaudhary D, Sainger M, et al. Sesame (Sesamum indicum L.). Methods Mol Biol 2015;1224:37-45. [PMID: 25416247 DOI: 10.1007/978-1-4939-1658-0_4]
- Hashempour-Baltork F, Torbati M, Azadmard-Damirchi S, Savage GP. Quality properties of sesame and olive oils incorporated with flaxseed oil. Adv Pharm Bull 2017;7(1):97. [PMID: 28507942 DOI: 10.15171/apb.2017.012]
- Gadri Y, Williams LE, Peleg Z. Tradeoffs between yield components promote crop stability in sesame. Plant Sci 2020;295:110105. [PMID: 32534624 DOI: 10.1016/j.plantsci.2019.03.018]
- Olaleye AA, Adamu YA, Lawan U. Effects of temperature change on the physicochemical properties of SSO. J Anal Chem 2019;7(1):13. [DOI: 10.11648/j.sjac.20190701.12]
- Mujtaba MA, Cho HM, Masjuki HH, Kalam MA, Ong HC, et al. Critical review on SSO and its methyl ester on cold flow and oxidation stability. Energy Rep 2020;6:40-54. [DOI: 10.1016/j.egyr.2019.11.160]
- Mishra R, Sharma HK. Effect of frying conditions on the physico-chemical properties of rice bran oil and its blended oil. J Food Sci Technol 2014;51:1076-84. [PMID: 24876639 DOI: 10.1007/s13197-011-0602-y]
- Nduka JKC, Omozuwa PO, Imanah OE. Effect of heating time on the physicochemical properties of selected vegetable oils. Arab J Chem 2021;14(4):103063. [DOI: 10.1016/j.arabjc.2021.103063]
- Songbo H, Frederike GHK, Thomas SK, Chandel A, Zhuorigebatu T, et al. Catalytic conversion of free fatty acids to bio-based aromatics: a model investigation using oleic acid and an H-ZSM-5/Al2O3 catalyst. ACS Sustain Chem Eng 2021;9(3):1128-1141. [DOI: 10.1021/acssuschemeng.0c06181]
- Negash YA, Amare DE, Bitew BD, Dagne H. Assessment of quality of edible vegetable oils accessed in Gondar City, Northwest Ethiopia. BMC Res Notes 2019;12(1):793. [PMID: 31801628 DOI: 10.1186/s13104-019-4831-x]
- Ahmed IA, Uslu N, Özcan MM, Juhaimi FA, Ghafoor K, et al. Effect of conventional oven roasting treatment on the physicochemical quality attributes of sesame seeds obtained from different locations. Food Chem 2021;338:128109. [PMID: 33091991 DOI: 10.1016/j.foodchem.2020.128109]
- AOCS OS. Official methods and recommended practices of the AOCS. Champaign: American Oil Chemists’ Society; 1998.
- Fonseca HM, Santos CO, Cruz LP, Arthur V, Freitas BC, et al. The effects of microwave application on the physicochemical properties of bacaba (Oenocarpus bacaba Mart.) oil. Acta Scientiarum Polonorum Technologia Alimentaria 2021;20(2):189-96. [DOI: 10.17306/J.AFS.0893]
- Zhang N, Li Y, Wen S, Sun Y, Chen J, et al. Analytical methods for determining the peroxide value of edible oils: a mini-review. Food Chem 2021;358:129834. [PMID: 33933972 DOI: 10.1016/j.foodchem.2021.129834]
- Zeng W, Endo Y. Lipid characteristics of camellia seed oil. J Oleo Sci 2019;68(7):649-58. [PMID: 31178460 DOI: 10.5650/jos.ess18234]
- Aparicio-Ruiz R, García-González DL, Morales MT, Lobo-Prieto A, Romero I. Comparison of two analytical methods validated for the determination of volatile compounds in virgin olive oil: GC-FID vs GC-MS. Talanta 2018;187:133-41. [DOI: 10.1016/j.talanta.2018.05.008]
- Zhao H, Li W, Zhao X, Li X, Yang D, et al. Cu/Zn superoxide dismutase (SOD) and catalase (CAT) response to crude oil exposure in the polychaete Perinereis aibuhitensis. Environ Sci Pollut Res Int 2017;24:616-27. [PMID: 27743327 DOI: 10.1007/s11356-016-7594-0]
- Pratap R, Pillai KK, Khanam R, Islam F, Ahmad SJ, et al. Protective effect of irbesartan, an angiotensin II receptor antagonist, alone and in combination with aspirin on middle cerebral artery occlusion model of focal cerebral ischemia in rats. Hum Exp Toxicol 2011;30(5):354-62. [DOI: 10.1177/0960327110371257]
- Venkata RP, Subramanyam R. Evaluation of the deleterious health effects of consumption of repeatedly heated vegetable oil. Toxicol Rep 2016;3:636-43. [PMID: 28959587 DOI: 10.1016/j.toxrep.2016.08.003]
- Hsu E, Parthasarathy S. Anti-inflammatory and antioxidant effects of sesame oil on atherosclerosis: a descriptive literature review. Cureus 2017;9(7):e1438. [PMID: 28924525 DOI: 10.7759/cureus.1438]
- Bhardwaj S, Passi SJ, Misra A, Pant KK, Anwar K, et al. Effect of heating/reheating of fats/oils, as used by Asian Indians, on trans fatty acid formation. Food chem 2016;212:663-70. [PMID: 27374582 DOI: 10.1016/j.foodchem.2016.06.021]
- Hageman G, Verhagen H, Schutte B, Kleinjans J. Biological effects of short-term feeding to rats of repeatedly used deep-frying fats in relation to fat mutagen content. Food Chem Toxicol 1991;29(10):689-98. [PMID: 1959823 DOI: 10.1016/0278-6915(91)90127-s]
- Bigoniya P, Nishad R, Singh CS. Preventive effect of sesame seed cake on hyperglycemia and obesity against high fructose-diet induced Type 2 diabetes in rats. Food chem 2012;133(4):1355-61. [DOI: 10.1016/j.foodchem.2012.01.112]
- Ng CY, Leong XF, Masbah N, Adam SK, Kamisah Y, et al. Heated vegetable oils and cardiovascular disease risk factors. Vascul Pharmacol 2014;61(1):1-9. [PMID: 24632108 DOI: 10.1016/j.vph.2014.02.004]
- Falade AO, Oboh G, Okoh AI. Potential health implications of the consumption of thermally-oxidized cooking oils – a review. Pol J Food Nutr Sci 2017;67(2):95-105. [DOI: 10.1515/pjfns-2016-0028]