Properties and Emerging Applications of Ruthenium Nanoclusters
1Department of Pharmaceutical Chemistry, S.M.B.T. College of Pharmacy, Dhamangaon, Nashik, Maharashtra 422403, India. Affiliated to Savitribai Phule Pune University, Pune
*Correspondence to: Vaibhavi Vijay Kshatriya, Department of Pharmaceutical Chemistry, S.M.B.T. College of Pharmacy, Dhamangaon, Nashik, Maharashtra 422403, India. Affiliated to Savitribai Phule Pune University, Pune, Tel.: +91-9762755260, E-mail: vaibhavikshatriya5086@gmail.com
Received: 24 February 2024; Revised: 29 March 2024; Accepted: 23 April 2024; Published Online: May 13 2024
Cite this paper:
Kshatriya VV, Kumbhare MR, Jadhav SV et al. Properties and Emerging Applications of Ruthenium Nanoclusters. BIO Integration 2024; 5: 1–12.
DOI: 10.15212/bioi-2024-0004. Available at: https://bio-integration.org/
Download citation
© 2024 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
Ruthenium nanoclusters have shown great promise as multifunctional nanomaterials in broad scientific and technological sectors. Owing to their distinct characteristics arising from their nanoscale size and tunable electrical configuration, ruthenium nanoclusters are highly useful in photocatalysis, biomedical, electronics, sensors, and energy storage applications. Ruthenium nanoclusters are an effective catalyst with remarkable activity and selectivity. Contact with reactants is facilitated by their large surface area. Size-dependent electronic characteristics enable ruthenium nanoclusters to detect gases and biomolecules with high sensitivity and selectivity. The creation of sophisticated materials for electronic devices, such as transistors, memory chips, and conductive coatings, is facilitated by ruthenium nanoclusters. These materials’ distinct electronic structures enable more effective and flexible electronic systems, and consequently improve device performance. Because of their stability and biocompatibility, ruthenium nanoclusters are used in the biomedical industry as drug delivery systems and imaging agents. Finally, ruthenium nanoclusters have shown photocatalytic efficiency in light-driven chemical processes, and thus may aid in solar energy conversion and environmental cleanup. Their roles in sustainable uses of solar energy may make these materials valuable for solving global problems.
Keywords
Biomedical fields, catalysis, electronics, energy storage, photocatalysis, ruthenium nanoclusters, sensors.
Introduction
The study of nanomaterials has led to a new area of scientific inquiry with enormous potential for breakthroughs in a variety of fields. Among these materials, ruthenium nanoclusters have drawn scientists’ attention because of their characteristics enabling a wide range of uses. The study of ruthenium nanoclusters, which are composed of a limited number of ruthenium atoms (Ru), is a rapidly developing area of nanotechnology. These materials’ nanoscale size endows them with unique characteristics making them highly adaptable to a wide range of applications. Ruthenium nanoclusters are highly effective in catalyzing chemical reactions because of their large surface area and tunable electronic structure (Figure 1). They are essential to sensor technologies, because their size-dependent electrical characteristics enable the sensitive detection of gases and biomolecules. Ruthenium nanoclusters, by aiding in the creation of novel materials, have found multiple applications in electronics, and have shown promise for use in memory devices, transistors, and conductive coatings. Researchers are investigating additional applications in batteries and supercapacitors to improve efficiency [1, 2].
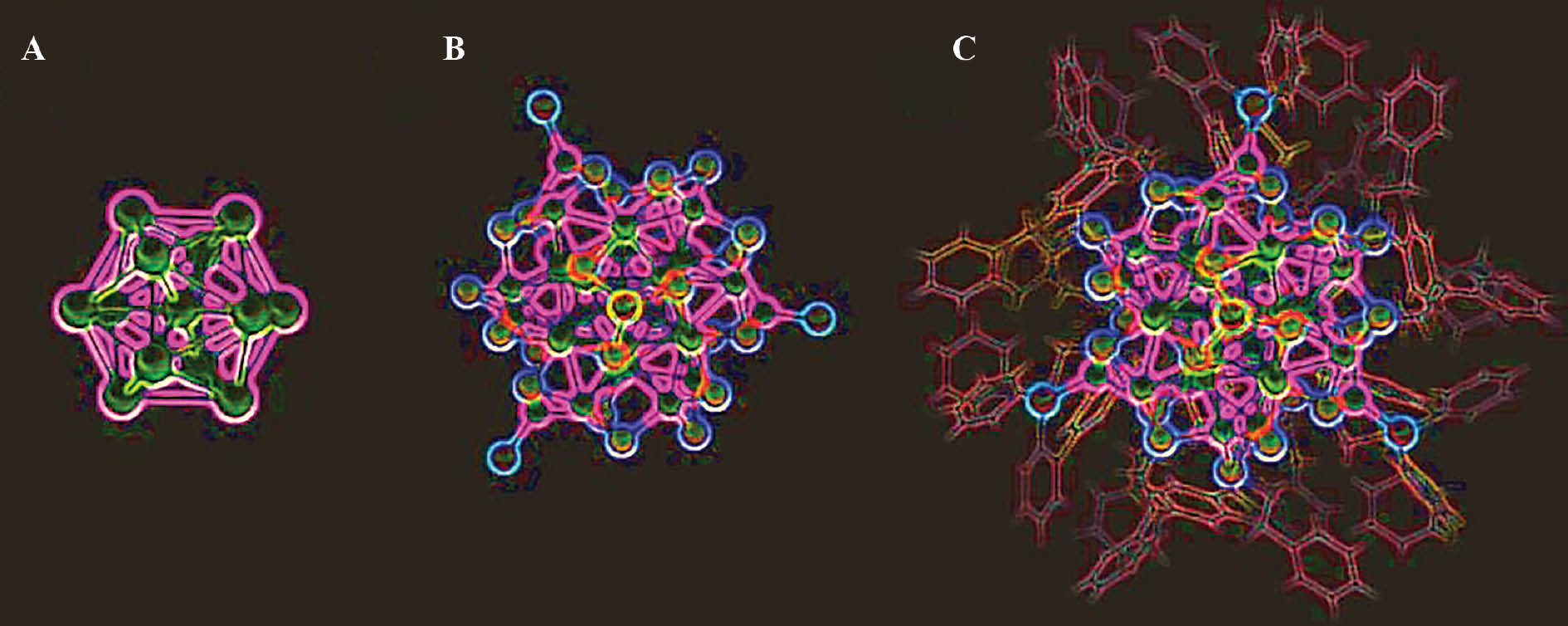
Figure 1 Structure of ruthenium nanoclusters.
Because of their biocompatibility and stability, ruthenium nanoclusters can potentially be used in biomedical applications such as drug delivery systems and imaging agents. Furthermore, these nanoclusters have shown promise in photocatalysis, a field of science involving light-driven chemical processes, and applications in environmental cleanup and renewable energy. Ruthenium nanoclusters may potentially influence the development of nanomaterials in many scientific fields, as researchers continue to elucidate their complex features [3].
Catalysis
That are changing the way that ruthenium nanocluster may be used in any reaction for improving the speed of reaction process. Their large surface area, arising from their nanoscale size, improves reactivity with substrates. This feature, together with ruthenium’s distinct electrical structure at the nanoscale, supports these materials’ exceptional catalytic activity and selectivity [4].
Research has focused on using ruthenium nanoclusters in a variety of catalytic applications. For example, these nanoclusters are remarkably efficient in hydrogenation processes, thereby providing a more environmentally friendly catalyst substitute than conventional materials. Ruthenium nanoclusters can also catalyze oxidation processes, thus expanding their potential use in the creation of useful chemical intermediates [5].
Sensors
Because of their size-dependent electrical characteristics, ruthenium nanoclusters are strong candidates for use in sensors. These characteristics have led to applications in the highly sensitive and selective detection of gases and biomolecules [6].
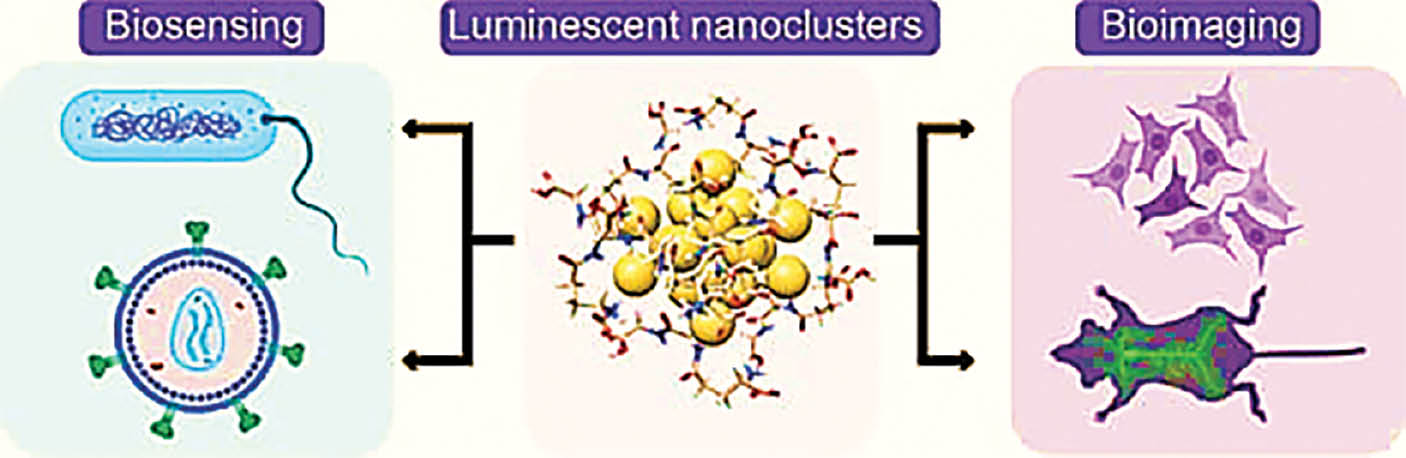
Ruthenium nanoclusters provide a flexible framework for detecting various gases in environmental monitoring, thus aiding in early detection of environmental contaminants. Furthermore, they are used in medical diagnostics, in which accurate biomarker identification is essential for early diagnosis of medical conditions. Ruthenium nanoclusters’ responsiveness is enhanced by their nanoscale size, which makes them ideal for creating advanced sensors with rapid detection times [7]. Figure 2 describes the ruthenium nanoclusters used in biosensing and bioimaging.
Figure 2 Schematic of ruthenium nanoclusters used in biosensing and bioimaging applications.
Electronics
In the electronics sector, ruthenium nanoclusters have gained attention for their roles in creating cutting-edge materials with enhanced electrical characteristics. Their electrical structure is influenced by quantum phenomena at the nanoscale; therefore, they are candidates of interest for use in conductive coatings, memory devices, and transistors [8].
Because of their qualities, ruthenium nanoclusters have been integrated into electrical devices to enhance device performance. These applications have enabled enhanced switching properties, elevated conductivity, and heightened stability. Current investigations of ruthenium nanoclusters in electronic applications have indicated a paradigm shift toward the use of nanoscale materials for next-generation electronic devices [9].
Energy storage
The search for cutting-edge materials for energy storage devices has prompted scientists to examine the possibilities of ruthenium nanoclusters. These nanoscale structures have notable characteristics, and may markedly advance the supercapacitor and battery industries [10].
Device efficiency and energy storage capacity can be enhanced in novel ways with ruthenium nanoclusters. Because of their large surface area and tunable characteristics, they are excellent choices for electrode materials that affect charge storage processes. Future developments in energy storage technology may be influenced by ruthenium nanoclusters, as the demand for effective and sustainable energy storage solutions continues to rise [11].
Current approaches to fabricating ruthenium nanoclusters
Several current approaches to fabricating silver nanoclusters, including their advantages and disadvantages, are summarized in Table 1.
Table 1 Summary of Current Approaches to Fabricating Ruthenium Nanoclusters
| Fabrication Method | Advantages | Disadvantages |
|---|---|---|
| Chemical Reduction | Relatively simple and scalable process | Requirement of toxic reagents (e.g., NaBH4) |
| High yield | Lack of precise control over size and shape | |
| Potential for tuning size and composition | Aggregation tendency | |
| Ability to be performed at moderate temperatures | Potential need for additional ligands for stability | |
| Solvent Thermal Method | Control over size and morphology | Limited scalability |
| High purity of resulting nanoclusters | High temperature requirement | |
| Potential for producing monodisperse nanoclusters | Energy-intensive process | |
| Ability to incorporate dopants or ligands | Complex synthesis procedures | |
| Relatively narrow size distribution | ||
| Microemulsion Method | Well-defined size and shape control | Limitation to specific nanocluster sizes |
| Facile scalability | Requirement of surfactants and co-surfactants | |
| High stability of resulting nanoclusters | Complex optimization for desired properties | |
| Potential for functionalization during synthesis | Potential multistep synthesis | |
| Homogeneous nucleation and growth | ||
| Electrochemical Method | Mild reaction conditions | Limited control over size and morphology |
| Continuous, controllable process | Requirement of specialized equipment and expertise | |
| High purity of resulting nanoclusters | Need for electrolyte selection for cluster stability | |
| Potential for large-scale production | Limited to specific electrolyte systems | |
| Potential for direct deposition onto substrates |
Biomedical applications
In biomedicine, ruthenium nanoclusters are versatile molecules with particular benefits for drug delivery systems and imaging applications. Their biocompatibility, stability, and tunable surface qualities make ruthenium nanoclusters attractive options in this ever-changing field [12].
Ruthenium nanoclusters are used in medication delivery to carry therapeutic substances and enable regulated and targeted drug release. Moreover, the imaging capabilities of ruthenium nanoclusters have enabled improvements in medical diagnostics and treatment monitoring in applications including fluorescence or magnetic resonance imaging (MRI) [13].
Photo catalysis
Ruthenium nanoclusters are essential to the development of photocatalysis, the use of light-driven chemical processes in a variety of applications. By serving as effective catalysts, these nanoclusters support environmentally friendly methods of energy conversion and environmental cleanup.
In photocatalysis, ruthenium nanoclusters capture solar radiation with exceptional efficacy. This characteristic is particularly important for the development of sustainable energy solutions, such as water splitting and manufacturing of solar fuel. Their use in environmental remediation procedures further emphasizes their potential for decreasing pollution and addressing global issues.
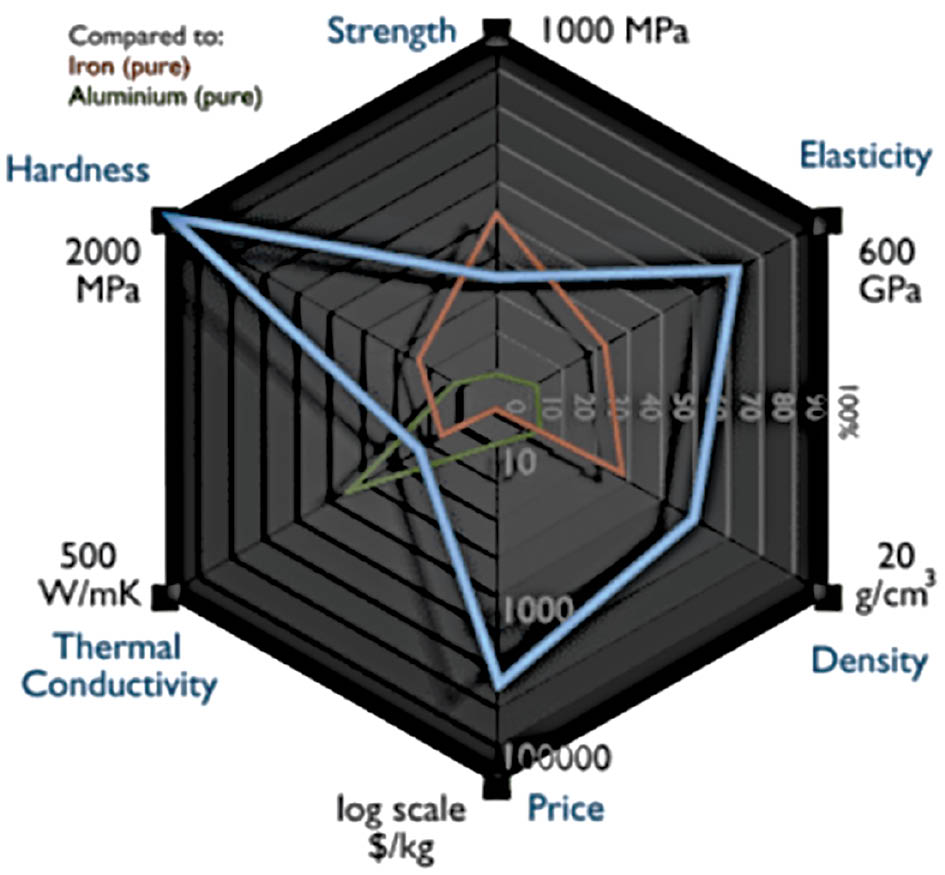
Because they enable many flexible applications in the disciplines of photocatalysis, energy storage, biomedical applications, sensors, electronics, and catalysis, ruthenium nanoclusters are at the forefront of nanomaterial innovation. This diversity of applications highlights nanocluster research as an active and evolving body of study with the potential to transform multiple scientific and technological fields. The profound effects of ruthenium nanoclusters on a range of sectors will be increasingly understood as further studies are conducted on these intricate structures. In the future, these nanoscale structures are expected to be essential for solving challenging problems and leading to innovation [14–16]. Figure 3 describes the various properties of ruthenium.
Figure 3 Various physical properties of ruthenium.
Literature review
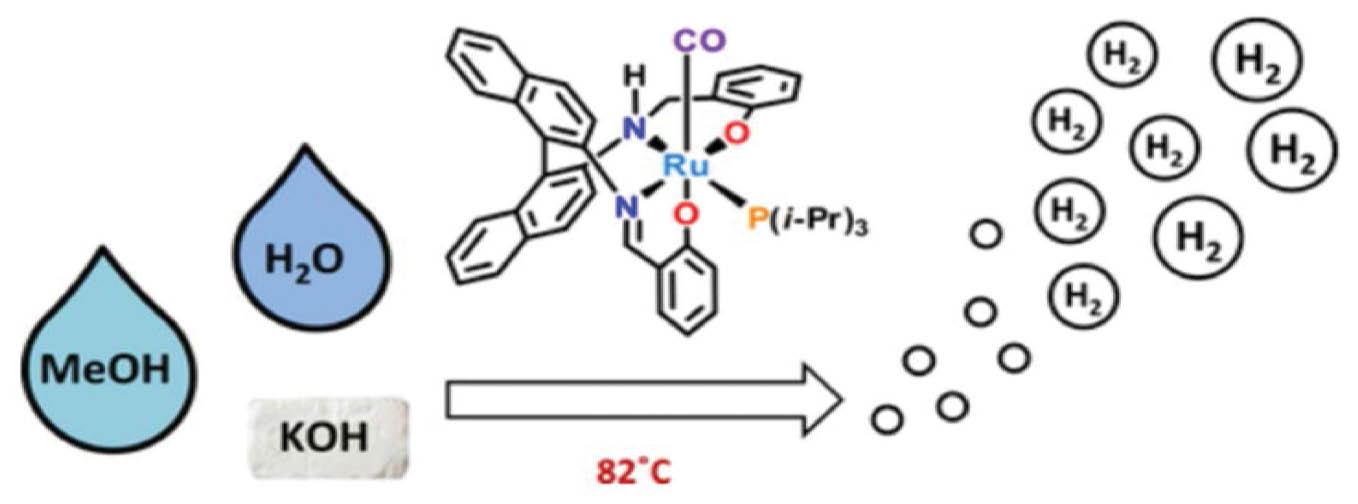
Because of their qualities providing clear benefits over conventional catalysts, ruthenium nanoclusters have become a major topic of study in catalysis. The many uses of ruthenium nanoclusters in catalysis are examined in this literature review, along with their selectivity, catalytic activity, and contributions to long-term chemical transformations. Ruthenium nanoclusters’ large surface area and nanoscale size endow them with remarkable catalytic activity. One notable study on magnetite-supported ruthenium nanoparticles has demonstrated the efficiency of these nanoparticles as recoverable catalysts for the mild hydrogenation of arenes. The ability of ruthenium nanoclusters to effectively catalyze these types of reactions suggests that they may find further use in environmentally friendly and sustainable chemical processes. Moreover, ruthenium nanoclusters have been demonstrated to be highly effective in oxidation processes. Their ability to serve as oxidation catalysts, thus contributing to the synthesis of valuable chemical intermediates, has been explored in multiple studies. The adaptability of ruthenium nanoclusters in catalyzing processes beyond hydrogenation has been demonstrated by research on ruthenium nanoparticle-decorated black phosphorus nanosheets. Ruthenium nanoclusters are important for catalysis because of their selectivity, which is essential for several chemical reactions. They are ideal for selective catalysis because of their tunable characteristics and distinct electronic structures at the nanoscale. This selectivity has been exploited in applications as diverse as precise chemical manufacturing and pharmaceutical synthesis [17–19]. Figure 4 describes dynamic hydrogen production from methanol by using a ruthenium catalyst.
Figure 4 Dynamic hydrogen production from methanol with ruthenium catalysts.
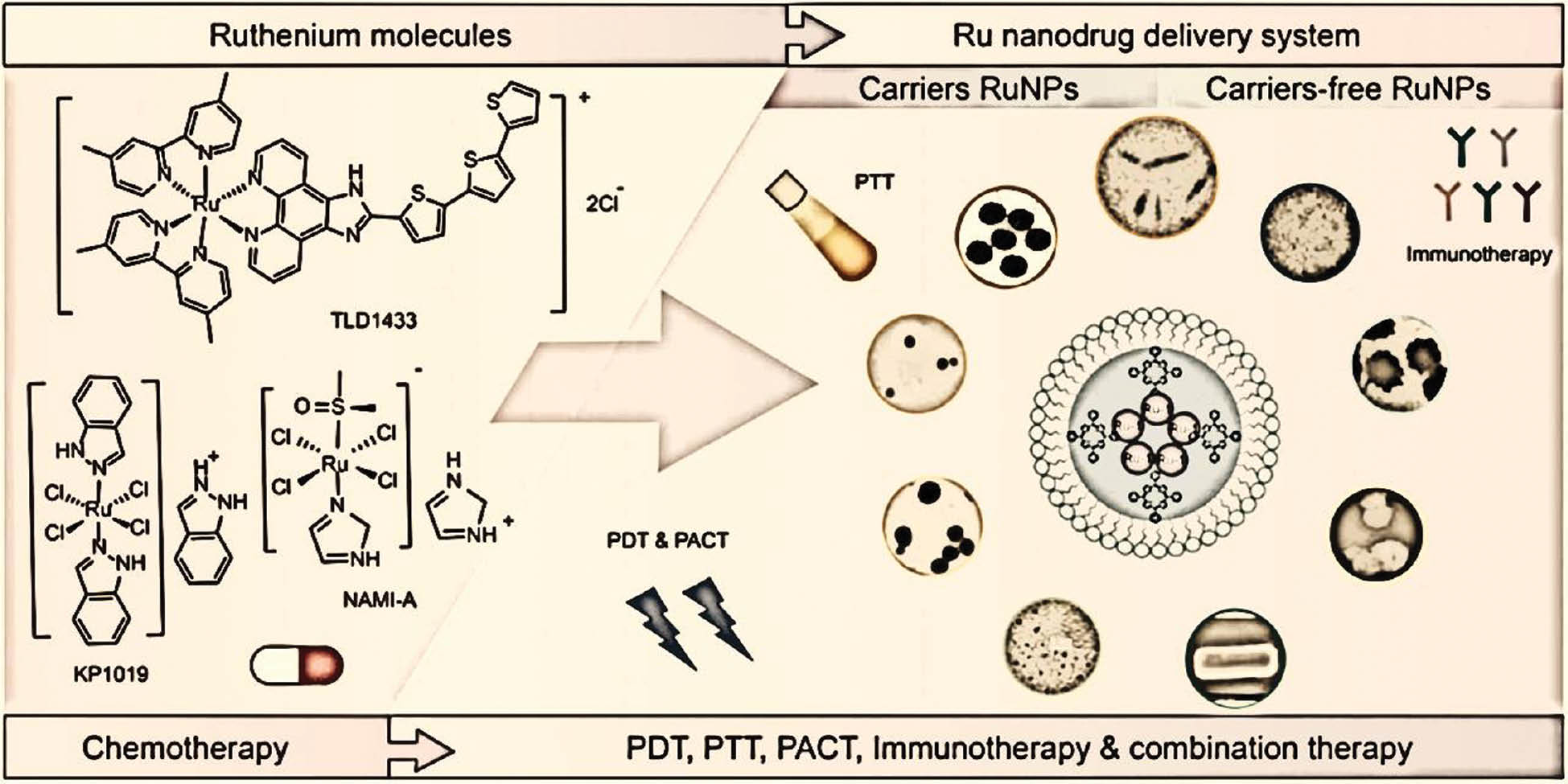
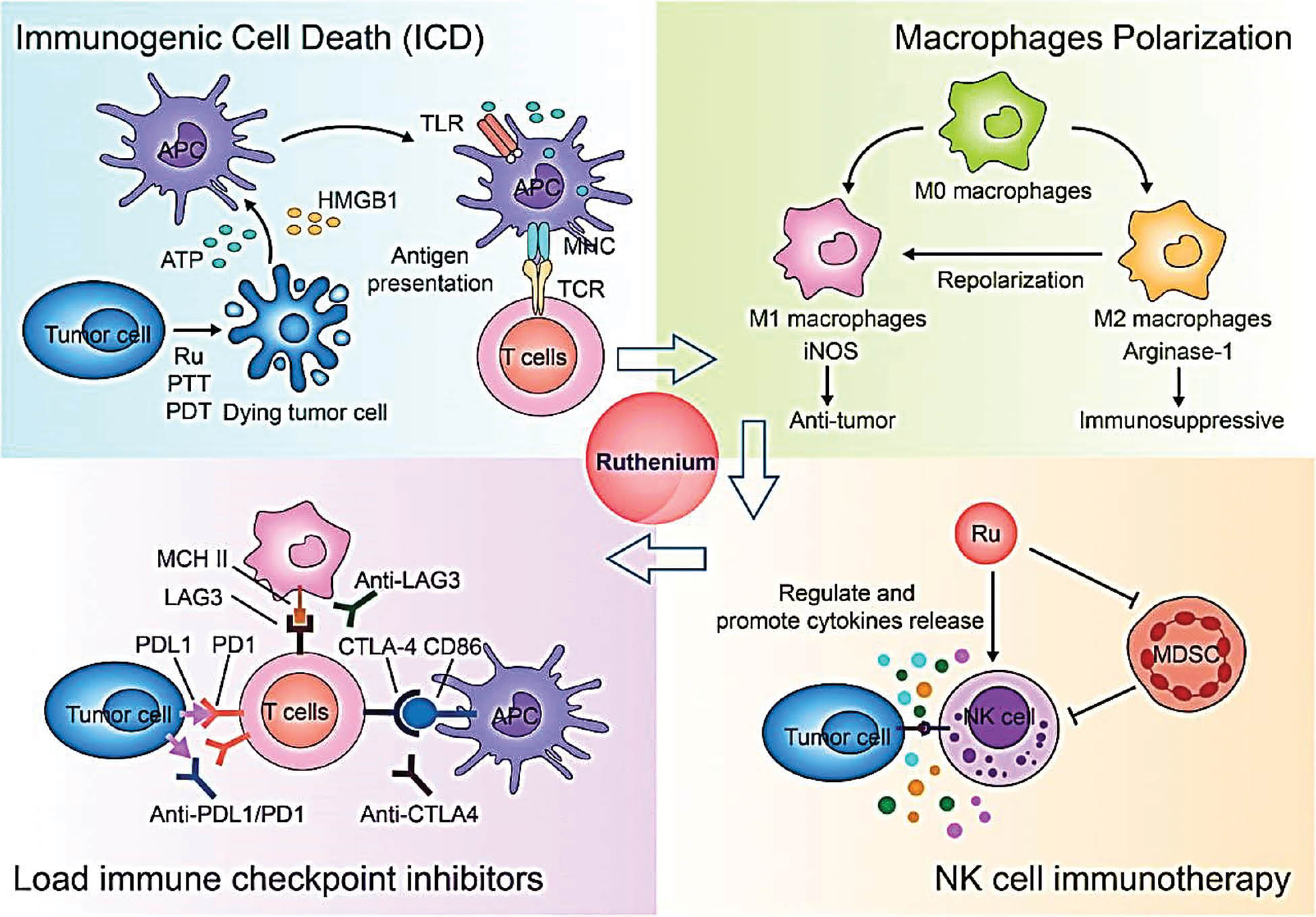
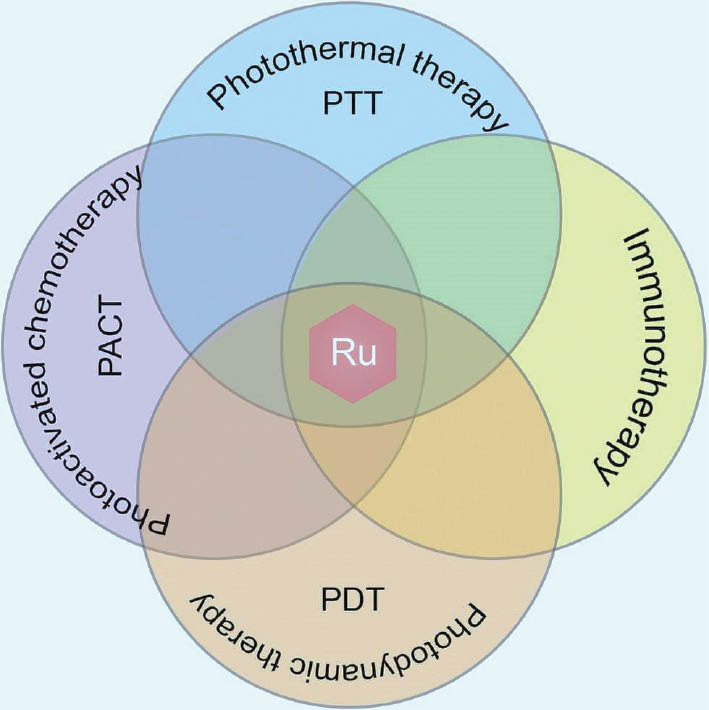
In intricate organic reactions, ruthenium nanoclusters have shown encouraging catalytic activity. Researchers have examined their catalysis of C-C and C-N bond formation processes, thus leading to the production of complex organic compounds. The pharmaceutical sector, in which precise control over chemical reactions is crucial for drug development, has found great value in these materials. Ruthenium nanoclusters are useful in heterogeneous catalysis as well as conventional homogeneous catalysis, because of their exceptional catalytic capabilities. To improve their stability and recyclability, and to create more sustainable catalytic systems, ruthenium nanoclusters can be immobilized on support materials. This aspect is important, because catalyst lifespan and reusability are critical considerations in industrial operations. Ruthenium nanoclusters are used catalytically in a broad range of chemical transformations. Their ability to catalyze a wide range of reactions makes them an invaluable tool for process engineers and synthetic chemists. This versatility demonstrates the dynamic properties of ruthenium nanoclusters as catalysis. In summary, research on ruthenium nanoclusters’ catalytic applications has emphasized their value in developing environmentally friendly and sustainable chemistry. The references cited herein provide insights into the wide range of reactions that ruthenium nanoclusters can catalyze, thus demonstrating their effectiveness in oxidation, hydrogenation, and other organic transformations. The potential of ruthenium nanocluster catalysis to influence chemical synthesis and industrial processes is becoming increasingly apparent as researchers continue to examine their complexities. The potential to create more effective, selective, and ecologically friendly catalytic systems is spurring research in the field [20–23]. Figure 5 shows immunotherapies and combination therapies using ruthenium nanoclusters.
Figure 5 Immunotherapy and combination therapy with ruthenium nanoclusters.
Because ruthenium nanoclusters have qualities that make them suitable for detecting gases and biomolecules, their use as sensors has attracted substantial attention. The applications of ruthenium nanoclusters in sensor technology are examined herein, with an emphasis on the materials’ sensitivity, selectivity, and potential to improve environmental monitoring and medical diagnostics. The exceptional performance of ruthenium nanoclusters as sensors is based on their size-dependent electrical characteristics. Their ability to selectively interact with particular analytes makes them useful for highly sensitive gas and biomolecule detection. Black phosphorus nanosheets adorned with ruthenium nanoparticles as a dual-mode biosensor have demonstrated the potential of ruthenium nanoclusters in sensing applications for improved hydrogen peroxide (H2O2) detection, as well as their flexibility and sensitivity. Ruthenium nanoclusters have been used in environmental monitoring to accurately identify various gasses. These clusters have a large surface area because of their nanoscale size, thus facilitating more interactions with gas molecules. This characteristic enhances the sensitivity of sensors based on ruthenium nanoclusters and makes them useful for the early identification of environmental contaminants. This application may influence industrial emissions control, air quality monitoring, and general environmental health. With the use of ruthenium nanoclusters, sensor technology in the biomedical industry has substantially advanced. Regarding the potential of ruthenium nanoclusters in medical diagnostics, a prior study5 has emphasized their use in high-performance MRI of cancer cells. Because of their stability and biocompatibility, ruthenium nanoclusters are a good choice for imaging applications in biological systems. Their adaptable surface characteristics further increase their utility in biomolecular detection sensor design, thereby advancing the field of illness diagnostics. Ruthenium nanoclusters can be used for purposes beyond conventional sensing. Their integration into wearable and portable sensor devices has the potential to revolutionize point-of-care diagnostics. The development of miniaturized and portable sensors using ruthenium nanoclusters has enabled real-time monitoring of biomarkers and environmental pollutants, thus offering rapid on-site detection capabilities. The tunable nature of ruthenium nanoclusters has also facilitated the development of chemosensors with selectivity toward specific analytes. This selectivity is crucial in medical diagnostics, in which precise detection of biomarkers is essential for early disease diagnosis. Ruthenium nanocluster-based chemosensors provide a platform for designing devices that can discriminate among analytes and enhance the specificity of the detection process. The literature on the application of ruthenium nanoclusters as sensors highlights their versatility and potential contributions to environmental monitoring and medical diagnostics. The cited references provide insights into the diverse applications of ruthenium nanoclusters in sensor platforms, including detecting gases and imaging cancer cells. As research in this field continues to evolve, the promise of ruthenium nanoclusters in developing advanced sensor technologies for a wide range of applications is becoming increasingly evident. Their unique electronic properties, coupled with advancements in nanotechnology, position ruthenium nanoclusters as key players in the future of sensor technology [24–27]. Figure 6 shows the antitumor effects of these materials.
Figure 6 Schematic of antitumor effects of ruthenium nanoclusters.
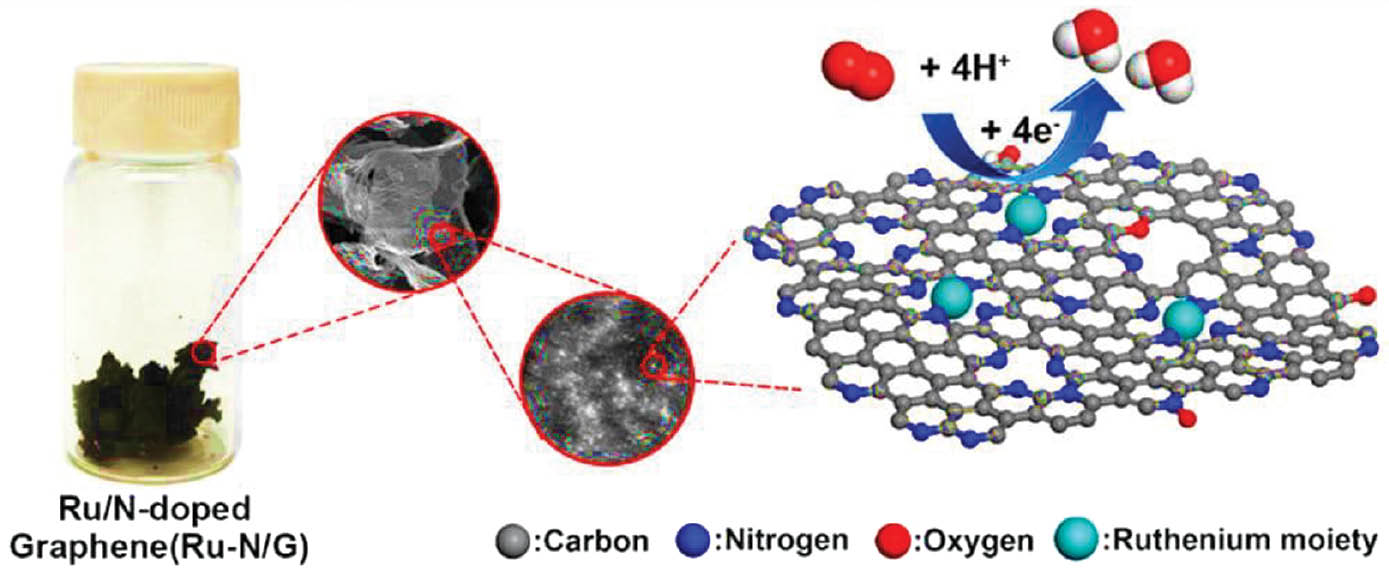
The potential substitution of alkaline water electrolysis occurs due to the lower water dissociation and it is similar to the hydrogen adsorption energy. To enhance the kinetics of water dissociation and accelerate the electrolysis process, a novel technique has been proposed in which local charge transfer is used to modify the electrical environment of ruthenium catalysts. Ru-N coordination polymers based on porphyrin and layered in two dimensions are assembled on nanocarbon substrates and then pyrolyzed to produce the final catalysts. These catalysts exhibit exceptional performance, owing to their distinct electronic environments, local charge transfer characteristics, and well-defined ruthenium nanocluster-Nx-coordination bonds. These catalysts demonstrate remarkable activity, with an overpotential of only 17 mV at 10 mA cm−2, and exhibit strong stability in water, surpassing that of even the most advanced ruthenium catalysts [28].
The synthesis of conductive and acid-stable IrRu@Te catalysts has been achieved through a hydrothermal one-pot process. These catalysts are made of an amorphous tellurium nanoparticle support loaded with ultrafine IrRu intermetallic nanoclusters. Additionally, they require overpotentials of only 220 and 303 mV to achieve 10 and 100 mA cm−2, respectively. Furthermore, IrRu@Te demonstrates substantial specific activity, thus showcasing its superior performance in contrast to that of other commercially available Ir- and ruthenium-based catalysts, as well as unsupported IrRu. Additionally, IrRu@Te exhibits an unprecedented mass activity, reaching 590AgIrRu−1 at a potential of 270 mV, surpassing that of most reported Ir- and ruthenium-based OER catalysts [29]. The synthesis process results in a highly monodispersed nanocluster bound to 9-ethynylphenanthrene and is achieved through a simple high-yield approach. By imitating the function of a conjugated organic polymer, this nanocluster has displayed an impressive ability to detect nitroaromatic explosives in solution through a luminescence quenching method, with a KSV value as high as 4.98×104 M−1 [30]. That study described the initial utilization of the RuCu bimetal in gas-sensitive material. By using a direct co-reduction method to modify SnO2 nanoparticle clusters, we have achieved successful synthesis of RuCu bimetallic nanoparticles with small sizes. The remarkable gas performance of the RuCu bimetal is attributable to its synergistic effects resulting in higher catalytic activity than that of the individual metals. Additionally, the chemical and electrical sensitization of the RuCu bimetal plays a important role [31]. Ammonia is synthesized more rapidly (at a rate of 5.557 mol gcat−1 h−1) through the catalysis of strained ruthenium nanoclusters in room-temperature nitrate electroreduction than through the Haber−Bosch process [32]. Prior research has underscored the importance of modifying the surrounding bonds to efficiently regulate the stability and catalytic activity of ruthenium clusters. These findings present an opportunity to create water electrolysis catalysts with improved longevity and performance. Previous studies have documented the use of ruthenium nanoparticles as catalysts, with a focus on ruthenium nanoclusters for expediting alkaline water electrolysis and ammonia electrosynthesis, and enhancing n-pentanol sensing performance through RuCu alloy nanoparticles. These catalysts and their potential for exceptional performance in these processes have been examined [33]. Applications of ruthenium nanoclusters are presented in Figure 7.
Figure 7 Applications of ruthenium nanoclusters.
Ru(o) nanoclusters with nitrogen-doped graphene exhibit remarkable performance as multifunctional catalysts for the oxygen reduction reaction and hydrogen evolution reaction. In alkaline solution, their activity is comparable to that of commercial Pt/C, thus highlighting their effectiveness as catalysts. The exceptional catalytic properties of Ru(o) nanoclusters have substantial implications for the advancement of sustainable energy conversion processes. Electrodes composed of Ru/MOC exhibit exceptional robustness and stability, and therefore are highly promising for practical applications in biosensing, catalysis, and energy storage. Furthermore, their production is both economically viable and environmentally benign [34]. A high-energy supercapacitor has been constructed by incorporating ruthenium and manganese oxide nanoparticles into reduced graphene oxide (RGO). The oxide nanoparticles, ranging in size from 2 to 10 nm, are uniformly distributed on 2D nanosheets of RGO. The presence of RM nanoparticles (NPs) expands the RGO interlayers and consequently increases the number of micro- and mesopores. Assembled as symmetric supercapacitor electrodes in a 0.5 M KOH electrolyte, these nanocomposites have an impressive energy density of 22.26 Wh kg−1 and a capacitance of 641 F g−1. The initial capacitance is maintained over 1000 cycles [35]. RuO2 nanoparticles have been synthesized through the instant technique, with Li2CO3 serving as a stabilizing agent, and subsequently subjected to microwave irradiation at 60°C, to investigate their supercapacitance properties. This high specific capacitance makes hydrous RuO2 a promising material for energy storage. However, as the crystallinity of RuO2 increases, the specific capacitance decreases. A reliable method has been described for producing RuO2 nanoparticles rapidly and consistently; these materials can subsequently be used in supercapacitors and water electrolysis applications [36]. This method is both scalable and straightforward, and therefore is an attractive option for future energy storage applications. The RGM system demonstrates excellent capacitive performance, electrochemical stability, and ease of preparation [37]. The effectiveness of ruthenium nanoparticles on carbon nanofibers as catalysts in the Li-CO2 reaction is truly exceptional. Consequently, our strategy offers a new and promising route for the development of Li-CO2 batteries with superior performance. Moreover, this approach has the potential to drive advancements in catalysis and other renewable energy storage technologies [38]. To create ruthenium nanoparticles (RuHT, RuPET, and RuPA), RuCl3 reduction has been performed with hydrazine hydrate. The nanoparticles were subsequently stabilized through the self-assembly of organic molecules (hexanethiol, phenylethanethiol, and phenylacetylene). The difference in capacitance may potentially be explained by the varying proportion of ruthenium in a higher valence state and the interfacial bonding between ruthenium and the outer layer of organic ligands [39]. Figure 8 describes the applications of ruthenium nanoclusters.
Figure 8 Applications of ruthenium nanoclusters.
To develop oxygenic hybrid ruthenium sulfide nanoclusters (RuSx NCs) with a high photothermal effect for photothermal therapy of tumors in living mice, the levels of sulfur flaws and oxygen concentrations must be precisely controlled. This control can be accomplished through a simple bottom-up approach that facilitates the manufacturing of RuSx NCs. A proposed defect engineering technique not only allows for the creation of RuSx NCs with exceptional photothermal properties but also presents an opportunity for investigating the potential of these nanoclusters in various biomedical applications in the future [40]. Ruthenium-quercetin conjugated nanoclusters have been synthesized a one-pot reflux process. To address the potential health hazards associated with inhaling heavy metal ions such as cobalt, researchers have developed a fluorescent probe enabling the precise measurement of Co(II) levels in both living cells and aqueous solutions. The hybrid nanoclusters within the probe, with an average size of 2 nm, are formed by combining the flavonoid quercetin with ruthenium(II) ions. The fluorescent probe exhibits a linear response to Co(II) concentrations ranging from 0.03 to 100 μM, with a detection limit of 9.28 nM. Nanoconjugates have been formed by coordinating pectin and dopamine with a ruthenium molecule through a polysaccharide-metal complex. These conjugates, measuring approximately 200 nm in size, exhibit selective suppression of the 786-O human renal cell cancer cell line. Multispectral data have confirmed the successful coordination of pectin and dopamine with the ruthenium complex leading to the self-assembly of regular nanospheres [41]. Ru1085, a Ru(II) metallacycle renowned for its profound optical penetration capability, has been fabricated and found to reach depths as high as 6 mm and to have high chemo-phototherapy effectiveness. This metallacycle is excited at 808 nm, and its emission extends beyond 1000 nm. Through precise NIR-II fluorescence imaging, Ru1085 enables monitoring of chemo-phototherapy against A549 tumors, and ensures minimal invasiveness and long-term efficacy. The development of long-wavelength emissive metallacycles is poised to introduce novel opportunities for the utilization of metal-based agents in biomedical applications [42]. The coordination complexes of ruthenium (II) and iridium (III) exhibit remarkable structural, photophysical, and biological attributes. These complexes have immense potential as agents for bioimaging and cancer treatment [43]. The effectiveness of transferrin-ruthenium nanoparticles in tumor eradication has been demonstrated through both in vivo and in vitro techniques. This effectiveness is attributable to their low toxicity and exceptional ability to destroy cells [44]. Manufactured Ru-CDs showcased promising results and a diverse range of biological uses, but the plasmid DNA was susceptible to harm solely in the presence of light and Ru-CDs (ruthenium-containing carbon dots). Hence, the manufactured ruthenium-containing carbon dots have shown promising results and may find diverse biological uses [45]. The combination of ruthenium complexes and selenium nanoparticles displays synergistic antimicrobial effects. Ruthenium complexes and Selenium nanoparticles displayed a synergistic antimicrobial effect this was compared with other synergistic effect. With their strong antibacterial action, remarkable selectivity, and good biocompatibility, Se@PEP-Ru NPs hold promise as a potential antimicrobial agent [46]. The use of RuTiO2 (Ru-doped TiO2) nanoparticles and multiwall carbon nanotubes as composite materials has posed challenges; however, the customized sensor has successfully shown effectiveness in the electrochemical analysis of clozapine [47]. Moreover, sRuNPs have shown notable therapeutic efficacy in mice with ALI, even when administered 6 hours after APAP intoxication. This innovative approach allows for precise adjustment of nanozymes’ catalytic activities, thus making them suitable for various biomedical applications [48]. Liquid exfoliation has enabled the creation of a CS/WS2/Ru composite with physiological activity. This composite effectively inhibits the growth of both S. aureus and E. coli bacteria. Furthermore, it has shown a combined anticancer effect against MCF-7 cancer cells. Prior studies have explored the unique biological consequences of this composite in the context of cancer applications [49–51]. Synthesized PEG-dBSA-RuS1.7 NCs exhibit exceptional photothermal conversion capability. Notably, these agents demonstrate prolonged blood circulation times and greater tumor-targeting efficiency in vivo than existing TMS-based photothermal therapy nanoagents. These enhancements are attributable to their suitable hydrodynamic diameter of approximately 70 nm and neutral charge of approximately 0 mV. With these advantages, the PEG-dBSA-RuS1.7 NCs effectively target specific tumor areas and efficiently eliminate cancer cells after exposure to near-infrared radiation [52]. Figure 9 describes the formation of the ruthenium moiety.
Figure 9 Schematic of formation of the ruthenium moiety.
Semiconductor-based photocatalysts modified by nanoclusters are a crucial focus. The incorporation of ruthenium nanoclusters as cocatalysts has been found to greatly enhance the photostability and photocatalytic activity of CdS photocatalysts. Moreover, compared with photo-induced ruthenium nanoparticle/CdS composite photocatalysts, the ruthenium nanocluster-modified hybrid CdS photocatalyst demonstrates enhanced stability and activity [53]. Both bare TiO2 and TiO2 nanoparticles doped with ruthenium at different molar concentrations have been synthesized through the precipitation approach. The presence of the ruthenium dopant has been hypothesized to be the underlying reason for the increased activity observed in the doped materials [54]. An antenna-reactor nanostructure has been developed through assembly of ultrathin ruthenium nanocluster shells onto plasmonic gold nanoantennas, thus ensuring the presence of the necessary catalytic activity. Within this nanostructure, the gold nanoantennas efficiently interact with light and result in the generation of hot carriers. Simultaneously, the ruthenium nanoclusters adsorb and activate N2, and facilitate its reduction to NH3 through the utilization of the hot electrons [55–57]. Metallic ruthenium-based nanoparticles work well as catalysts in photo- and electro-catalytic hydrogen evolution reaction processes. Ruthenium-based bimetallic nanomaterials, pure ruthenium nanocrystals, and ruthenium/non-metal nanocomposites are among the other ruthenium-based nanomaterials that have been created [58–60]. The ultra-low loading of Ru6 clusters results in an activity more than 20 times that of bare g-C3N4, owing to synergistic effects. In-depth examinations of the catalyst’s photoluminescence and photo-electrochemical properties have demonstrated that the presence of enhanced charge migration, inhibition of electron-hole pair recombination, and surface-area expansion through exfoliation are factors contributing to these effects [61]. Figure 10 describes the 2D and 3D surface structures of ruthenium nanoclusters, and Figure 11 shows comprehensive examinations of the various applications of ruthenium nanoclusters from 2014 to 2019.
Figure 10 Appropriate 2D and 3D surface structures of ruthenium nanoclusters.
Figure 11 Comprehensive examination of various applications of ruthenium nanoclusters from 2014 to 2019.
Patents related to ruthenium nanoclusters
Table 2 lists patents reported for ruthenium nanoclusters [62].
Table 2 Patents on Ruthenium Nanoclusters
| Sr. No. | Patent Number | Patent Title |
|---|---|---|
| 1 | JP7151984B2 | Solid solution nanoparticles, method for producing the same, and catalyst |
| 2 | JP7157456B2 | PdRu solid solution nanoparticles, manufacturing method and catalyst therefor, method for controlling crystal structure of PtRu solid solution nanoparticles, and AuRu solid solution nanoparticles and manufacturing method therefor |
| 3 | KR101113632B1 | KR1 synthesis methods of nano-sized transition metal catalyst on a carbon support |
| 4 | JP2017177094A | Metal-containing nanoparticle-carrying catalyst and carbon dioxide reduction apparatus |
| 5 | CN105431230B | Method for forming noble metal nanoparticles on a support |
Clinical trials involving ruthenium nanoclusters/nanoparticles
Table 3 lists clinical trials on ruthenium nanoclusters [63].
Table 3 Clinical Trials of Ruthenium Nanoclusters
| Sr. No. | Trial Number | Study Title | Sponsor |
|---|---|---|---|
| 1 | NCT05893654 | Melphalan chemoreduction for ocular melanoma (MELCOM) | Hospital das Clínicas de Ribeirão Preto |
| 2 | NCT01415297 | Dose escalation study of NKP-1339 to treat advanced solid tumors | Niiki Pharma Inc. |
| 3 | NCT04421820 | BOLD-100 in combination with folfox for the treatment of advanced solid tumours | Bold Therapeutics, Inc. |
| 4 | NCT00079417 | Neoadjuvant carboplatin and vincristine and standard local ophthalmic therapy in treating patients with intraocular retinoblastoma | Children’s Oncology Group |
Conclusion
In this analysis, we have explored the use of ruthenium nanoclusters in diverse fields, encompassing biomedical, electronics, sensors, energy storage, and photocatalysis. Based on the 41.2% publication data the ruthenium nanocluster is used extensively in catalysis. Additionally, the commonly used as a sensor has been 61.4% for sensors, 57.4% for electronics, 51.6% for energy storage, 43.2% for biomedical applications, and 49.6% for photocatalysis. These findings unequivocally demonstrate the extensive applicability of ruthenium nanoclusters across various disciplines, and the scientific underpinnings of their frequent utilization in different domains.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships.
Acknowledgements
The authors acknowledge the support of various open-access data-resource websites and journals, which aided in easy reference gathering. Because this is a short review, no patient or animal data were used directly in this article.
References
- Khashab NM, Gawande MB, Zboril RS, Varma RS. Magnetite-supported ruthenium nanoparticles as a recoverable catalyst for the hydrogenation of arenes under mild conditions. Green Chem 2015;17:4135.
- Zhao S, Zhang W, Liu Q, Han J, Liu B. Ruthenium nanoparticle-decorated black phosphorus nanosheets as a dual-mode biosensor for enhanced detection of H2O2. Nanoscale 2018;10:15617.
- Zhang J, Huang G, Yang S. Ruthenium nanoclusters supported on N-Doped graphene as an efficient catalyst for hydrogen evolution reaction. Chem Eur J 2015;21:9504.
- Du P, Fu HC, Zhang XX, Guo YB. Ruthenium nanoclusters confined in carbon nanotube-grafted nitrogen-doped graphene for high-performance supercapacitors. Small 2019;15:1805168.
- Zhang Y, Zhang Y, Zheng Y, Chen J. Ruthenium nanoclusters-based nanoprobes for high-performance magnetic resonance imaging of cancer cells. Analyst 2019;144:3807.
- Wang L, Han Q, Zhao J. Ruthenium nanoclusters as photocatalysts for water splitting and environmental remediation. Adv Mater Interfaces 2018;5:1701627.
- Kolimi P, Narala S, Youssef AA, Nyavanandi D, Dudhipala N. A systemic review on development of mesoporous nanoparticles as a vehicle for transdermal drug delivery. Nanotheranostics 2023;7:70-89. [PMID: 36593800 DOI: 10.7150/ntno.77395]
- Xu J, Lian Z, Wei B, Li Y, Bondarchuk O, et al. Strong electronic coupling between Ultrafine Iridium−Ruthenium nanoclusters and conductive, acid-stable Tellurium nanoparticle support for efficient and durable oxygen evolution in acidic and neutral media. ACS Catal 2020;10:3571-9. [DOI: 10.1021/acscatal.9b05611]
- Dave P, Agrawal B, Thakarda J, Bhowmik S, Maity P. An organometallic ruthenium nanocluster with conjugated aromatic ligand skeleton for explosive sensing. J Chem Sci 2019;131:14. [DOI: 10.1007/s12039-018-1589-y]
- Wang C, Bai J, Wang H, Li Y, Li Y, et al. Enhanced n-pentanol sensing performance by RuCu alloy nanoparticles decorated SnO2 nanoclusters. Sens Actuators B Chem 2022;351:130900. [DOI: 10.1016/j.snb.2021.130900]
- Li J, Zhan G, Yang J, Quan F, Mao C, et al. Efficient ammonia electrosynthesis from nitrate on strained ruthenium nanoclusters. J Am Chem Soc 2020;142:7036-46. [DOI: 10.1021/jacs.0c00418]
- Ye R, Liu Y, Peng Z, Wang T, Jalilov AS, et al. Tour. High performance electrocatalytic reaction of hydrogen and oxygen on ruthenium nanoclusters. ACS Appl Mater Interfaces 2017;9(4):3785-91. [PMID: 28055176 DOI: 10.1021/acsami.6b15725]
- Lou BS, Veerakumar P, Chen SM, Veeramani V, Madhu R, et al. Ruthenium nanoparticles decorated curl-like porous carbons for high performance supercapacitors. Sci Rep 2016;6:19949. [PMID: 26818461 DOI: 10.1038/srep19949]
- Annamalai KP, Zheng X, Gao J, Chen T, Tao Y. Nanoporous ruthenium and manganese oxide nanoparticles/reduced graphene oxide for high-energy symmetric supercapacitors. Carbon 2019;144:185-92. [DOI: 10.1016/j.carbon.2018.11.073]
- Devadas A, Baranton S, Napporn TW, Coutanceau C. Tailoring of RuO2 nanoparticles by microwave assisted “Instant method” for energy storage applications. J Power Sources 2011;196(8):4044-53. [DOI: 10.1016/j.jpowsour.2010.11.149]
- Wang W, Guo S, Lee I, Ahmed K, Zhong J, et al. Hydrous ruthenium oxide nanoparticles anchored to graphene and carbon nanotube hybrid foam for Supercapacitors. Sci Rep 2014;4:4452. [PMID: 24663242 DOI: 10.1038/srep04452]
- Qiao Y, Xu S, Liu Y, Dai J, Xie H, et al. Transient, in situ synthesis of ultrafine ruthenium nanoparticles for a high-rate Li–CO2 battery. Energy Environ Sci 2019;12:1100-7. [DOI: 10.1039/C8EE03506G]
- Guo Y, Zhang W, Sun Y, Dai M. Ruthenium nanoparticles stabilized by mercaptan and acetylene derivatives with supercapacitor application. Electrochimica Acta 2018;270:284-93. [DOI: 10.1016/j.electacta.2018.03.037]
- Zhu H, Li Z, Ye E, Leong DT. Oxygenic enrichment in hybrid ruthenium sulfide nanoclusters for an optimized photothermal effect. ACS Appl Mater Interfaces 2021;13:50. [PMID: 34874695 DOI: 10.1021/acsami.1c17608]
- Lakshmi BA, Bae J-Y, An JH, Kim S. Nanoclusters prepared from ruthenium(II) and quercetin for fluorometric detection of cobalt(II), and a method for screening their anticancer drug activity. Microchim Acta 2019;186:539. [PMID: 31317334 DOI: 10.1007/s00604-019-3657-5]
- Diao J, Bai F, Wang Y, Han Q, Xu X, et al. Engineering of pectin-dopamine nano-conjugates for carrying ruthenium complex: A potential tool for biomedical applications. J Inorg Biochem 2019;191:135-42. [PMID: 30521965 DOI: 10.1016/j.jinorgbio.2018.11.016]
- Xu Y, Li C, Lu S, Wang Z, Liu S, et al. Construction of emissive ruthenium(II) metallacycle over 1000 nm wavelength for in vivo biomedical applications. Nat Commun 2022;13:2009. [PMID: 35422104 DOI: 10.1038/s41467-022-29572-2]
- Shen J, Rees TW, Ji L, Chao H. Recent advances in ruthenium(II) and iridium(III) complexes containing nanosystems for cancer treatment and bioimaging. Coord Chem Rev 2021;443:214016. [DOI: 10.1016/j.ccr.2021.214016]
- Junnuthula V, Kolimi P, Nyavanandi D, Sampathi S, Vora LK, et al. Polymeric micelles for breast cancer therapy: recent updates, clinical translation and regulatory considerations. Pharmaceutics 2022;14:1860. [PMID: 36145608 DOI: 10.3390/pharmaceutics14091860]
- Yue L, Li H, Sun Q, Zhang J, Luo X, et al. Red-emissive ruthenium-containing carbon dots for bioimaging and photodynamic cancer therapy. ACS Appl Nano Mater 2020;3(1):869-76. [DOI: 10.1021/acsanm.9b02394]
- Huang N, Chen X, Zhu X, Xu M, Liu J. Ruthenium complexes/polypeptide self-assembled nanoparticles for identification of bacterial infection and targeted antibacterial research. Biomaterials 2017;141:296-313. [PMID: 28709020 DOI: 10.1016/j.biomaterials.2017.07.005]
- Shetti NP, Nayak DS, Malode SJ, Kulkarni RM. Fabrication of MWCNTs and Ru doped TiO2 nanoparticles composite carbon sensor for biomedical application. ECS J Solid State Sci Technol 2018;7:Q3070. [DOI: 10.1149/2.0101807jss]
- Xia F, Hu X, Zhang B, Wang X, Guan Y, et al. Ultrasmall ruthenium nanoparticles with boosted antioxidant activity upregulate regulatory T cells for highly efficient liver injury therapy. Nano Micro Small 2022;18:2201558. [DOI: 10.1002/smll.202201558]
- Kasinathan K, Marimuthu K, Murugesan B, Sathaiah M, Subramanian P, et al. Fabrication of eco-friendly chitosan functionalized few-layered WS2 nanocomposite implanted with ruthenium nanoparticles for in vitro antibacterial and anticancer activity: Synthesis, characterization, and pharmaceutical applications. Int J Biol Macromol 2021;190:520-32. [PMID: 34480908 DOI: 10.1016/j.ijbiomac.2021.08.153]
- Lu Z, Huang Fy, Cao R, Zhang L, Tan GH, et al. Long blood residence and large tumor uptake of ruthenium sulfide nanoclusters for highly efficient cancer photothermal therapy. Sci Rep 2017;7:41571. [PMID: 28139763 DOI: 10.1038/srep41571]
- Wang X, Yu B, Wang Q, Caoq J, Wang M, et al. L-cysteine-protected ruthenium nanoclusters on CdS as efficient and reusable photocatalysts for hydrogen production. Int J Hydrogen Energy 2023;48(77):30006-17. [DOI: 10.1016/j.ijhydene.2023.04.199]
- Ismael M. Highly effective ruthenium-doped TiO2 nanoparticles photocatalyst for visible-light-driven photocatalytic hydrogen production. New J Chem 2019;43:9596-605. [DOI: 10.1039/C9NJ02226K]
- Jia H, Yang Y, Dou Y, Li F, Zhaoa M, Zhang CY. (Plasmonic gold core)@(ultrathin ruthenium shell) nanostructures as antenna-reactor photocatalysts toward nitrogen photofixation. Chem Commun 2022;58:1013-6. [DOI: 10.1039/D1CC06014G]
- Han S, Yun Q, Tu S, Zhu L, Cao W, Lu Q. Metallic ruthenium-based nanomaterials for electrocatalytic and photocatalytic hydrogen evolution. J Mater Chem A 2019;7:24691-714. [DOI: 10.1039/C9TA06178A]
- Mori K, Osaka R, Naka K, Tatsumi D, Yamashita H. Ultra-low loading of Ru clusters over graphitic carbon nitride: a drastic enhancement in photocatalytic hydrogen evolution activity. ChemCatChem 2019;11:1963-9. [DOI: 10.1002/cctc.201900073]
- Vidanapathirana AK, Pullen BJ, Zhang R, Duong MN, Goyne JM, et al. A novel ruthenium-based molecular sensor to detect endothelial nitric oxide. Sci Rep 2019;9:1720. [DOI: 10.1038/s41598-019-39123-3]
- Singh A, Barman P. Recent advances in schiff base ruthenium metal complexes: synthesis and applications. Top Curr Chem (Cham) 2021;379:29. [PMID: 34109453 DOI: 10.1007/s41061-021-00342-w]
- Jain A. Multifunctional, heterometallic ruthenium-platinum complexes with medicinal applications. Coord Chem Rev 2019;401:213067. [DOI: 10.1016/j.ccr.2019.213067]
- Ye C-X, Meggers E. Chiral-at-ruthenium catalysts for nitrene-mediated asymmetric C–H functionalizations. Acc Chem Res 2023;56(9):1128-41. [PMID: 37071874 DOI: 10.1021/acs.accounts.3c00081]
- Riccardi C, Musumeci D, Trifuoggi M, Irace C, Paduano L, et al. Anticancer ruthenium(III) complexes and Ru(III)-containing nanoformulations: an update on the mechanism of action and biological activity. Pharmaceuticals 2019;12:146. [PMID: 31561546 DOI: 10.3390/ph12040146]
- Sanchez-Lecuona G, Vazquez-Nunez MC, Donnadieu B, Muñoz-Hernández MA, Montiel-Palma V. Reactivity of ruthenium complexes towards organogallium reagents: gallium as a Z-type ligand or as a gallate counterion. Polyhedron 2024;247:116703. [DOI: 10.1016/j.poly.2023.116703]
- Pandey G, Marimuthu M, Kanagavalli P, Ravichandiran V, Balamurugan K, et al. Chitosanylated MoO3–Ruthenium(II) nanocomposite as biocompatible probe for bioimaging and herbaceutical detection. ACS Biomater Sci Eng 2019;5(7):3606-17. [PMID: 33405742 DOI: 10.1021/acsbiomaterials.9b00575]
- Zasypalov G, Vutolkina A, Klimovsky V, Abramov E, Vinokurov V, et al. Hydrodeoxygenation of guaiacol over halloysite nanotubes decorated with Ru nanoparticles: effect of alumina acid etching on catalytic behavior and reaction pathways. Appl Catal B Environ 2024;342:123425. [DOI: 10.1016/j.apcatb.2023.123425]
- Xu G, Tu Z, Hu X, Zhang X, Wu Y. Supported Ruthenium catalysts with electronic effect and acidity-basicity for efficient reductive amination of biomass-based carbonyl compounds. Chem Eng J 2024;481:148704. [DOI: 10.1016/j.cej.2024.148704]
- Ali I, Kashyout AEHB, Tayel M, Shokry Hassan H, Rizk M. Ruthenium (Ru) doped zinc oxide nanostructure-based radio frequency identification (RFID) gas sensors for NH3 detection. J Mater Res Technol 2020;9(6):15693-704. [DOI: 10.1016/j.jmrt.2020.11.033]
- Ristić-Djurović JL, Fernández-Izquierdo L, Hadžić B, Jiménez-Hernández L, Díaz-García AM, et al. Raman spectroscopy of zinc oxide nanoplatelets modified with ruthenium (II) complexes. J Raman Spectrosc 2019;50:1829-38. [DOI: 10.1002/jrs.5718]
- Liu J, Lai H, Xiong Z, Chenb B, Chen T. Functionalization and cancer-targeting design of ruthenium complexes for precise cancer therapy. Chem Commun 2019;55:9904-14. [DOI: 10.1039/C9CC04098F]
- Amirthaganesan K, Vadivel T, Dhamodaran M, Chandraboss VL. In vitro antifungal studies of Ruthenium (III) complex derived from chitosan Schiff bases. Mater Proc 2022;60(3):1716-20. [DOI: 10.1016/j.matpr.2021.12.265]
- Lee L-C, Lo KK-W. Strategic design of luminescent Rhenium(I), Ruthenium(II), and Iridium(III) complexes as activity-based probes for bioimaging and biosensing. Chem Asian J 2022;17(22):e202200840. [PMID: 36131616 DOI: 10.1002/asia.202200840]
- Khairnar SV, Pagare P, Thakre A, Nambiar AR, Junnuthula V, et al. Review on the scale-up methods for the preparation of solid lipid nanoparticles. Pharmaceutics 2022;14:1886. [PMID: 36145632 DOI: 10.3390/pharmaceutics14091886]
- Azharuddin M, Zhu GH, Das D, Ozgur E, Uzun L, et al. A repertoire of biomedical applications of noble metal nanoparticles. Chem Commun 2019;55:6964-96. [DOI: 10.1039/C9CC01741K]
- Li Q, Liu Y, Zhao B, Lei J, Lu S, et al. A single-molecular ruthenium(ii) complex-based NIR-II fluorophore for enhanced chemo-photothermal therapy. Chem Commun 2022;58:6546-9. [DOI: 10.1039/D2CC00082B]
- Youf R, Nasir A, Müller M, Thétiot F, Haute T, et al. Ruthenium(II) polypyridyl complexes for antimicrobial photodynamic therapy: prospects for application in cystic fibrosis lung airways. Pharmaceutics 2022;14:1664. [PMID: 36015290 DOI: 10.3390/pharmaceutics14081664]
- Kaya SI, Kurbanoglu S, Yavuz E, Demiroglu Mustafov S, Sen F, et al. Carbon-based ruthenium nanomaterial-based electroanalytical sensors for the detection of anticancer drug Idarubicin. Sci Rep 2020;10:11057. [PMID: 32632278 DOI: 10.1038/s41598-020-68055-6]
- Sivan SE, Oh KR, Yoon JW, Yoo C, Hwang YK. Immobilization of a trimeric ruthenium cluster in mesoporous chromium terephthalate and its catalytic application. Dalton Trans 2022;51:13189-94. [DOI: 10.1039/D2DT01462A]
- Komaty S, Özçelik H, Zaarour M, Ferre A, Valable S, et al. Ruthenium tris(2,2′-bipyridyl) complex encapsulated in nanosized faujasite zeolite as intracellular localization tracer. J Colloid Interface Sci 2021;581:919-27. [PMID: 32956911 DOI: 10.1016/j.jcis.2020.08.117]
- Nehru S, Veeralakshmi S, Kalaiselvam S, Subin David SP, Sandhya J, et al. DNA binding, antibacterial, hemolytic and anticancer studies of some fluorescent emissive surfactant-ruthenium(II) complexes. J Biomol Struct Dyn 2020:2242-56. [DOI: 10.1080/07391102.2020.1747547]
- Li S, Xu G, Zhu Y, Zhao J, Gou S. Bifunctional ruthenium(ii) polypyridyl complexes of curcumin as potential anticancer agents. Dalton Trans 2020;49:9454-63. [DOI: 10.1039/D0DT01040E]
- Rodríguez-Prieto T, Michlewska S, Hołota M, Ionov M, Javier de la Mata F, et al. Organometallic dendrimers based on Ruthenium(II) N-heterocyclic carbenes and their implication as delivery systems of anticancer small interfering RNA. J Inorg Biochem 2021;223:111540. [PMID: 34273717 DOI: 10.1016/j.jinorgbio.2021.111540]
- Chen X, Zhang Y, Chen C, Li H, Lin Y, et al. Atomically dispersed ruthenium catalysts with open hollow structure for lithium–oxygen batteries. Nano-Micro Lett 2023;16:27. [PMID: 37989893 DOI: 10.1007/s40820-023-01240-0]
- Han X, Kong X, Wang D, Li X, Dong L. Hydrous ruthenium oxide quantum dots anchored on carbon nanocages for Zn-ion hybrid capacitors. Chem Eng J 2023;477:147078. [DOI: 10.1016/j.cej.2023.147078]
- https://patents.google.com/patent/WO2016059238A1/en17. [Last accessed on 28 Mar 2024].
- ClinicalTrials.gov. [Last accessed on 28 Mar 2024].