Updated Review on Recent Advances in Silver Nanoclusters in Bioanalytical and Biomedical Applications
1Department of Pharmaceutical Chemistry, S.M.B.T. College of Pharmacy Dhamangaon, Nashik, Maharashtra 422403, India Affiliated to Savitribai Phule Pune University, Pune
*Correspondence to: Department of Pharmaceutical Chemistry, S.M.B.T. College of Pharmacy Dhamangaon, Nashik, Maharashtra 422403, India Affiliated to Savitribai Phule Pune University, Pune, Tel.: +91-9762755260, E-mail: vaibhavikshatriya5086@gmail.com
Received: February 24 2024; Revised: March 28 2024; Accepted: April 1 2024; Published Online: April 25 2024
Cite this paper:
Vaibhavi Vijay Kshatriya, Manoj Ramesh Kumbhare, Shraddha Vikas Jadhav, Prajakta Jaywant Thorat and Rushikesh Gajanan Bhambarge. Updated Review on Recent Advances in Silver Nanoclusters in Bioanalytical and Biomedical Applications. BIO Integration 2024; 5: e992.
DOI: 10.15212/bioi-2024-0003. Available at: https://bio-integration.org/
Download citation
© 2024 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
Silver nanoclusters (AgNCs) have emerged as highly adaptable nanomaterials with vast potential in theranostic applications, by integrating therapeutic and diagnostic capabilities within a single platform. This review summarizes current developments in the synthesis, characterization, and use of AgNCs for theranostics. AgNC synthesis has substantially advanced, and a variety of techniques such as chemical reduction, green synthesis, and templated methods are being used to manage stability, size, and form. AgNCs’ optical characteristics, including high fluorescence and surface-enhanced Raman scattering (SERS) signals, make them ideal for bioimaging and diagnostic applications. Furthermore, AgNCs’ surface chemistry enables simple functionalization with therapeutic drugs and targeting ligands, thus improving effectiveness and selectivity. AgNCs have been used in several diagnostic imaging modalities, including photoacoustic imaging, fluorescence imaging, and SERS-based sensing. They are suitable for both in vitro and in vivo imaging applications because of their exceptional photostability and biocompatibility, which enables real-time tracking of disease progression and therapy response.
Keywords
AgNCs, bioimaging, biosensors, targeted therapy, theranostics, nanomedicine, personalized medicine.
Introduction
Fundamentals of metal nanoclusters
Between atomic-scale and plasmonic metal nanoparticles, atomically precise metal nanoclusters (NCs) are minuscule particles with a core diameter smaller than 2 nm [1–3]. These metal NCs are hard molecules and have unique electrical and optical properties, including strong photoluminescence (PL) and superior catalytic properties. Noble metal NCs have attracted substantial research interest because of their distinctive structures and broad potential uses [4–7]. Organic ligands, including thiolates, phosphines, and alkynyls, are commonly used to coat the surfaces of certain gold (Au) NCs and silver NCs (AgNCs) to inhibit aggregation and aid in their separation. These ligands affect the mechanisms through which Au NCs and AgNCs develop, but they also have a consequence influence on the sizes, shapes, and final characteristics of these particles [8–16].
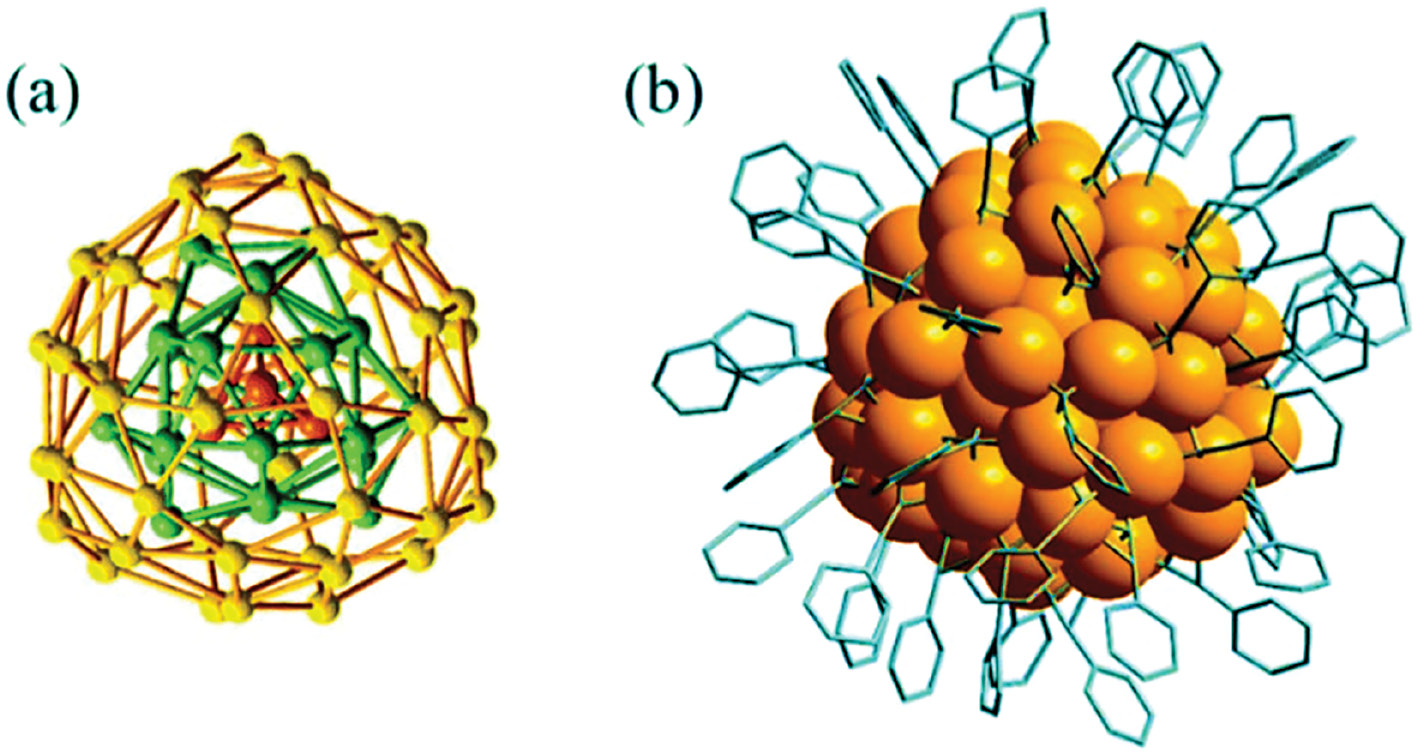
The field of nanotechnology has emerged as a revolutionary discipline with wide-ranging applications. Among the plethora of nanomaterials, AgNCs have captured substantial interest because of their distinctive size-dependent characteristics and adaptability. AgNCs, comprising nanoscale silver particles, typically consisting of several to several tens of atoms, have unique electronic and optical properties. Their customizable size and shape render them of particular interest to researchers in diverse fields, including materials science, chemistry, biomedicine, and electronics. Figure 1 shows the AgNC structure.
Figure 1 Structure of silver nanoclusters.
The architecture of AgNCs is intricate. Among the noble metal NCs investigated to date, AgNCs are notable for their unique physical properties, such as robust luminescence and diminutive size. These traits provide exciting opportunities for developing luminescent probes tailored to bio-imaging and sensing applications. However, silver in its zero-valent state exhibits higher reactivity and susceptibility to oxidation than Au. This heightened reactivity poses challenges in the preparation and examination of AgNCs, in contrast to their extensively studied Au counterparts. Consequently, ensuring the availability of high-quality AgNCs with accurately defined size, structure, and surface properties is crucial for both fundamental scientific investigations and practical applications.
Properties and features of AgNCs
The notable characteristics of AgNCs include their outstanding optical attributes. These NCs display absorption and fluorescence properties dependent on their size, thus resulting in vibrant and adjustable photoluminescence [17]. This feature has been harnessed in diverse fields including bioimaging, sensing, and optoelectronics. The robust fluorescence emission of AgNCs, combined with their exceptional photostability, has made them invaluable in cellular and molecular imaging, in which they facilitate detailed exploration of biological phenomena at the nanoscale. Beyond their optical characteristics, AgNCs have excellent catalytic and antimicrobial properties that make them suitable for applications in catalysis, environmental remediation, and treatment of microbial infections [18]. Their ability to generate reactive oxygen species under light irradiation has led to the development of photodynamic therapy (PDT) platforms, thus providing a non-invasive, targeted approach to cancer treatment.
Recently, a plethora of efficient techniques have been developed to fabricate AgNCs with tailor-made physical and chemical attributes, in quantities suitable for practical applications. By carefully designing synthesis approaches, such as direct reduction, chemical etching, and ligand exchange, well-established processes can be used to produce high-quality AgNCs with novel and sometimes unprecedented properties. Additionally, various techniques such as UV-visible absorption spectroscopy, PL emission spectroscopy, electrospray ionization mass spectrometry, and single-crystal X-ray crystallography have been used to analyze the physical and chemical properties, and elucidate the complete structures, of AgNCs, thereby establishing a central avenue of nanoscience research. The precise atomic-level configurations of AgNCs enable investigation of the correlation between their structure and properties, which may potentially enable performance increases. Among these properties, luminescence is particularly valuable in biological contexts. Studies in the past 5 years have emphasized the ability to adjust the photoluminescent properties of AgNCs by regulating both the core size and the ligand type [19, 20].
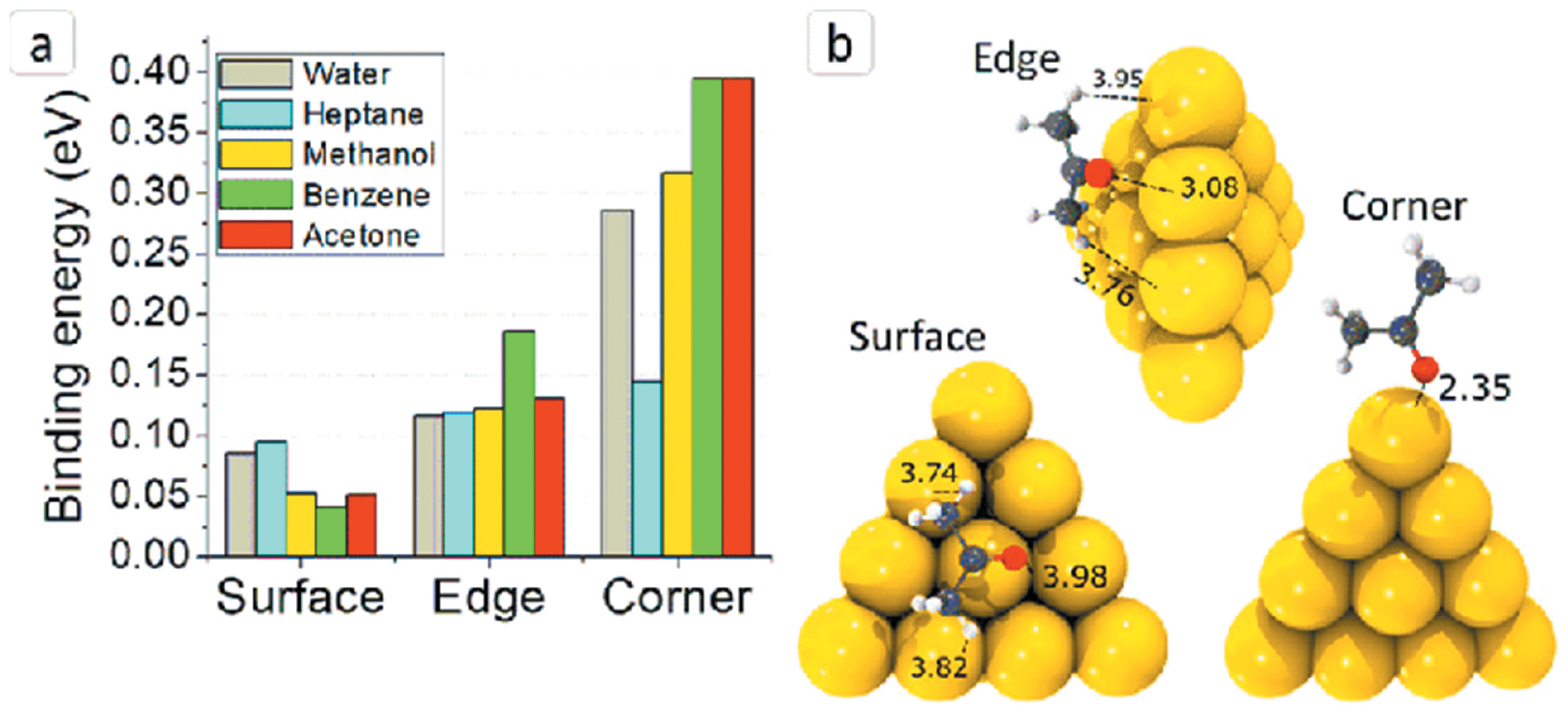
Figure 2 shows the binding energy of AgNCs with various solvents, such as water, heptane, methanol, benzene, and acetone. The above figure also describes the AgNC surface, edge, and corner dimensions. This introductory explanation lays the groundwork for extensive examination of AgNCs, including their synthesis, structural characteristics, distinct optical qualities, and diverse applications spanning multiple scientific fields. As scientists delve deeper into the capabilities of AgNCs, these minute silver structures are becoming increasingly critical in uncovering leading edge solutions in nanotechnology, biomedicine, and beyond.
Figure 2 Binding energy of silver nanoclusters.
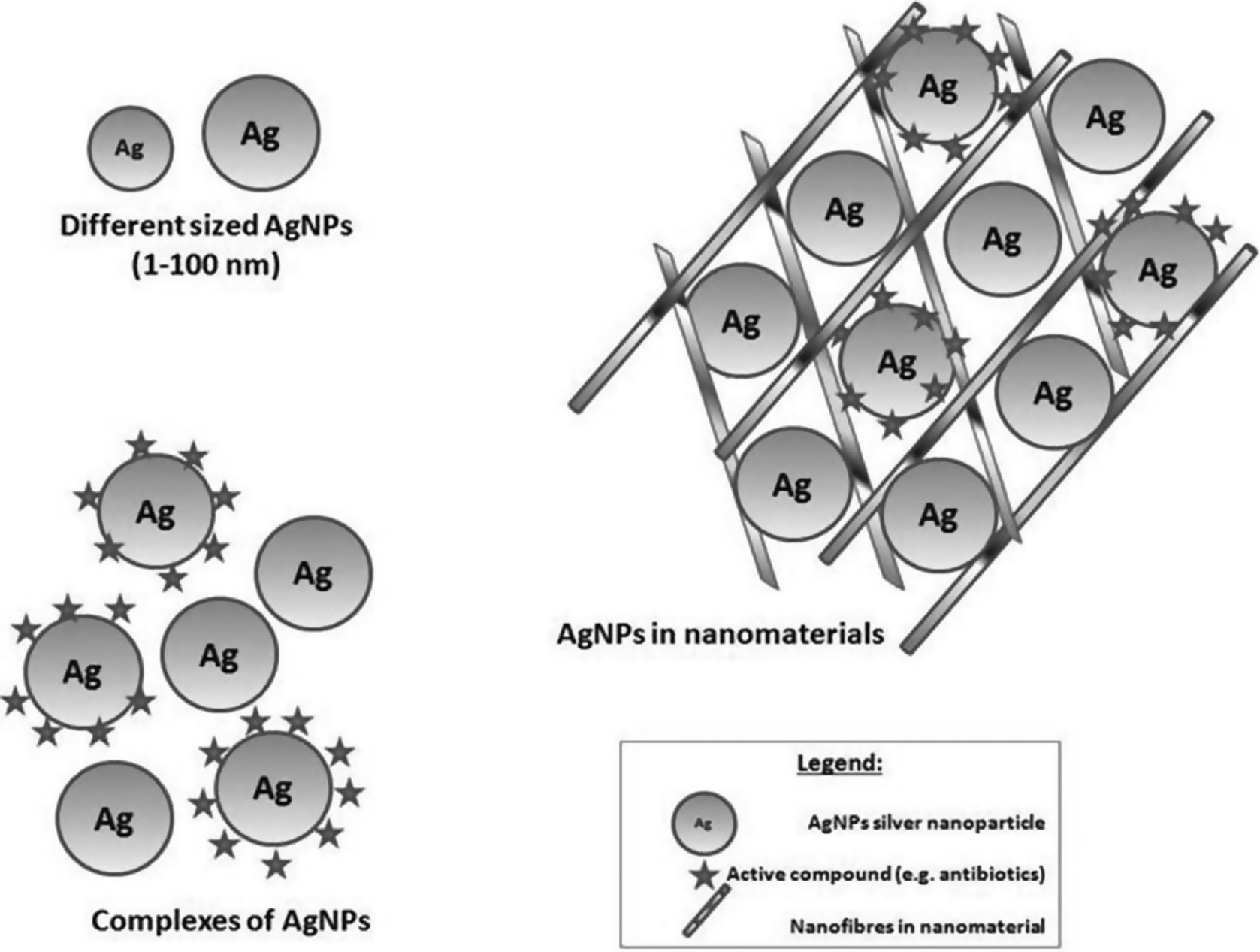
Figure 3 shows how NCs arise from nanoparticles of different sizes forming a complexes of silver nanoparticles (AgNPs).
Figure 3 Silver-nanoparticle-forming nanoclusters.
AgNC synthesis approaches
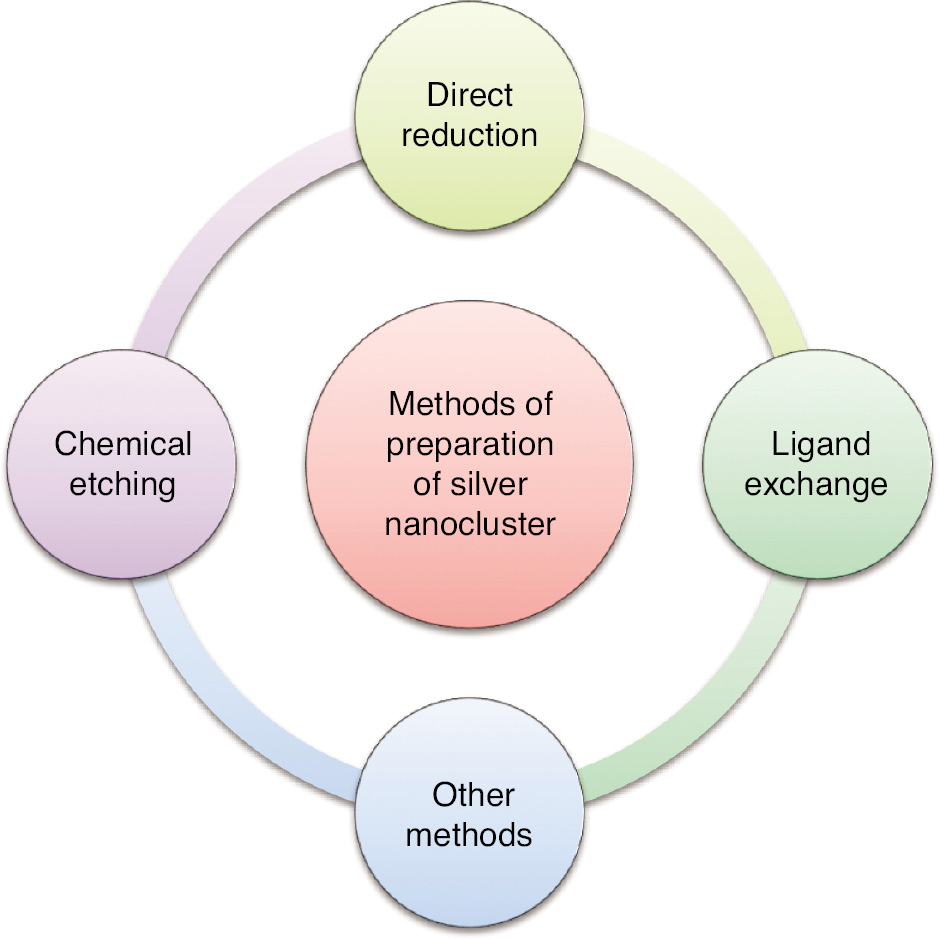
Various synthesis methods, including chemical reduction, ligand-assisted growth, and template-directed approaches, have been developed to tailor the size, shape, and surface chemistry of AgNCs. These advances have unlocked numerous possibilities for harnessing AgNCs in a wide array of applications. The synthesis of AgNCs is a more intricate process than that of Au NCs, because of AgNCs’ high sensitivity to environmental conditions in solution. Consequently, achieving precise control is essential for generating AgNCs with well-defined compositions [21]. Recent reports have documented successful endeavors in this regard. The production of AgNCs can be broadly classified into three primary approaches: direct reduction of silver precursors, facilitated by specific ligands; chemical etching; and post-synthetic ligand exchange [22].
Figure 4 describes methods for AgNC synthesis, including direct reduction, ligand exchange, and chemical etching.
Figure 4 Method of preparation of silver nanoclusters.
Chemical etching techniques
Some AgNCs can be produced through a chemical etching procedure, whereby larger AgNPs are selectively degraded to yield smaller AgNCs. The chemical etching process has had fewer documented successes than the direct reduction method, primarily because chemical etching is inherently time-consuming and tends to produce AgNCs in small quantities. However, these limitations can be mitigated to some extent by optimizing the etching conditions, such as the etching duration, reaction temperature, and ratio of the etching agent to Ag precursors. An effective synthesis approach requires a gentle etching environment that enables controlled AgNC formation within the reaction mixture [23–25].
Direct reduction techniques
The direct reduction method is efficacious in synthesizing AgNCs in both organic and aqueous solutions. This synthetic approach entails rapid formation of intermediate AgNCs via reduction, followed by gradual fine-tuning of their size to yield monodisperse AgNCs within a reducing agent. NaBH4, a commonly used reducing agent for the synthesis of AgNCs, can be used alongside various ligands, such as thiolates, alkynyls, DNAs, peptides, proteins, and polymers. Several AgNCs, including thiol-protected and alkynyl-protected AgNCs, have been successfully created with this method. However, NaBH4 is known for its rapid reduction kinetics, which can result in the formation of polydisperse AgNCs. To counteract this formation, multiple approaches can be used to slow the reduction kinetics of NaBH4. For example, the solution’s pH, the concentration of reducing agents, and the choice of solvent can be adjusted to modulate NaBH4’s reducing capability. To slow the rate of AgNC formation, an effective strategy involves replacing NaBH4 with gentler reducing agents, such as formic acid and N,N-Dimethylformamide. Moreover, alternative methods including light exposure, ultrasonication, and electrical processes can establish a mild reducing environment favorable for AgNC synthesis [26–28].
Ligand exchange technique
In recent years, the procedure of initiating size and structure alterations via ligand exchange has gained considerable significance. Peripheral organic ligands have critical roles in determining characteristics such as nuclearity, geometry, bonding, and electronic transitions within metal NCs. Depending on the particular metal NC, ligand exchange can be either partial or complete, and its effects may or may not extend to the metal core itself. In 2014, Bakr and colleagues introduced a method for rapidly and completely exchanging thiolate ligands with other thiolates in Ag44(SR)30 clusters. Subsequently, the researchers discovered that the ligand exchange process can efficiently and directly convert Ag35(SG)18 clusters (where SG denotes glutathionate) into Ag44(4-FTP)30 clusters (where 4-FTP denotes 4-fluorothiophenol), whereas the reverse transformation occurs slowly and involves intermediate cluster sizes [29, 30].
Other techniques
Other methods for synthesizing AgNCs involve performing reactions under solid-state conditions or within a gel medium. For example, the Pradeep research group has developed a technique to fabricate red-emitting Ag9(H2MSA)7 NCs through a solid-state approach. This method can also synthesize other types of thiolated AgNCs, including Ag32(SG)1956 and Ag152(PET)60 (where PET denotes phenylethanethiol), as well as selenolate-protected Ag44(SePh)30 NCs. Furthermore, Chakraborty and colleagues have used a gel-based approach to form thiolated Ag25(SG)18 NCs with intense red luminescence. Detailed information on the synthesis of AgNCs can be found in recent reviews [31–33].
Current approaches to fabricating AgNCs
Several current approaches to fabricating AgNCs, along with their advantages and disadvantages, are summarized in Table 1.
Table 1 Summary of Current Approaches for Fabricating Silver Nanoclusters
| Method | Advantages | Disadvantages |
|---|---|---|
| Chemical reduction | Relatively simple and cost-effective | May involve toxic chemicals |
| High yield | Lack of precise control of size and shape | |
| Scalable | Aggregation tendency | |
| Allows for surface modification | ||
| Can be performed under mild conditions | ||
| Tunable optical properties | ||
| Electrochemical method | Control of size and shape | Need for specialized equipment |
| High purity | Properties affected by electrolyte composition | |
| Continuous production possible | Limited scalability | |
| Tunable optical properties | Complexity in controlling parameters | |
| Environmentally friendly | Risk of dendrite formation in certain cases | |
| Photochemical method | High precision in size and shape control | Requirement of specific light sources |
| Low-temperature processing | Limitation to certain types of silver precursors | |
| Narrow size distribution | Potential requirement for complex ligands for stabilization | |
| Control of surface chemistry | Limited scalability | |
| In situ monitoring ability | Photobleaching of stabilizing ligands | |
| Green synthesis | Environmentally friendly | Potentially varying reaction conditions |
| Biocompatible | Slow reaction kinetics | |
| Sustainable | Limited control of size and shape | |
| Low cost | Variable product purity | |
| Uses natural resources | Physical properties are less manipulated as compare to chemicals |
Properties of AgNCs
Various properties of AgNCs are summarized in Table 2.
Table 2 Properties of Silver Nanoclusters.
| Properties | Description |
|---|---|
| Fluorescence | Fluorescence exhibited by silver nanoclusters is a distinct form of photoluminescence involving emission of light with longer wavelengths (lower energy) after the absorption of photons from a higher-energy source, such as ultraviolet (UV) or blue light. |
| Photoluminescence | Photoluminescence observed in silver nanoclusters entails emission of light after exposure to external light sources, including UV or visible light, by silver nanoparticles or nanoclusters. |
| UV-visible absorption | A prominent feature of silver nanoclusters is the phenomenon of surface plasmon resonance, distinguished by unique optical absorption patterns. |
| Biological | The biological properties of silver nanoclusters pertain to their interactions with living organisms and biological molecules, and are of interest for potential applications in fields such as medicine and biology. |
| Structural | In silver nanoclusters, structural properties encompass the physical and chemical attributes that dictate the arrangement of silver atoms within these materials at the nanoscale. |
Photoluminescence
PL is a captivating property of nanomaterials, because of its vast array of potential applications. Upon excitation from their ground state, AgNCs emit excess energy as they revert to their ground state, thus giving rise to PL. However, AgNCs typically exhibit low quantum yield, and unresolved questions remain regarding the fundamental aspects of their PL properties. Several factors influence the PL of AgNCs, including the cluster size, type of protective ligands, and presence of heterometal atoms. Furthermore, the PL behavior is highly sensitive to factors including the number of valence electrons, metal oxidation state, crystal structure, temperature, and pH. Each of these factors plays a crucial role in modulating the photoluminescence characteristics [34–36].
UV-visible absorption
The UV-visible absorption spectra of AgNPs prominently exhibit a phenomenon called surface plasmon resonance, distinguished by unique optical absorption patterns, as illustrated in Table 1. [37–39]. Typically, the surface plasmon resonance peak for AgNPs appears around 400 nm. In contrast, AgNCs exhibit several discernible absorption peaks within the UV-visible range. The unique optical absorption profiles of AgNCs and AgNPs arise from various factors and manifest at different wavelengths. Spectral information can confirm the effective synthesis of AgNCs and the conversion of small AgNCs into larger plasmonic AgNPs. Modifications in protective ligands and cluster size, both of which affect behavior in the excited state, result in alterations in electronic transitions [40].
Fluorescence
Fluorescence, an intriguing trait of AgNCs, has received considerable interest in the fields of nanomaterials and nanophotonics. These clusters have distinctive and adjustable fluorescence characteristics, and thus may be have promise in diverse applications [41–45].
Intense emission
AgNCs are recognized for their potent and precise fluorescence emission, which is typically characterized by a distinctively narrow emission peak. This high luminosity is advantageous in fluorescence-based assays and bioimaging applications, by facilitating the detection and tracking of biomolecules and cellular structures with enhanced signal strength. Figure 5 describes AgNC sensors.
Figure 5 AgNCs as sensors [45].
Size-dependent fluorescence
The fluorescence characteristics of AgNCs are intricately associated with their size and structure. Changes in NC size correspondingly alter the fluorescence emission wavelength. This phenomenon, known as size-dependent fluorescence, arises from quantum confinement effects, wherein the confinement of electrons and holes within NCs creates discrete energy levels and consequently distinct emission wavelengths. This adjustable emission capability has led to versatile AgNC applications in fluorescence-based sensing and imaging.
Environment sensitivity
The fluorescence of AgNCs exhibits remarkable sensitivity to their surrounding environment, influenced by factors such as temperature, pH, and the presence of particular molecules or ions. This inherent sensitivity can be used in sensing applications, in which variations in the environment induce detectable shifts in the fluorescence emission spectra of AgNCs.
Biocompatibility and photostability
AgNCs readily undergo functionalization with biocompatible ligands, thereby enabling their use in biological contexts. Their small size and outstanding fluorescence characteristics render them appropriate for labeling and imaging of diverse biological structures and processes, within controlled laboratory settings or living organisms. Notably, AgNCs have impressive photostability, thus ensuring prolonged fluorescence emission without photobleaching or degradation. This resilience is particularly advantageous in extended imaging and tracking experiments, by ensuring the sustained reliability of fluorescence signals over time.
Diverse applications
Owing to their distinctive fluorescence characteristics, AgNCs have been used across broad fields, including biological imaging, environmental monitoring sensors, disease diagnosis tools, and materials science advancements. Additionally, their potential applications extend to optoelectronic devices and integration as components within nanoscale photonic systems. Thus, the fluorescence properties of AgNCs make them a fascinating and valuable class of nanomaterials. Their tunable emission, brightness, photostability, and sensitivity to environmental changes facilitate diverse applications, such as advanced imaging techniques and cutting-edge sensing technologies.
Structural properties of AgNCs
AgNCs have a broad array of structural attributes depending on their size, composition, and surrounding ligand environment Figure 8. Understanding these properties is critical for customizing their functionality and potential applications [19, 20, 46–48].
Ligand-induced structures
The selection of ligands is critical in shaping the structural properties of AgNCs. Organic ligands stabilize the clusters and can also determine their morphology. For example, thiolate ligands promote the development of symmetric and precisely defined structures, whereas alternative ligands may result in varied cluster geometries.
Lattice imperfections
AgNCs can possess lattice imperfections, such as stacking faults, vacancies, or twinning, which affect their electronic and optical properties. These imperfections can arise from growth processes or ligand-induced effects, and contribute to the unique properties of individual AgNCs.
Core-shell structures
Some AgNCs have core-shell structures in which a silver core is surrounded by a layer of ligands. The core-shell architecture can give rise to interesting optical and electronic properties. For example, Ag2(SR)2 NCs consist of a silver dimer core protected by two thiolate ligands. Figure 6 describes the AgNC surface structure.
Figure 6 Surface structure of AgNCs.
Size-dependent structures
A prominent structural property of AgNCs is their size-dependent atomic arrangement. With changes in the number of silver atoms in the cluster, the arrangement of these atoms can transition from compact to hollow structures, or even from planar to three-dimensional geometries. For example, Ag25(SG)18 NCs, where SG denotes glutathionate, exhibit a well-defined icosahedral geometry with a central Ag13 core surrounded by a shell of Ag12 atoms.
Surface atoms
The properties of AgNCs are often dominated by their surface atoms. These atoms have a high surface-to-volume ratio, thus leading to unique chemical reactivity and optical behavior. Surface atoms have crucial roles in the interaction of AgNCs with other molecules and materials.
Chirality
Some AgNCs exhibit chirality, a structural property associated with their handedness. Chiral AgNCs have been of particular interest, because of their potential applications in chiral sensing and catalysis.
Biological properties of AgNCs
AgNCs have attracted considerable attention in biomedicine and biology, because of their distinct biological attributes, such as biocompatibility, fluorescence, and promising prospects for both therapeutic interventions and imaging techniques [49–51].
Fluorescence imaging
AgNCs exhibit strong and tunable fluorescence emission, and therefore are valuable in biological imaging. Their fluorescence properties, including quantum yield and emission wavelength, can be controlled by adjustment of the size and ligands of the NCs. For instance, Ag2(SR)2 NCs have been used for cellular imaging, because of their bright and stable fluorescence.
Sensing and detection
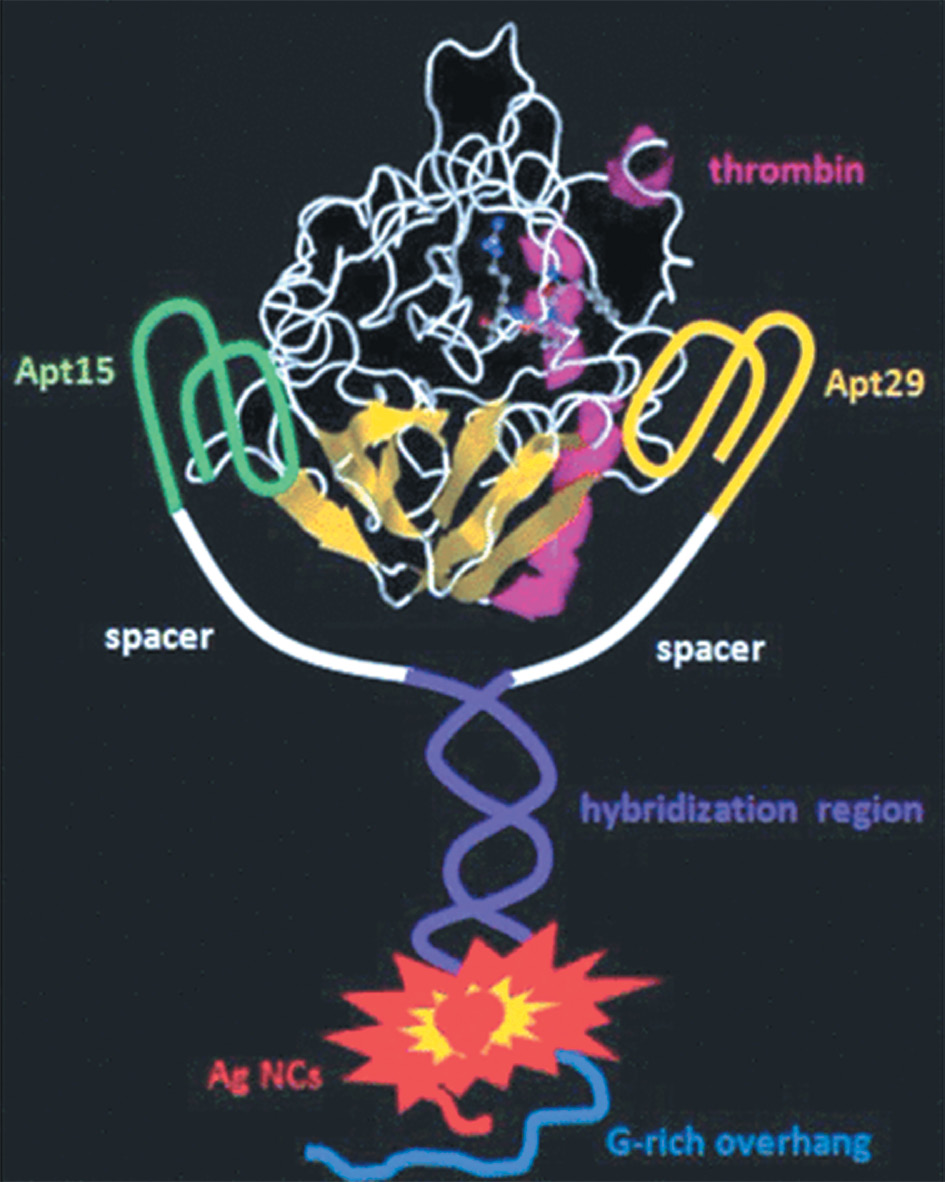
AgNCs have been used as biosensors for the detection of various biomolecules and ions. Their fluorescence properties are highly sensitive to changes in their local environment, thus enabling detection of specific analytes. Functionalized AgNCs have been used for the detection of DNA, proteins, and metal ions in biological samples. Aptamers, which are oligonucleotides capable of protein binding, have been designed and used in association with DNA-AgNC to develop half-life sensors for protein detection. A similar type of control containing a DNA probe with thrombin aptamer and C-rich fragment was used for such protein detection with a limit of detection of 1 nM. Silver ions bind to cytosines, which enables the fluorescent AgNC to be selectively formed on C-rich fragment. These clusters show good photostability and stability in bioassay conditions despite being quenched in fluorescence in the presence of thrombin. This system exhibits good specificity when compared to the other four proteins used as controls that indicated the presence of any quenching effect. The quenching mechanism was also studied and has been attributed to the AgNC’s structural change induced by protein binding to the aptamer. The same protein can also be detected using a turn-on strategy.
In this case, the presence of thrombin at 1 nM promotes the fluorescence intensity of G-rich AgNCs due to the proximity of a G-rich protein. This results in a shift in fluorescence from weak green emission to a strong red emission, as shown in Figure 7. The specificity of the method was tested by comparing it with other four proteins. In all cases, the changes in fluorescence were lower than those observed for thrombin even at 10-fold higher concentrations.
Figure 7 Principle of a binding-induced fluorescence turn-on assay for protein detection.
Biocompatibility
AgNCs are widely recognized for their biocompatibility, which renders them suitable for multiple biological applications. They demonstrate minimal cytotoxicity and can be easily functionalized with biocompatible ligands, thereby facilitating applications in both in vivo and in vitro investigations. For instance, AgNCs stabilized with bovine serum albumin have been demonstrated to be biocompatible and effective in cellular imaging.
Antimicrobial activity
Silver ions released from AgNCs have intrinsic antimicrobial properties. AgNCs have been investigated for their potential as antimicrobial agents to treat bacterial infections and as coatings for medical devices to decrease microbial contamination.
Drug delivery
AgNCs can serve as carriers for drugs in targeted delivery systems. Their small size and surface functionalization capability enable the attachment of therapeutic agents, thus facilitating controlled and precise release. This attribute holds promise for applications in cancer therapy and various other medical treatments.
Application of AgNCs
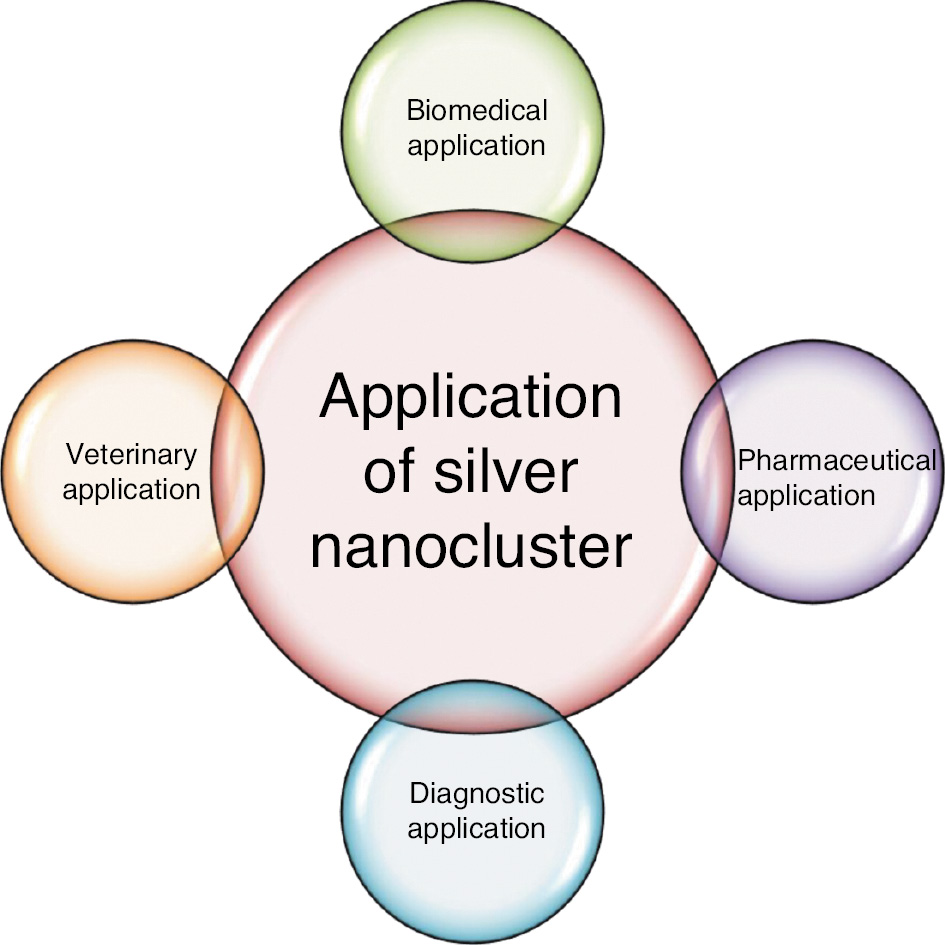
Figure 8 shows various applications of AgNCs.
Figure 8 Application of silver nanoclusters.
Biomedical applications
AgNCs readily interact with and effectively stabilize single-stranded DNA in experiments [52]. After interaction with rhodamine 6G, these structures form self-assembled complexes emitting light in the blue spectrum. However, the presence of fluorescent dye marginally decreases the intrinsic fluorescence of the clusters. Intriguingly, this fluorescence further decreases after addition of melamine into the aqueous suspension, thus ultimately disrupting the self-assembled complex [53, 54]. One biosensor designed to detect a crucial RNA protein comprises two distinct sections of a DNA tetrahedral framework. These sections are composed of sequences of four nucleic acids, thereby forming a scaffold that can be easily switched, along with a photo-induced electron-transfer pair serving as a fluorescence converter. When coupled with an Ag cluster, this DNA complex, with or without the protein target, exhibits fluorescence exhibits fluorescence in combination pattern as there is presence of 2 distinct models. This pioneering sensor has enabled detection of the target protein, even within individual cells, for the diagnosis of HIV [55].
A novel approach for modulating the fluorescence intensity of AgNP aggregates by using DNA has been applied to identify hepatitis B virus in samples. The fluorescence intensity is significantly amplified when multilayer complexes form between the nanoparticles and the target DNA, thus subsequently immobilizing the captured samples.
Moreover, a novel bimetallic electrochemical auto sensor has been described, featuring an outer shell comprising Au nanoparticles acting as a conduit for transmitting signals from AgNCs to Au NCs. This innovative sensor has remarkable capabilities for ultra-sensitive and highly selective detection, with a low detection threshold for two distinct types of microRNA potentially associated with sarcoma. The sensor platform integrates a covalent-organic lattice constructed from nanowires, synthesized via the condensation of 1,3,6,8-tetra(4-carboxyphenyl)pyrene and melamine. This lattice has also been used for capturing single strands of DNA helices, thus facilitating hybridization alongside complementary probes targeting these microRNA variants [56].
A hydrogel made from poly(ethylene glycol) that emits fluorescence has been effectively used as a advance method of highly sensitive microRNA detection. In this scenario, the introduction of Ag+ into the hydrogel triggers a consequence cation exchange process while also promoting the hybridization of DNA strands. Notably, the hydrated hydrogel medium itself mitigates the risk of fluorescence quenching, thus streamlining the experimental setup [57].
Another remarkably selective method for rapid detection of microRNA in complex biological solutions has harnessed the fluorescence properties of AgNCs. The response of AgNC for detection of presence of microRNA shows high specificity.
Pharmaceutical application
Drug delivery systems
AgNCs have vast potential as drug delivery systems in the pharmaceutical sector. With their small size, extensive surface area, and ability to transport diverse payloads, AgNCs have emerged as ideal candidates for encapsulating and delivering drugs to precise target locations. AgNCs can enhance the stability and bioavailability of pharmaceutical compounds, thereby increasing their therapeutic effectiveness while simultaneously decreasing potential adverse effects. In a study by Sengupta et al., AgNPs, including AgNCs, have been used as drug carriers for the delivery of antimicrobial agents. The researchers have demonstrated the synergistic potential of AgNCs in combination with pharmaceutical compounds, thus highlighting their role in enhancing drug delivery systems [58].
Antimicrobial agents
Utilization as antimicrobial agents is a well-established application of AgNCs in pharmaceuticals. AgNCs have high antimicrobial efficacy against a wide array of microorganisms, including bacteria, viruses, and fungi. This activity is based on AgNCs’ capability to liberate silver ions, which obstruct cell membrane, disrupt DNA replication, and instigate oxidative stress in microorganisms. Pharmaceutical formulations have incorporated AgNCs to formulate potent antimicrobial agents. For example, researchers have demonstrated the use of AgNCs in wound dressings to prevent and treat bacterial infections. Additionally, AgNCs have been used in the development of antimicrobial coatings for medical devices, thus decreasing the risk of healthcare-associated infections [59].
Cancer therapeutics
AgNCs have emerged as promising contenders in cancer therapeutics, and have received attention for their distinctive attributes, which make them attractive candidates for selective targeting of cancer cells. Engineered AgNCs can be tailored to preferentially accumulate in tumor tissues, thereby facilitating the precise eradication of cancer cells while preserving healthy cells. Beyond targeted drug delivery, AgNCs are being investigated for their potential in photothermal therapy. After exposure to near-infrared light, AgNCs absorb energy and produce heat, thus providing a means to selectively eliminate cancer cells via hyperthermia [60].
Gene delivery
AgNCs have shown potential as gene delivery carriers—a critical element of gene therapy in pharmaceutical applications. Through functionalization, therapeutic genes can be loaded onto the surfaces of AgNCs, which enable precise gene delivery into designated cells or tissues. This targeted gene delivery strategy has substantial promise for addressing genetic disorders and various other diseases. A comprehensive review by Li et al. has discussed the synthesis and properties of AgNPs, including AgNCs, in gene delivery applications, and has provided insights into their potential in pharmaceutical gene therapy [61].
Radiation enhancers
AgNCs have been investigated as radiation enhancers in cancer therapy. When used alongside radiation therapy, AgNCs can boost therapeutic efficacy by increasing radiation damage to cancer cells. This approach enhances the therapeutic results of radiation therapy while mitigating harm to neighboring healthy tissues. Zhang et al., in a study on the size-dependent radiosensitization of Au nanoparticles, have highlighted the potential of similar approaches using AgNCs in cancer radiation therapy [62].
Anti-inflammatory agents
The anti-inflammatory attributes of AgNCs have opened new avenues in pharmaceutical exploration. Inflammation is a critical factor in numerous diseases, such as rheumatoid arthritis and inflammatory bowel disease. AgNCs have the potential to be integrated into pharmaceutical formulations to mitigate inflammation and provide relief to affected individuals. Li et al. have emphasized the anti-inflammatory potential of AgNCs in a review article, including their roles in decreasing inflammation in various disease contexts. Therefore, AgNCs are promising candidates for the development of anti-inflammatory pharmaceutical products [63].
Veterinary application
In veterinary science, AgNCs are used primarily as potent antimicrobial agents. Their robust antibacterial, antiviral, and antifungal properties make them invaluable in preventing and managing infectious diseases in animals. These nanoparticles, which can target diverse pathogens, including drug-resistant strains, have crucial roles in veterinary medicine. Wound management is important in animal healthcare, and AgNCs have been applied to expedite wound healing in animals, because of their antimicrobial characteristics and ability to promote tissue regeneration. AgNCs can be integrated into wound dressings and gels to provide an environment conducive to wound closure. In addition, AgNCs have found applications in veterinary diagnostics. AgNCs can be functionalized with tailored ligands or antibodies for the detection of pathogens, biomarkers, or antibodies in animals. These customized AgNCs have been applied in assays and point-of-care diagnostic tests, thus facilitating rapid and precise disease detection with high sensitivity [64].
Diagnostic application
Functionalized AgNCs have emerged as valuable diagnostic tools in pharmaceutical research. Through modification with specific ligands or antibodies, AgNCs can be customized to detect disease-associated biomarkers. This adaptability enables the creation of rapid and highly sensitive diagnostic tests that facilitate early disease detection and monitoring. In pharmaceutical analysis, AgNCs have been incorporated into aptamer-based fluorescent biosensors. These biosensors enable the detection of specific biological molecules, thus offering valuable insights into disease diagnosis and pharmaceutical research [65].
Patents associated with AgNCs
Table 3 lists patents for AgNCs [66].
Table 3 Patents on Silver Nanoclusters
| Sr. No. | Patent Number | Patent Title |
|---|---|---|
| 1 | CN108372312B | Green fluorescent silver nanocluster and preparation method and application thereof |
| 2 | CN108641708B | The preparation method of melamine ratio fluorescent probe based on silver nanoclusters compound |
| 3 | CN106018366B | A kind of fluorescent DNA-silver nanoclusters and preparation method thereof and application |
| 4 | US8105847B2 | Nano-sized optical fluorescence labels and uses thereof |
| 5 | US8536119B2 | Synthesis of fluorescent metal nanoclusters |
Clinical trials associated with AgNCs/AgNPs
Table 4 lists clinical trials of AgNCs [67].
Table 4 Clinical Trials of Silver Nanoclusters
| Sr. No. | Trial Number | Title of the Study | Sponsor |
|---|---|---|---|
| 1 | NCT05902975 | Evaluation of the Antimicrobial Fiber Reinforced Composite Resin Space Maintainer Modified With Silver Nano Particles | Al-Azhar University |
| 2 | NCT02761525 | Topical Application of Silver Nanoparticles and Oral Pathogens in Ill Patients | Universidad Autonoma de San Luis Potosí |
| 3 | NCT01950546 | Nanosilver Fluoride to Prevent Dental Biofilms Growth (NSFCT) | University of Pernambuco |
| 4 | NCT05231330 | Clinical Evaluation of Silver Nanoparticles in Comparison to Silver Diamine Fluoride in Management of Deep Carious Lesions | Suez Canal University |
| 5 | NCT04894409 | Evaluation of Silver Nanoparticles for the Prevention of COVID-19 (COVID-19) | Cluster de Bioeconomia de Baja California, A.C |
Conclusion
AgNCs are promising in theranostic applications, because of their tunable optical properties and biocompatibility supporting integrated diagnostics and therapy. Advanced synthesis techniques allow for precise control of size, shape, and surface chemistry, thus enhancing AgNC versatility. AgNCs enable sensitive imaging for early disease detection and real-time treatment monitoring. Moreover, they exhibit antimicrobial properties and efficacy in PDT and targeted drug delivery, and consequently improve therapeutic outcomes while minimizing adverse effects. Challenges include rigorous biocompatibility assessment, long-term toxicity studies, and regulatory considerations for clinical translation. Balancing therapeutic efficacy and cytotoxicity is crucial. AgNCs have potential in personalized medicine, but safety concerns must be addressed before clinical use. Future research should optimize AgNC-based platforms and advance clinical translation to realize their full therapeutic potential in disease management.
References
- Schmid G. Large clusters and colloids. Metals in the embryonic state. Chem Rev 1992;92:1709-27. [DOI: 10.1021/cr00016a002]
- Daniel M-C, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 2004;104:293-346. [PMID: 14719978 DOI: 10.1021/cr030698+]
- Chopade N, More M, Pardeshi S, Puri A, Naik J, et al. Designing bilayer lipid encapsulated mesoporous Silica nanostructures: review on structural and functional features of protocell. Int J Nano Dimens 2023;14:203-11. [DOI: 10.22034/IJND.2023.1981974.2208]
- Lu Y, Chen W. Sub-nanometre sized metal clusters: from synthetic challenges to the unique property discoveries. Chem Soc Rev 2012;41:3594-623. [PMID: 22441327 DOI: 10.1039/c2cs15325d]
- Qian H, Zhu M, Wu Z, Jin R. Quantum sized gold nanoclusters with atomic precision. Acc Chem Res 2012;45:1470-9. [PMID: 22720781 DOI: 10.1021/ar200331z]
- Kolimi P, Narala S, Youssef AA, Nyavanandi D, Dudhipala N. A systemic review on development of mesoporous nanoparticles as a vehicle for transdermal drug delivery. Nanotheranostics 2023;7:70-89. [PMID: 36593800 DOI: 10.7150/ntno.77395]
- Chakraborty I, Pradeep T. Atomically precise clusters of noble metals: emerging link between atoms and nanoparticles. Chem Rev 2017;117:8208-71. [PMID: 28586213 DOI: 10.1021/acs.chemrev.6b00769]
- Aikens CM. Electronic structure of ligand-passivated gold and silver nanoclusters. J Phys Chem Lett 2011;2:99-104. [PMID: 26295527 DOI: 10.1021/jz101499g]
- Patil A, Patil P, Pardeshi S, Shrimal P, Rebello N, et al. Combined microfluidics and drying processes for the continuous production of micro-/nanoparticles for drug delivery: a review. Dry Technol 2023;41:1533-68. [DOI: 10.1080/07373937.2023.2167827]
- Fernando A, Weerawardene KLDM, Karimova NV, Aikens CM. Quantum mechanical studies of large metal, metal oxide, and metal chalcogenide nanoparticles and clusters. Chem Rev 2015;115:6112-216. [PMID: 25898274 DOI: 10.1021/cr500506r]
- Dı´az SA, Hastman DA, Medintz IL, Oh E. Understanding energy transfer with luminescent gold nanoclusters: a promising new transduction modality for biorelated applications. J Mater Chem B 2017;5:7907-7926. [PMID: 32264193 DOI: 10.1039/c7tb01654a]
- Maity S, Bain D, Patra A. An overview on the current understanding of the photophysical properties of metal nanoclusters and their potential applications. Nanoscale 2019;11:22685-723. [PMID: 31774095 DOI: 10.1039/c9nr07963g]
- Bain D, Maity S, Patra A. Opportunities and challenges in energy and electron transfer of nanocluster based hybrid materials and their sensing applications. Phys Chem Chem Phys 2019;21:5863-81. [PMID: 30534715 DOI: 10.1039/c8cp06188b]
- Du Y, Sheng H, Astruc D, Zhu M. Atomically precise noble metal nanoclusters as efficient catalysts: a bridge between structure and properties. Chem Rev 2020;120:526-622. [PMID: 30901198 DOI: 10.1021/acs.chemrev.8b00726]
- Jin R. Atomically precise metal nanoclusters: stable sizes and optical properties. Nanoscale 2015;7:1549-65. [PMID: 25532730 DOI: 10.1039/c4nr05794e]
- Udayabhaskararao T, Pradeep T. New protocols for the synthesis of stable Ag and Au nanocluster molecules. J Phys Chem Lett 2013;4:1553-64. [PMID: 26282314 DOI: 10.1021/jz400332g]
- Zheng K, Yuan X, Goswami N, Zhang Q, Xie J. Recent advances in the synthesis, characterization, and biomedical applications of ultrasmall thiolated silver nanoclusters. RSC Adv 2014;4:60581-96. [DOI: 10.1039/C4RA12054J]
- Yang J, Jin R. New advances in atomically precise silver nanoclusters. ACS Mater Lett 2019;1:482-89. [DOI: 10.1021/acsmaterialslett.9b00246]
- Kang X, Zhu M. Tailoring the photoluminescence of atomically precise nanoclusters. Chem Soc Rev 2019;48:2422-57. [PMID: 30838373 DOI: 10.1039/c8cs00800k]
- Yu H, Rao B, Jiang W, Yang S, Zhu M. The photoluminescent metal nanoclusters with atomic precision. Coord Chem Rev 2019;378:595-617. [DOI: 10.1016/j.ccr.2017.12.005]
- Yao Q, Chen T, Yuan X, Xie J. Toward total synthesis of thiolate-protected metal nanoclusters. Acc Chem Res 2018;51:1338-48. [PMID: 29792422 DOI: 10.1021/acs.accounts.8b00065]
- Junnuthula V, Kolimi P, Nyavanandi D, Sampathi S, Vora LK, et al. Polymeric micelles for breast cancer therapy: recent updates, clinical translation and regulatory considerations. Pharmaceutics 2022;14:1860. [PMID: 36145608 DOI: 10.3390/pharmaceutics14091860]
- Chakraborty I, Udayabhaskararao T, Pradeep T. High temperature nucleation and growth of glutathione protectedB Ag75 clusters. Chem Commun 2012;48:6788-90. [PMID: 22648389 DOI: 10.1039/c2cc33099g]
- Wang Z, Gupta RK, Luo G-G, Sun D. Design and development of highly efficient light-emitting layers in OLEDs with dimesitylboranes: an updated review. Chem Rec 2019;19:1-15. [PMID: 31642593 DOI: 10.1002/tcr.201900068]
- Chakraborty I, Udayabhaskararao T, Deepesh GK, Pradeep T. Sunlight mediated synthesis and antibacterial properties of monolayer protected silver clusters. J Mater Chem B 2013;1:4059-64. [PMID: 32260958 DOI: 10.1039/c3tb20603c]
- Muhammed MA, Aldeek F, Palui G, Trapiella-Alfonso L, Mattoussi H. Growth of in situ functionalized luminescent silver nanoclusters by direct reduction and size focusing. ACS Nano 2012;6:8950-61. [PMID: 22957671 DOI: 10.1021/nn302954n]
- Adhikari B, Banerjee A. Facile synthesis of watersoluble fluorescent silver nanoclusters and HgII sensing. Chem Mater 2010;22:4364-71. [DOI: 10.1021/cm1001253]
- Wu Z, Lanni E, Chen W, Bier ME, Ly D, et al. High yield, large scale synthesis of thiolate-protected Ag7 clusters. J Am Chem Soc 2009;131:16672-4. [PMID: 19886625 DOI: 10.1021/ja907627f]
- Zhou T, Rong M, Cai Z, Yang CJ, Chen X. Sonochemical synthesis of highly fluorescent glutathionestabilized Ag nanoclusters and S2 sensing. Nanoscale 2012;4:4103-6. [PMID: 22635158 DOI: 10.1039/c2nr30718a]
- González BS, Blanco M, López-Quintela MA. Single step electrochemical synthesis of hydrophilic/hydrophobic Ag5 and Ag6 blue luminescent clusters. Nanoscale 2012;4:7632-5. [PMID: 23064311 DOI: 10.1039/c2nr31994b]
- Le Guével X, Spies C, Daum N, Jung G, Schneider M. Highly fluorescent silver nanoclusters stabilized by glutathione: a promising fluorescent label for bioimaging. Nano Res 2012;5:379-87. [DOI: 10.1007/s12274-012-0218-1]
- Yuan X, Setyawati MI, Tan AS, Ong CN, Leong DT, et al. Highly luminescent silver nanoclusters with tunable emissions: cyclic reduction-decomposition synthesis and antimicrobial properties. NPG Asia Mater 2013;5:e39. [DOI: 10.1038/am.2013.3]
- Mrudula KV, Udayabhaskararao T, Pradeep T. Interfacial synthesis of luminescent 7 kDa silver clusters. J Mater Chem 2009;19:4335-42. [DOI: 10.1039/b900025a]
- Khairnar SV, Pagare P, Thakre A, Nambiar AR, Junnuthula V, et al. Review on the scale-up methods for the preparation of solid lipid nanoparticles. Pharmaceutics 2022;14:1886. [PMID: 36145632 DOI: 10.3390/pharmaceutics14091886]
- Chakraborty I, Govindarajan A, Erusappan J, Ghosh A, Pradeep T, et al. The superstable 25 kDa monolayer protected silver nanoparticle: measurements and interpretation as an icosahedral Ag152(SCH2CH2Ph)60 cluster. Nano Lett 2012;12:5861-6. [PMID: 23094944 DOI: 10.1021/nl303220x]
- Chakraborty I, Udayabhaskararao T, Pradeep T. Luminescent sub-nanometer clusters for metal ion sensing: a new direction in nanosensors. J Hazard Mater 2012;211-212:396-403. [PMID: 22221854 DOI: 10.1016/j.jhazmat.2011.12.032]
- Udayabhaskararao T, Pradeep T. Luminescent Ag7 and Ag8 clusters by interfacial synthesis. Angew Chem Int Ed 2010;49:3925-9. [PMID: 20408149 DOI: 10.1002/anie.200907120]
- Dhanalakshmi L, Udayabhaskararao T, Pradeep T. Conversion of double layer charge-stabilized Ag@citrate colloids to thiol passivated luminescent quantum clusters. Chem Commun 2012;48:859-61. [PMID: 22121500 DOI: 10.1039/c1cc15604g]
- AbdulHalim LG, Kothalawala N, Sinatra L, Dass A, Bakr OM. Neat and complete: thiolate-ligand exchange on a silver molecular nanoparticle. J Am Chem Soc 2014;136:15865-8. [PMID: 25345688 DOI: 10.1021/ja508860b]
- Bootharaju MS, Burlakov VM, Besong TMD, Joshi CP, AbdulHalim LG, et al. Reversible size control of silver nanoclusters via ligand-exchange. Chem Mater 2015;27:4289-97. [DOI: 10.1021/acs.chemmater.5b00650]
- Jiang R, Li B, Fang C, Wang J. Unraveling the evolution and nature of the plasmons in (Au core)-(Ag shell) nanorods. Adv Mater 2014;26:5274-309. [PMID: 22714684 DOI: 10.1002/adma.201201896]
- Liz-Marzán LM, Murphy CJ, Wang J. Nanoplasmonics. Chem Soc Rev 2014;43:3820-2. [PMID: 24728184 DOI: 10.1039/c4cs90026j]
- Zhang QB, Tan YN, Xie JP, Lee JY. Colloidal synthesis of plasmonic metallic nanoparticles. Plasmonics 2009;4:9-22. [DOI: 10.1007/s11468-008-9067-x]
- Huang J, Zhu Y, Lin M, Wang Q, Zhao L, et al. Site-specific growth of Au-Pd alloy horns on Au nanorods: a platform for highly sensitive monitoring of catalytic reactions by surface enhancement Raman spectroscopy. J Am Chem Soc 2013;135:8552-61. [PMID: 23675958 DOI: 10.1021/ja4004602]
- Lee TH, Dickson RM. Discrete two-terminal single nanocluster quantum optoelectronic logic operations at room temperature. Proc Natl Acad Sci U S A 2003;100:3043-6. [PMID: 12621154 DOI: 10.1073/pnas.0635474100]
- Chakraborty I, Erusappan J, Govindarajan A, Sugi KS, Udayabhaskararao T, et al. Emergence of metallicity in silver clusters in the 150 atom regime: a study of differently sized silver clusters. Nanoscale 2014;6:8024-31. [PMID: 24905949 DOI: 10.1039/c4nr00679h]
- Jin R. Quantum sized, thiolate-protected gold nanoclusters. Nanoscale 2010;2(3):343-62. [PMID: 20644816 DOI: 10.1039/b9nr00160c]
- Xu W, Jin R. Unraveling the structures of cluster-assembled materials with X-ray crystallography. Acc Chem Res 2008;41(11):1629-40.
- Rong Z, Zhang Z. Biomedical applications of aggregation-induced emission fluorogens. Small 2017;13(6):1603578.
- Kang X, et al. AIEgens for dark through-bond energy transfer: design, synthesis, and applications in fluorescence imaging. Chem Commun 2015;51(31):6772-5.
- Lan GY, et al. Highly selective detection of Hg2+ and l-cysteine based on DNA-templated copper/silver nanoclusters. Analytica Chimica Acta 2016;936:69-74.
- Vijayan VM, Raji V. Design and development of drug delivery systems based on silver nanoparticles. Adv Drug Deliv Rev 2020;153:37-70.
- Rai M, et al. Antibiotic resistance and antibacterial activity of silver nanoparticles against methicillin-resistant staphylococcus aureus (MRSA). Nanomedicine 2009;5(4):452-6.
- Fu Y, Jin H, Bu X, Gui R. Melamine-induced decomposition and anti-FRET effect from a self-assembled complex of rhodamine 6G and DNA-stabilized silver nanoclusters used for dual-emitting ratiometric and naked-eye-visible fluorescence detection. J Agric Food Chem 2018;66(37):9819-27. [PMID: 30160493 DOI: 10.1021/acs.jafc.8b03402]
- Zhang K, Huang W, Huang Y, Li H, Wang K, et al. DNA tetrahedron based biosensor for argonaute2 assay in single cells and human immunodeficiency virus type-1 related ribonuclease H detection in vitro. Anal Chem 2019;91(11):7086-96. [PMID: 31050888 DOI: 10.1021/acs.analchem.9b00011]
- Wu L, Wang Y, He R, Zhang Y, He Y, et al. Fluorescence hydrogel array based on interfacial cation exchange amplification for highly sensitive microRNA detection. Anal Chim Acta 2019;1080:206-14. [PMID: 31409471 DOI: 10.1016/j.aca.2019.07.024]
- Jin F, Li H, Xu D. Enzyme-free fluorescence microarray for determination of hepatitis B virus DNA based on silver nanoparticle aggregates-assisted signal amplification. Anal Chim Acta 2019;1077:297-304. [PMID: 31307722 DOI: 10.1016/j.aca.2019.05.066]
- Cao ZM, Duan FH, Huang XY, Liu Y, Zhou N, et al. A multiple aptasensor for ultrasensitive detection of miRNAs by using covalent-organic framework nanowire as platform and shell-encoded gold nanoparticles as signal labels. Anal Chim Acta 2019;1082:176-85. [PMID: 31472706 DOI: 10.1016/j.aca.2019.07.062]
- Li Y, Yu C, Zhao C, Ren C, Zhang X. Catalytic hairpin assembly induced dual signal enhancement for rapid detection of miRNA using fluorescence light-up silver nanocluster. Anal Chim Acta 2019;1084:93-8. [PMID: 31519239 DOI: 10.1016/j.aca.2019.08.007]
- Rai M, Kon K, Ingle A, Duran N, Galdiero S, et al. Broad-spectrum bioactivities of silver nanoparticles: the emerging trends and future prospects. Appl Microbiol Biotechnol 2015;98(5):1951-61. [PMID: 24407450 DOI: 10.1007/s00253-013-5473-x]
- Wei L, Lu J, Xu H, Patel A, Chen ZS, et al. Silver nanoparticles: synthesis, properties, and therapeutic applications. Drug Discovery Today 2015;20(5):595-601. [PMID: 25543008 DOI: 10.1016/j.drudis.2014.11.014]
- Gurunathan S, Jeong J-K, Han JW, et al. Antiangiogenic properties of silver nanoparticles. Biomaterials 2015;34(33):1010-20.
- Sengupta S, Patra CR, Bhattacharya S. Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents. Int J Nanomedicine 2017;12:3535-51.
- Iravani S, Korbekandi H, Mirmohammadi SV, Zolfaghari B. Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci 2014;9:385-406. [PMID: 26339255]
- Ding Y, Li J, Chen L. Aptamer-based fluorescent biosensors for rapid detection of biological molecules in pharmaceutical analysis. Trends Anal Chem 2019;117:194-205.
- https://patents.google.com/patent/WO2016059238A1/en17 [Last accessed 28 Mar 2024].
- ClinicalTrials.gov [Last accessed 28 Mar 2024].