Targeting the CD24-Siglec10 Axis: A Potential Strategy for Cancer Immunotherapy
1Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Medical Research Center, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, P. R. China
2Guangzhou Key Laboratory of Medical Nanomaterials, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, P. R. China
3Cellular and Molecular Diagnostics Center, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, P. R. China
4Department of Dermatology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, P. R. China
5Department of Ultrasound, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, P. R. China
6Department of Chemistry, University of Georgia, Athens, GA 30602, USA
aThese authors contributed equally to this work.
*Correspondence to: Nengtai Ouyang, Email: ouynt@mail.sysu.edu.cn; Phei Er Saw, caipeie@mail.sysu.edu.cn; Wei Yang, wei.yang@uga.edu
Received: 18 December 2023; Revised: 20 January 2024; Accepted: 25 January 2024; Published Online: 19 February 2024
Cite this paper:
Junyue Fang, Li Lin, Yuan Cao, Jiabao Tan, Yixia Liang, Xiaoyun Xiao, Nengtai Ouyang, Phei Er Saw and Wei Yang. Targeting the CD24-Siglec10 Axis: A Potential Strategy for Cancer Immunotherapy. BIO Integration 2024; 5: e997.
DOI: 10.15212/bioi-2023-0022. Available at: https://bio-integration.org/
Download citation
© 2024 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
CD24, also known as heat-stable protein, is a highly glycosylated glycosylphosphatidylinositol junction membrane protein. CD24 specifically binds sialic-acid-binding Ig-like lectin 10 (Siglec10) on macrophages and serves as a “don’t eat me” signal, thus blocking the phagocytosis of tumor cells by macrophages and triggering tumor immune escape. Blocking the CD24-Siglec10 axis to reprogram the tumor immune microenvironment is a current research hotspot in cancer immunotherapy. Targeting the CD24-Siglec10 axis has received widespread attention, because of the high expression of CD24 on a variety of tumor cells and absence of blood toxicity. Targeting the CD24-Siglec10 axis as a cancer immunotherapy has shown favorable results and progress in preclinical studies. In this review, we summarize the discovery and functions of the CD24-Siglec10 axis, and review the roles and effects of this axis as a novel immune checkpoint in cancer immunotherapy. We also highlight recent advances in nanoparticle-mediated treatments targeting the CD24-Siglec10 axis for enhancing cancer immunotherapy.
Keywords
Cancer immunotherapy, CD24-Siglec10 axis, immune checkpoints, nanoparticles (NPs).
Introduction
Cancer is a complex, variable cellular ecosystem in which malignant cells co-exist and interact with other host immune cells in their tumor microenvironment (TME) [1]. The TME plays a crucial role in cancer occurrence and development, and targeting TME remains a major aspect of cancer immunotherapy [2, 3]. Unlike direct targeted killing of tumor cells, cancer immunotherapy primarily recognizes and attacks cancer cells by activating the host’s immune system, regulating the TME, and enhancing host immunity, thus facilitating treatment of cancers. This tumor treatment strategy is clearly the most promising method currently available.
Tumor-associated macrophages (TAMs) are the most abundant innate immune infiltrating cells in the TME [4]. In the past decade, with continued exploration of immune checkpoints, substantial progress has been made in the application of cancer immunotherapy (e.g., immune checkpoint blockade therapy). The best-described innate immune checkpoints associated with macrophages are the “don’t eat me” signals, including the CD47-SIRPα axis and CD24-Siglec10 axis. Cancer cells can evade macrophage clearance and achieve tumor immune escape by overexpressing the “don’t eat me” signal (including CD47 and CD24) and binding anti-phagocytic surface proteins (including SIRPα and Siglec10) on macrophages [5, 6]. Therefore, blocking this signaling axis to restore the immune and phagocytic activity of natural immune cells against tumor cells is a key step in tumor immune blockade therapy.
CD24 was initially described as a pre-B lymphocyte marker [7]. Currently, CD24 targeting is an emerging cancer therapy with great potential as a new-generation immune checkpoint for tumor immunotherapy. Compared with CD47, CD24 has a more limited distribution in healthy tissues and higher expression in tumor tissues. Notably, CD24 is not expressed on human red blood cells, thus decreasing the targeted anti-tumor toxicity of the CD24 targeted therapy [6]. Therefore, targeting CD24 does not produce blood-related adverse effects similar to those with CD47 targeting, and has a larger safety window. CD24 exhibits differential expression profiles with PD-L1 in various tumors, thus supporting potential future clinical applications in combination with PD-1/L1 inhibitors [8, 9]. At present, in preclinical studies, CD24-Siglec10 axis target therapies have focused primarily on monoclonal antibodies (mAbs), antibody-drug conjugates, target gene silencing by RNA interference (RNAi) technology, and chimeric antigen receptor T-cell (CART) therapy [10, 11], which have shown favorable prospects in cancer immunotherapy. However, this highly promising targeted immune checkpoint therapy is accompanied by well-known adverse effects denoted immune-related adverse events [12]. Therefore, the development and optimization of new effective therapeutic strategies with minimal adverse effects are necessary to support cancer immunotherapy.
The development and application of immune checkpoint inhibitors (ICBs) have ushered in a new era of cancer immunotherapy. However, the use of free protein/peptide or nucleic acid drugs for cancer immunotherapy is often inefficient, because drugs are easily metabolized or even degraded under physiological conditions. The emergence of nanomedicine has provided vast possibilities to overcome these shortcomings. Using nanoparticles (NPs) as carriers to deliver ICBs for cancer therapy has become a highly promising strategy [13–16]. Herein, we systematically review the origin and function of the CD24-Siglec10 axis, and discuss the effects of targeting this axis in cancer immunotherapy. We also highlight recent advances in NP-mediated treatments targeting the CD24-Siglec10 axis for collaborative cancer immunotherapy.
Overview of the CD24-Siglec10 axis
CD24 was discovered in 1978 and was initially referred to as a heat-stable antigen [17]. In 1990, the CD24 genes of mice and humans were successfully cloned [18, 19]. Comparison of CD24 antigens between mice and humans has indicated differences in the expression profiles during B cell development [18]. CD24, a highly glycosylated adhesive molecule with a relative molecular weight of approximately 35–45 kDa, is connected to the cell membrane by glycosyl phosphatidylinositol [20]. As a membrane glycoprotein, CD24 has O-linked glycosylation sites located on the N-terminal side, and it forms dense dextran chains at the top of the molecule, thus enabling more glycosylation sites to be arranged on the membrane surface [18, 21]. These features provide the main structural basis for CD24’s roles in adhesion and metastasis. CD24 is also a signaling molecule that mediates signal transduction by recruiting Src family protein tyrosine kinases, which in turn initiate the activation of downstream signaling cascades [22]. CD24 affects signal transducer and activator of transcription 3 (STAT3) phosphorylation and alters the expression of STAT3-reliant genes via Src activation [23]. CD24 interacts with the Wnt pathway by activating β-catenin [24]. Lyn, a crucial member of the Src family kinases, is involved in CD24-induced ERK1/2 activation [25]. At the post-transcriptional level, microRNAs (miRNAs), including miR-146a [26], miR-125b [27], miR-34a [28], miR-1185-1 [29], and miR-3064-5p [30], play crucial roles in modulating the expression of CD24 in cancer cells.
In 2001, a new member of the Siglec family of sialic-acid-binding Ig-like lectins, called Siglec10, was first reported [31]. Siglec10 is a single-channel transmembrane protein with an extracellular structural domain containing one V-set Ig-like and three C2-set structural domains, and a cytoplasmic structural domain containing an immunoreceptor tyrosine-based inhibitor motif (ITIM) [32]. After tyrosine phosphorylation, the ITIM recruits SH2 family phosphatases, such as SHP-1 and PTPN6, thereby blocking signal transduction [33]. Siglec10 has an inhibitory motif based on immune receptor tyrosine in the cytoplasmic domain, and it also serves as a ligand for the mAb alemtuzumab target, CD52 [34]. Siglec10 is involved in the negative regulation of B cell antigen receptor signaling. A recent study has indicated that the increase in CD19+ Siglec10+ B cells correlates with the activity of SLE [35]. Thus, Siglec10 may play important roles in the pathogenesis and progression of SLE [35]. Increasing research is investigating the biological functions of CD24 and Siglec10. However, the interaction of CD24 with Siglec10 was not identified until 2009, in a study demonstrating that CD24 interacts with Siglec10 molecules on innate immune cells, transmits immunosuppressive signals, and inhibits inflammatory responses [36].
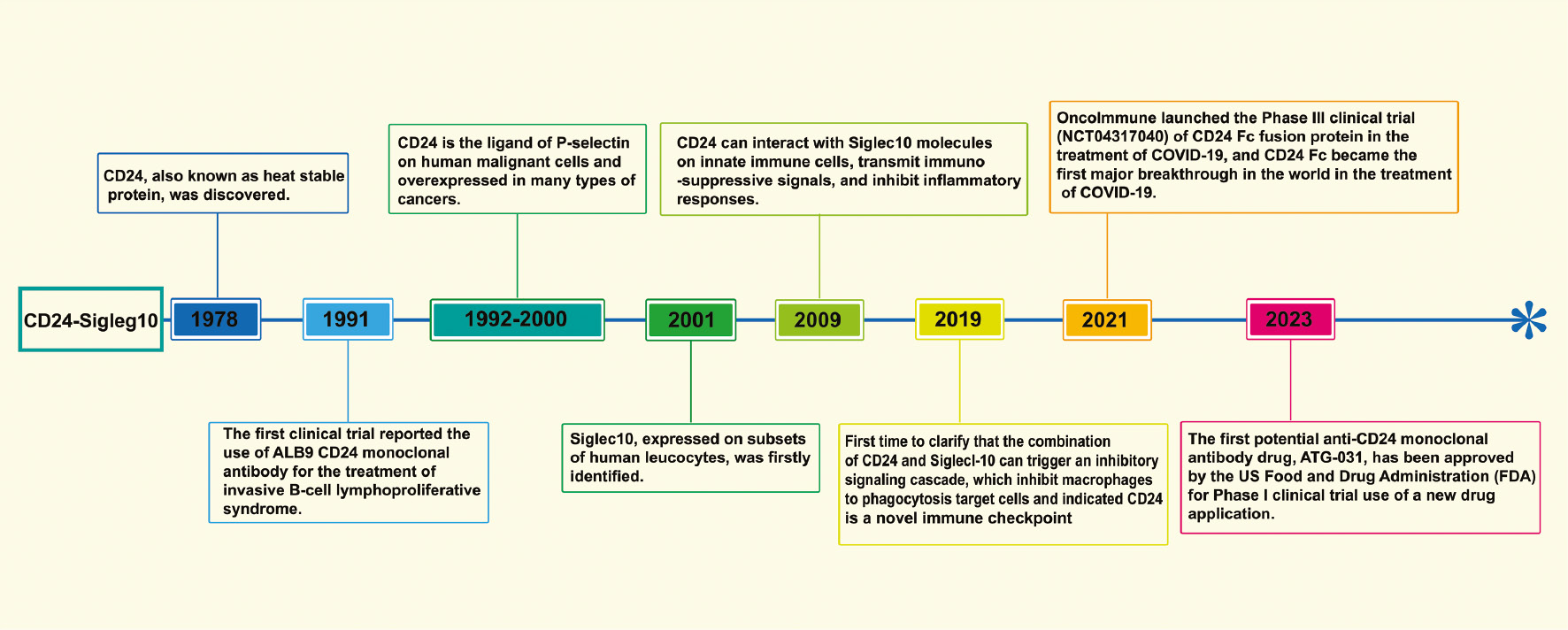
CD24 is extensively expressed by various human cells, including T cells, B cells, monocytes, macrophages, and keratinocytes [18, 37–40]. Similarly, Siglec10 is broadly expressed on dendritic cells, B cells, and macrophage subsets [41]. In early research, CD24 was used primarily as a biomarker for immune characterization of the blood system. For example, loss of the CD24 anchor protein can be used to evaluate the occurrence of paroxysmal nocturnal hemoglobinuria through cytofluorography [22]. Associations between CD24 and cancer were suggested by experts and researchers more than a decade after the discovery of the CD24. Most solid tumors, such as triple-negative breast cancer (TNBC) and ovarian cancer [6, 42, 43], have CD24 overexpression. CD24 expression is negatively correlated with the survival time of patients with cancer and can serve as a prognostic indicator for cancer treatment [27, 44, 45]. In 2019, a major study proposed that CD24 might serve as a new-generation immune checkpoint: CD24 binds Siglec10 on macrophages, activates SHP-1/SHP-2 mediated inhibitory signaling pathways, inhibits tumor cells from being engulfed by macrophages, and thus exerts immune escape effects [6]. This research led to CD24’s becoming a major target after PD-1 and CD47, and also started a new chapter regarding CD24 in cancer immunotherapy. With the rapid development of more powerful sequencing technologies (e.g., single-cell RNA sequencing), more signaling axes associated with immunotherapy are expected to be discovered. For example, a recent study using single-cell RNA sequencing has revealed that the intratumoral immunomodulatory effects of CD73 inhibition differ from those of PD-1 inhibition, and thus may serve as a new type of colorectal cancer anti-cancer immunotherapy through synergistic effects with PD-1 blockers [46]. The history of research regarding the CD24-Siglec10 axis and related breakthrough studies are summarized in Figure 1.
Figure 1 Timeline of the history of CD24-Siglec10 axis research and related breakthrough studies.
Pro-tumorigenic role of the CD24-Siglec10 axis
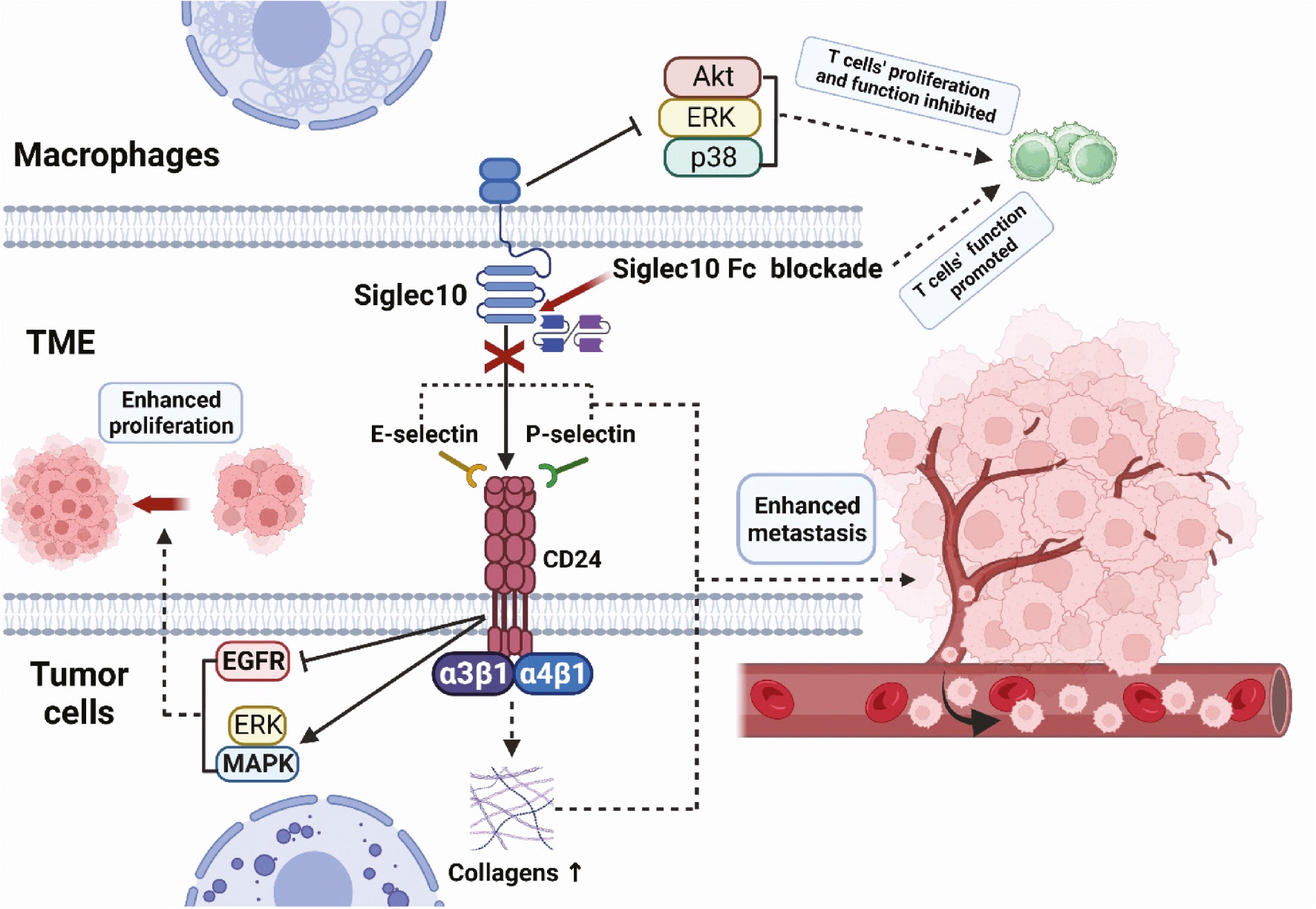
Malignant proliferation and metastasis of tumor cells are key features of cancers. The CD24-Siglec10 axis is involved in tumor proliferation and metastasis through various pathways (Figure 2). E-selectin and P-selectin play important roles in tumor metastasis. Binding of CD24 to E-selectin mediates rolling and extravasation of tumor cells on the endothelial surfaces of blood vessels, thus leading to tumor cell metastasis [47]. Similarly, tumor cells attach to P-selectin via CD24, thereby increasing tumor cells’ adhesion to the vascular endothelium and platelets under static conditions and rolling downstream conditions, and consequently enhancing their migratory and metastatic activity [48, 49]. In addition, CD24 promotes the binding of tumor cells to fibronectin and other extracellular matrix components (e.g., collagens) through the activation of integrin subunits (primarily a3β1 and a4β1), thereby increasing tumor cell mobility and metastasis [50]. CD24 induces activation of mitogen activated protein kinase (MAPK) and ERK signals, which in turn promote tumor cell proliferation both in vivo and in vitro [51]. Moreover, CD24 inhibits the internalization and degradation of epidermal growth factor receptor, thereby promoting the proliferation of tumor cells [52]. CD24 is a regulator of cell migration, invasion, and proliferation; its expression is associated with poor prognosis, and it is also used as a cancer stemness marker [53]. In contrast, members of the Siglec family are involved in the regulation of immune cell activation, proliferation, and apoptosis, and play crucial roles in tumorigenesis and development. A recent study has found that Siglec10 inhibits the proliferation and function of tumor-infiltrating CD8+ T cells through the Akt/P38/ERK signaling pathway, and in vitro experiments have shown that blockade of Siglec10 promotes CD8+ T cell function [54].
Figure 2 Pro-tumorigenic role of the CD24-Siglec10 axis. Tumor cells attach to P-selectin and E-selectin via CD24, thereby increasing the adhesion of tumor cells to the vascular endothelium and platelets. CD24 promotes the binding of tumor cells to fibronectin and collagens through the activation of a3β1 and a4β1, thereby increasing tumor cell mobility and metastasis. CD24 induces activation of MAPK and ERK signals, which promote tumor cell proliferation both in vivo and in vitro. Siglec10 inhibits the proliferation and function of tumor-infiltrating CD8+ T cells through the Akt/P38/ERK signaling pathway. The blockade of Siglec10 promotes the function of CD8+ T cells.
Effects of targeting the CD24-Siglec10 axis in cancer immunotherapy
Targeting CD24-Siglec10 axis has widespread effects in cancer immunotherapy, because CD24/Siglec10 is expressed not only on tumor cells but also on various immune cells. However, CD24 has completely opposite effects in physiological and pathological states: in physiological states, CD24 inhibits tissue growth, whereas CD24 promotes cell proliferation in pathological conditions. Targeting the CD24-Siglec10 axis for cancer immunotherapy can also cause multiple immune cell interactions in the TME, which play critical roles in enhancing tumor immune efficacy. First, blockade of the CD24-Siglec10 axis interaction and inhibition of SHP-1 activation enhance the activating phosphorylation of Src kinase, thus leading to macrophage phagocytosis [6]. Second, owing to the critical roles of CD24 in interacting with various immune cells, blocking CD24-Siglec10 axis interaction enhances CD4+ and CD8+ T cell responses and activates T-cell receptor (TCR)- and B-cell receptor (BCR)-related kinases, thus inhibiting tumor immune escape [55]. Third, blockade of the CD24-Siglec10 axis increases NK cell cytotoxicity, thus promoting direct killing of cancer cells [56].
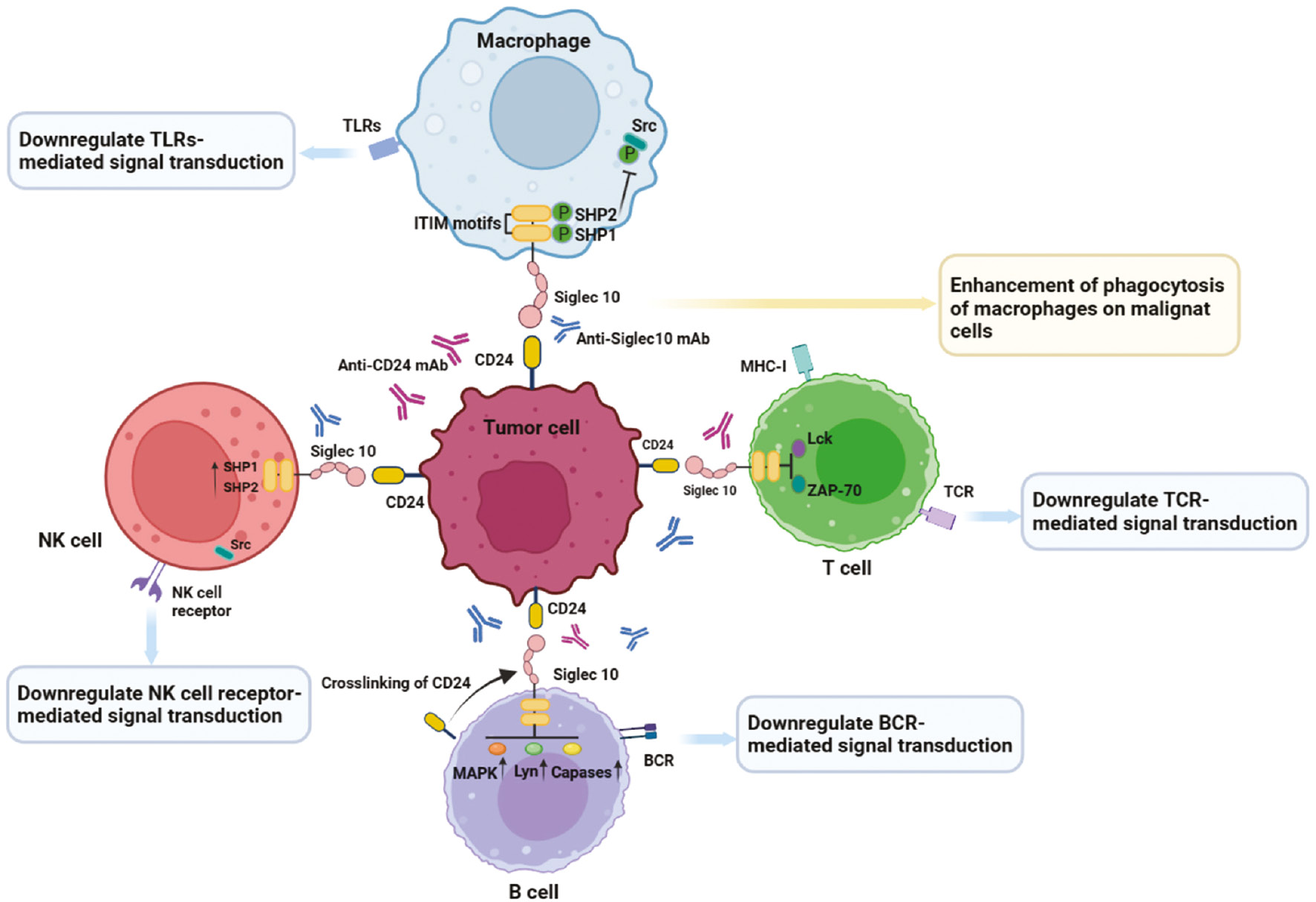
Therapies targeting the CD24-Siglec10 axis are available for the enhancement of phagocytosis of malignant cells by macrophages, which exert antitumor effect through a variety of mechanisms (Figure 3). Initially, research on the CD24-Siglec10 axis focused on anti-infection treatments and graft-versus-host disease (GvHD). CD24 interacts with Siglec10 on innate immune cells, thus inhibiting destructive inflammatory responses to infection, sepsis, liver injury, and GvHD [36, 57, 58]. The CD24-Siglec10 axis interaction negatively regulates the activity of NF-κB through the intracellular ITIM domain associated with SHP-1, thereby inhibiting the secretion of TNF-a, IL-1β, and IL-6 (major targets in autoimmune diseases and cancer) [59]. The interaction between the CD24-Siglec10 axis and various immune cells plays an important role in tumor immunotherapy. Siglec10 is expressed in T cells and acts as an inhibitory receptor by blocking TCR activation and inducing immunosuppression. Mechanistically, Siglec10 achieves immunosuppressive effects by inhibiting the formation of T cell MHC-I peptide complexes and the phosphorylation of T cell receptor-associated kinases (e.g., Lck and ZAP-70) [56, 60]. Targeting the CD24-Siglec10 axis promotes T cell activation mediated by TCR and inhibits tumor immune escape for cancer immunotherapy. B cells, such as B1 cells, also express the inhibitory receptor Siglec10 on their cell surfaces [31]. However, the specific mechanism through which Siglec10 regulates the immune activity of B cells is currently unclear. Siglec10 is expressed not only in human B cells but also in mouse B cells (in which it is called SiglecG). Siglec10 and SiglecG are highly homologous, and both bind CD24, and consequently hinder host inflammation and immune responses induced by danger-versus pathogen-associated molecular patterns [36]. CD24 affects BCR signal transduction by cross-linking with BCR, thereby affecting B cell function. When CD24 is overexpressed, SiglecG inhibits BCR-mediated calcium ion channel signal transduction in B1 cells by recruiting growth factor receptor binding protein 2 (Grb2) and ITIM binding protein SHP-1 [41]. Simultaneously, CD24 cross-linking activates multiple signaling pathways (e.g., MAPK) that mediate intracellular signal transduction, thereby leading to B cell apoptosis [61]. Little is known regarding the roles and effects of NK cells in targeted CD24-Siglec10 axis tumor immunotherapy. Siglec10 is expressed primarily on NK cells in liver tumor environments, and the abundance of Siglec10+ NK cells in tumor tissue is higher than that in adjacent non-tumor tissues [56]. Notably, high expression of Siglec10 on NK cells can undermine NK cell function in hepatocellular carcinoma [56]. The binding between CD24 expressed on hepatocellular carcinoma cells and Siglec10 expressed on NK cells, similarly to that on macrophages, may be conducive to cancer escape from the killing effects of NK cells and may increase tumor immune escape. Therefore, blocking the CD24-Siglec10 axis may reactivate NK cell function and promote the killing of tumor cells.
Figure 3 Effects of targeting the CD24-Siglec10 axis in cancer immunotherapy. Highly expressed CD24 on tumor cells interacts with Siglec10 expressed by various immune cells (e.g., macrophages, T cells, B cells, and NK cells). Interaction of Siglec10 on macrophages with CD24 on tumor cells triggers SHP-1-mediated inhibitory signals, which prevent phagocytosis by macrophages. Siglec10 on T cell surfaces inhibits T cell activation and phosphorylation of Lck and ZAP-70. Siglec10 on B cell surfaces downregulates BCR mediated signal transduction by cross-linking with CD24. NK cell receptor-mediated signal transduction is inhibited by the CD24-Siglec10 axis. Anti-CD24 mAbs or anti-Siglec10 mAbs block the CD24-Siglec10 axis, restore the anti-tumor function of immune cells, and enhance tumor immune efficacy.
Therapeutic strategies targeting the CD24-Siglec10 axis for cancer immunotherapy
The CD24-Siglec10 axis is a promising therapeutic target, because of its overexpression in many types of cancers and its close association with tumor progression. Inhibition of the CD24-Siglec10 axis with anti-CD24 or anti-Siglec10 mAbs causes phagocytosis of cancer cells by macrophages. Multiple immunotherapies targeting the CD24-Siglec10 axis are under extensive exploration in diverse developmental phases.
Monoclonal-antibody-mediated cancer immunotherapy
Currently, a widely used strategy for targeting the CD24-Siglec10 axis in cancer immunotherapy uses anti-CD24 mAbs. Anti-CD24 mAbs reactivate macrophage phagocytosis of tumor cells by blocking the CD24-Siglec10 axis. Antibody-drug conjugate (ADC) and CART immunotherapy are also important extensions of the CD24 mAbs. Because CD24 is expressed not only on tumor cells but also on various immune cells, it may cause harmful adverse effects (e.g., off-target effects). However, many attempts have been made to evaluate the therapeutic efficacy of anti-CD24 mAbs against tumors. Studies have used anti-CD24 mAbs in the treatment of cancers including bladder cancer [62], breast cancer [63], lung cancer [23], and ovarian cancer [64], and have shown strong anti-tumor therapeutic effects in a time- and dose-dependent manner. A recent study has indicated that blocking CD24 with anti-CD24 mAbs promotes the anti-tumor immune effects of oral squamous cell carcinoma [65]. Early preclinical studies attempted to analyze the anti-tumor efficacy of anti-CD24 mAbs. One study has indicated that anti-CD24 mAb (SWA11) targeting of tumor cells in a SCID mouse model successfully retards tumor growth [64]. ALB9 has been used to treat human bladder cancer cells, and found to decrease tumor growth and metastasis [62]. Anti-CD24-CAR has been used to enhance the killing ability of effector cells through targeting CD24+ ovarian cancer cells in vivo [66]. Anti-CD24-ADC (HN-01) exhibits effective internalization in vitro, significantly increases the release of NO in liver cancer cells, and effectively inhibits the growth of liver cancer [67]. Moreover, single-chain variable fragments, recombinant antibodies targeting CD24, have received U.S. Food and Drug Administration (FDA) approval for treating malignancies (e.g., blinatumomab for acute lymphoblastic leukemia) [68]. Similarly, blocking Siglec10 with mAbs or genetically knocking out Siglec10 from macrophages also augments macrophages’ ability to engulf tumor cells [6]. A recent study has used Siglec10 mAb to block Siglec10 in THP1 cells, and has observed the enhancement of phagocytosis of HepG2 cells by macrophages [69]. Furthermore, high infiltration of Siglec10 hi TAMs is associated with impaired CD8+ T cell function in hepatocellular carcinoma (HCC) [70]. Notably, blockade of Siglec10 with the competitive binding antibody Siglec10 Fc decreases the expression of immunosuppressive molecules, increases the cytotoxicity of CD8+ T cells against HCC cells, and promotes the antitumor efficacy of PD-L1 inhibitors [70]. Many pharmaceutical companies have embarked on anti-CD24 mAbs research and development. However, to date, only a few types of drugs are in preclinical research or drug discovery stages, for example, engineered cancer specific anti-CD24 mAb (ONC-781), chimeric antigen receptor CART (ONC-782), T cell mediated bispecific antibody CD3xCD24 (ONC-783), antibody conjugates (ONC-784) (https://www.oncoc4.com), bispecific antibody target CD24+SIRPα (IMM-4701), CD24 molecule inhibitors (IMM-47), and bispecific antibody target CD24+PDL1 (IMM-2547) (http://immuneonco.com/). Current new drug research and discovery targeting the CD24-Siglec10 axis is summarized in Table 1.
Table 1 Current Drug Research and Discovery Targeting the CD24-Siglec10 Axis for Cancer Therapy
| Drug name | Indication | Target | Mechanism | Status |
|---|---|---|---|---|
| ATG-031 | Hematological malignancies, solid tumors | CD24 | Block CD24 [71] | Phase I clinical trial demonstrated by FDA |
| BCG-002 | Solid tumors | CD24 | Block CD24a | Preclinical |
| ONC-781 | Neoplasms | CD24 | Block CD24b | Preclinical |
| ONC-782 | Neoplasms | CD24 | Block CD24b | Preclinical |
| ONC-783 | Neoplasms | CD24 | Block CD24b | Preclinical |
| ONC-784 | Neoplasms | CD24 | Block CD24b | Drug discovery |
| IMM-47 | Solid tumors | CD24 | Block CD24 [72] | Preclinical |
| IMM-4701 | Solid tumors | CD24, CD47 | Block CD24 and CD47c | Preclinical |
| IMM-2547 | Solid tumors | CD24, PDL1 | Block CD24 and PDL1c | Drug discovery |
| rG7S-MICA | Colorectal carcinoma | CD24, NKG2D | Block CD24 [73] | Unknown |
Note: aThe most recent research can be found at www.biocytogen.com.cn; bThe most recent research can be found at www.oncoc4.com; cThe most recent research can be found at immuneonco.com/.
RNA-interference-mediated cancer immunotherapy
RNAi has become a new strategy for tumor gene therapy, because of its unique advantages. siRNAs targeting specific target genes in tumor cells inhibit tumors, induce apoptosis, and enhance sensitivity to chemotherapy or radiotherapy, thereby exerting anti-cancer effects. Because of the inevitable immune response and high production cost of humanized antibodies, researchers have also attempted to knock down CD24 in tumor cells by using siRNAs or shRNAs targeting CD24, and have evaluated their anti-tumor efficacy. CD24 siRNA transfection significantly inhibits invasion and cell viability by knocking down the expression of CD24, thus suggesting that CD24 siRNA knockdown may serve as a potential for cancer therapy [74]. Wild-type or CD24 gene knockout (ΔCD24) cells have been cultured with macrophages expressing Siglec-10, and the latter have been found to be more easily engulfed and degraded by macrophages [6]. Moreover, Siglec10 knockout in donor-derived macrophages markedly exacerbates the phagocytosis of wild-type MCF-7 cells [6]. Additionally, shRNA knockdown of CD24 has shown anti-cancer efficacy in ovarian cancer and lung cancer bone metastasis models [75, 76]. Knockdown of Siglec10 to promote macrophage phagocytosis appears to be a potential strategy. Although Siglec10 knockdown can be easily achieved with a Siglec10 CRISPR plasmid, no reports or experiments have evaluated the specific anti-cancer effects of Siglec10 knockdown. Current research has focused on cells or mouse models, and no clinical trials have examined CD24 or Siglec10 nucleic acid drugs. However, we believe that the application of RNAi technology still has great potential for targeting the CD24-Siglec10 axis in cancer treatment.
ICB-mediated combination therapy
Immune checkpoints, a class of immunosuppressive molecules, are involved in many inhibitory pathways of the immune system that are crucial for maintaining self-tolerance, and regulating the duration and magnitude of physiological immune responses in peripheral tissues, to minimize collateral tissue damage [77]. One promising way to activate therapeutic anti-tumor immunity is the blockade of immune checkpoints. Recent basic research evidence suggests that the clearance and detection of cancer cells by phagocytosis induced by innate immune checkpoints play crucial roles in tumor-mediated immune escape [78]. The well-known and most clearly described innate immune checkpoint is the “don’t eat me” signal, including the MHC-I/LILRB1 axis, PD-1/PD-L1 axis, CD47-SIRPα axis, and CD24/Siglec-10 axis.
Immunotherapy drugs represented by ICBs have achieved great success in the treatment of various tumors, thereby greatly increasing the survival rate and quality of life of some patients with tumors. However, the benefits of single ICB therapy for patients with cancer are limited. For example, although PD-1/L1 mAb has been approved for more than 20 types of cancer treatments, only several tumor species (such as classical Hodgkin lymphoma and melanoma) respond well [79, 80]. Recently, a global medical research service organization has released a report entitled “Global Oncology Trends 2023: Outlook to 2027” (https://www.iqvia.com/), indicating that oncology research and development are increasingly focusing on drugs targeting solid tumors. Notably, the report indicates that transcending the limitations of single-ICB methods will require the use of combination therapy. To date, the FDA has approved several combination therapies for various types of cancer. The combination of first-line nivolumab and ipilimumab has been shown increase the overall survival rate in patients with advanced non-small cell lung cancer [81]. Cabozantinib plus nivolumab has significant benefits over sunitinib in terms of progression overall survival, free survival, and response probability of patients with advanced renal cell carcinoma (NCT03141177) [82]. Blockade of CD47 increases the tumor infiltration of CD8+ T lymphocytes in human pancreatic ductal adenocarcinoma, and anti CD47 and anti PDL1 combination therapy significantly inhibits the growth of pancreatic ductal adenocarcinoma (in the MPC-83 model) [83]. In a recent study, the combination of anti PD-1/anti CTLA-4 has achieved favorable response and survival rates for metastatic melanoma [84].
The CD24-Siglec10 axis, a “do not eat me” signal axis discovered in recent years, provides a crucial promising direction for ICB therapy in tumor treatment. CD24 is functionally complementary to CD47 and PD-L1; consequently, dual treatment with CD24 and CD47 antibodies might increase phagocytosis and thus enhance antitumor effects [6]. However, patients exhibit varying responses to anti-PD-L1/PD-1 immunotherapies, and CD24 blockade might be used as an alternative strategy [6]. CD47/CD24 bispecific antibodies have been found to effectively activate the medullary immune system in the brain, decrease the occurrence of immune related adverse reactions, and have synergistic anti-tumor effects [85]. In contrast, the function of the PD-1/L1 axis, a classical T cell immune checkpoint, has been well elucidated in T cells and found to play a critical role in anti-tumor therapy. However, a recent study has shown that the PD-1/L1 axis also functions in the regulation of the phagocytosis by TAMs [86]. PD-1 on macrophages may also activate immunosuppressive signals that hinder phagocytic activity by macrophages [87]. This finding also suggests that combination therapies reversing the phagocytic activity of macrophages through PD1 inhibitors combined with anti-CD24 or CD47 mAbs blocking the “don’t eat me” signal axis may greatly benefit patients with cancer. Multiple immune checkpoint blocking therapies remain a novel and strategy worthy of exploration. For example, IMM-4701, an inhibitor simultaneously targeting CD24 and CD47, is currently in preclinical stages of application to solid tumors. IMM-2547 simultaneously targets CD24 and PDL1, and is currently in the drug discovery stage (Table 1). ICB-mediated combination therapy will be a future trend in tumor immunotherapy. The introduction of small-molecule drugs, dual antibodies, CART, and other emerging immunotherapy approaches, as well as the launch of multiple immune combination regimens, will bring hope in patient treatment.
Clinical trials targeting the CD24-Siglec10 axis
Although many clinical studies have shown that targeting the CD24-Siglec10 axis for tumor immunotherapy can have good anti-tumor effects, few clinical trials have targeted the CD24-Siglec10 axis to date. In 1991, the first anti-CD24 mAb (ALB9) was used in a clinical trial for the treatment of invasive B lymphoproliferative syndrome [88]. Overall, the clinical trial indicated good tolerability and favorable survival rates. However, treatment efficacy in the central nervous system is very limited, and further follow-up and research have not been conducted. Given the important roles of CD24 in autoimmune diseases, subsequent clinical trials on CD24 targets have focused on anti-inflammatory effects and GvHD, particularly the development and testing of soluble CD24 (CD24-Fc). Several clinical trials examining CD24-Fc in immune diseases including GvHD and multiple sclerosis are in progress (http://www.oncoimmune.com). Recently, AI-071, an agonist targeting Siglecs, passed phase I clinical trials (ACTRN12622000643774). However, AI-071 is focused on infectious diseases or other respiratory disorders/diseases rather than cancer. Interestingly, a new clinical trial (NCT04317040) has shown that CD24-Fc significantly decreases systemic inflammation and restores immune balance in SARS-CoV-2-infected individuals [89]. Additionally, CD24-Fc is being explored for therapy targeting the CD24 immune checkpoint in melanoma or advanced solid tumors (NCT04060407, NCT04552704). However, these two clinical trials have not achieved the expected results: one was terminated, and the other was withdrawn. The most recent (June 2023) recorded clinical trial is currently in recruitment stage (NCT05888701), and is aimed at improving anti-cancer immunity in anti-CD24 mAb treatment of mantle-cell lymphoma and B cell chronic lymphocytic leukemia. Unexpectedly, in May 2023, the phase I clinical trial application (IND) for anti-CD24 mAb (ATG-031) for the treatment of advanced solid tumors and B cell non-Hodgkin’s lymphoma was approved by the FDA. ATG-031 is the first anti-CD24 mAb to enter the clinical development stage in the field of tumor therapy worldwide. Sufficient preclinical data have shown that ATG-031 blocks the CD24-Siglec10 axis in the TME [71]. The specially designed ATG-031 enhances the phagocytosis of tumor cells by macrophages, and promotes the infiltration of CD8+ T cells in the TME via blocking the interaction between CD24 and Siglec10. Clinical trials of agents targeting the CD24-Siglec10 axis are summarized in Table 2.
Table 2 Clinical Trials Targeting the CD24-Siglec10 Axis
| Trial Number | Condition or Disease | Agent | Study Type | Start Date | End Date | Phase | Status |
|---|---|---|---|---|---|---|---|
| NCT04060407 | Metastatic melanoma | CD24 Fc, lpilimumab, nivolumab | Interventional | June 15, 2021 | December 30, 2023 | I/II | Withdrawn |
| NCT04552704 | Advanced malignant solid neoplasm | CD24 Fc | Interventional | October 30, 2020 | January 26, 2022 | I/II | Terminated |
| NCT05888701 | Mantle-cell lymphoma, B cell chronic lymphocytic leukemia | Anti-CD24, anti-CD47, anti-CD20 | Observational | September 8, 2022 | Dec-24 | Pre-clinical | Recruiting |
| NCT04747574 | SARS-CoV-2 | EXO-CD24 | Interventional | September 25, 2020 | March 25, 2021 | I | Unknown |
| NCT04969172 | COVID-19 | EXO-CD24 | Interventional | July 11, 2021 | July 11, 2022 | II | Active, not recruiting |
| NCT03960541 | HIV infections, dyslipidemias | Efprezimod alfa | Interventional | August 31, 2020 | May 27, 2021 | II | Terminated |
| NCT02663622 | GvHD, hematopoietic stem cell transplantation, leukemia | Efprezimod alfa, methotrexate, tacrolimus, placebo | Interventional | September 19, 2016 | May 18, 2021 | II | Completed |
| NCT04317040 | COVID-19 | Efprezimod alfa, placebo | Interventional | April 24, 2020 | October 20, 2020 | III | Completed |
Nanoparticle-mediated targeting of the CD24-Siglec10 axis
Treatments targeting the cancer immune system are now a clinical reality and have achieved substantial success. The most notable examples are ICB and CART therapy. However, the development of new immunotherapies or combination therapies to improve patient treatment benefits continues to encounter major challenges. The adverse effects triggered by “off-target” effects after systemic administration remain hurdles in cancer immunotherapy. Targeted delivery of tumor immunotherapeutic drugs may be a potential solution, which not only maximizes treatment effectiveness but also minimizes adverse effects. In the past two decades, NP-mediated drug delivery platforms have been increasingly used for cancer immunotherapy. These platforms can (i) improve drug stability (ii) improve the enrichment of drugs at tumor sites, and (iii) decrease “off target” effects and toxic adverse effects. Because of these advantages, many NPs have been designed and developed to provide multiple types of therapeutic drugs for tumor immunotherapy. Regarding the strategy of targeting the CD24-Siglec10 axis by using NPs for cancer immunotherapy, preclinical studies are currently focused on the CD24 target rather than Siglec10.
NP-mediated delivery of monoclonal antibodies (for targeting) for cancer therapy
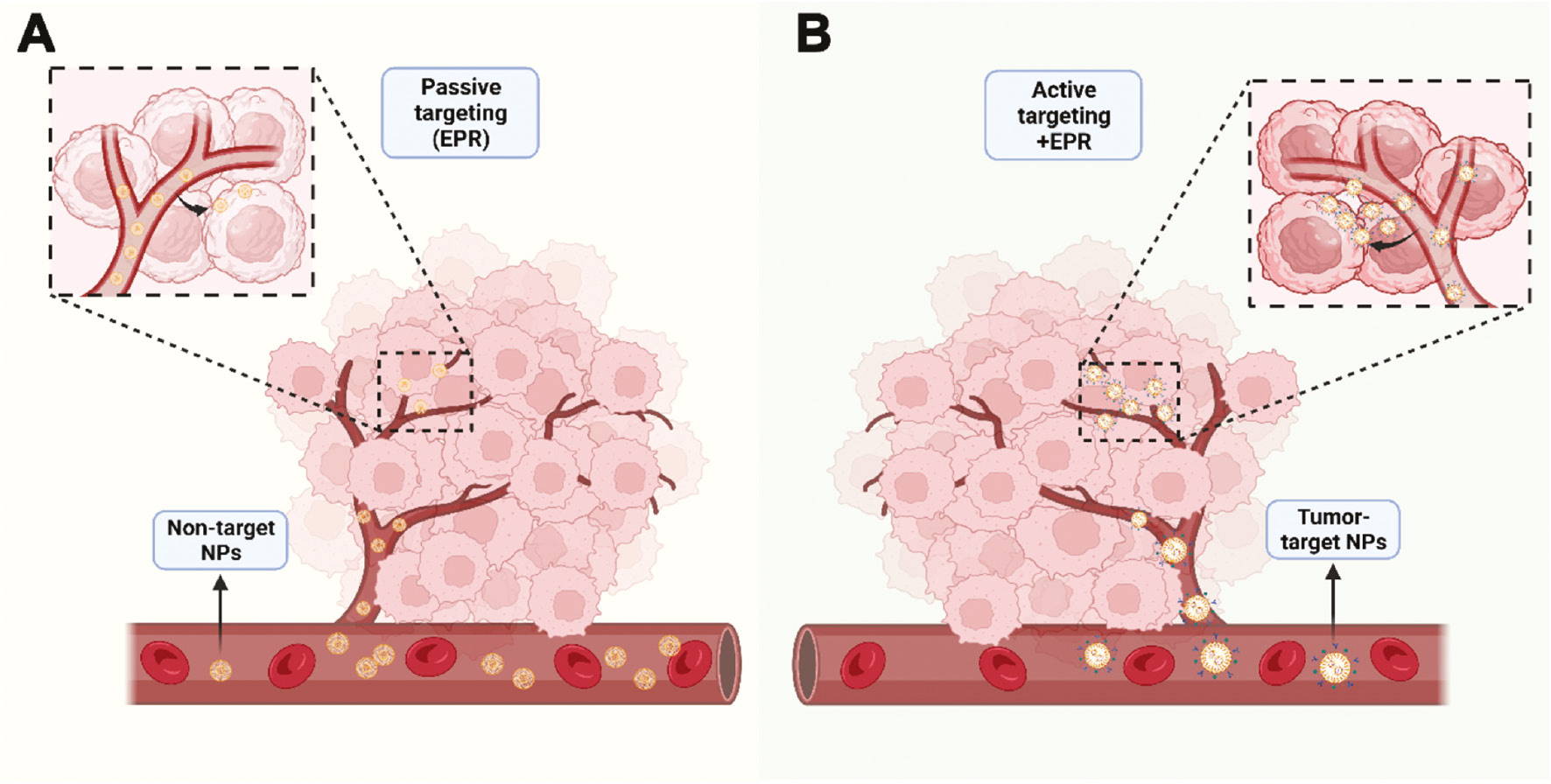
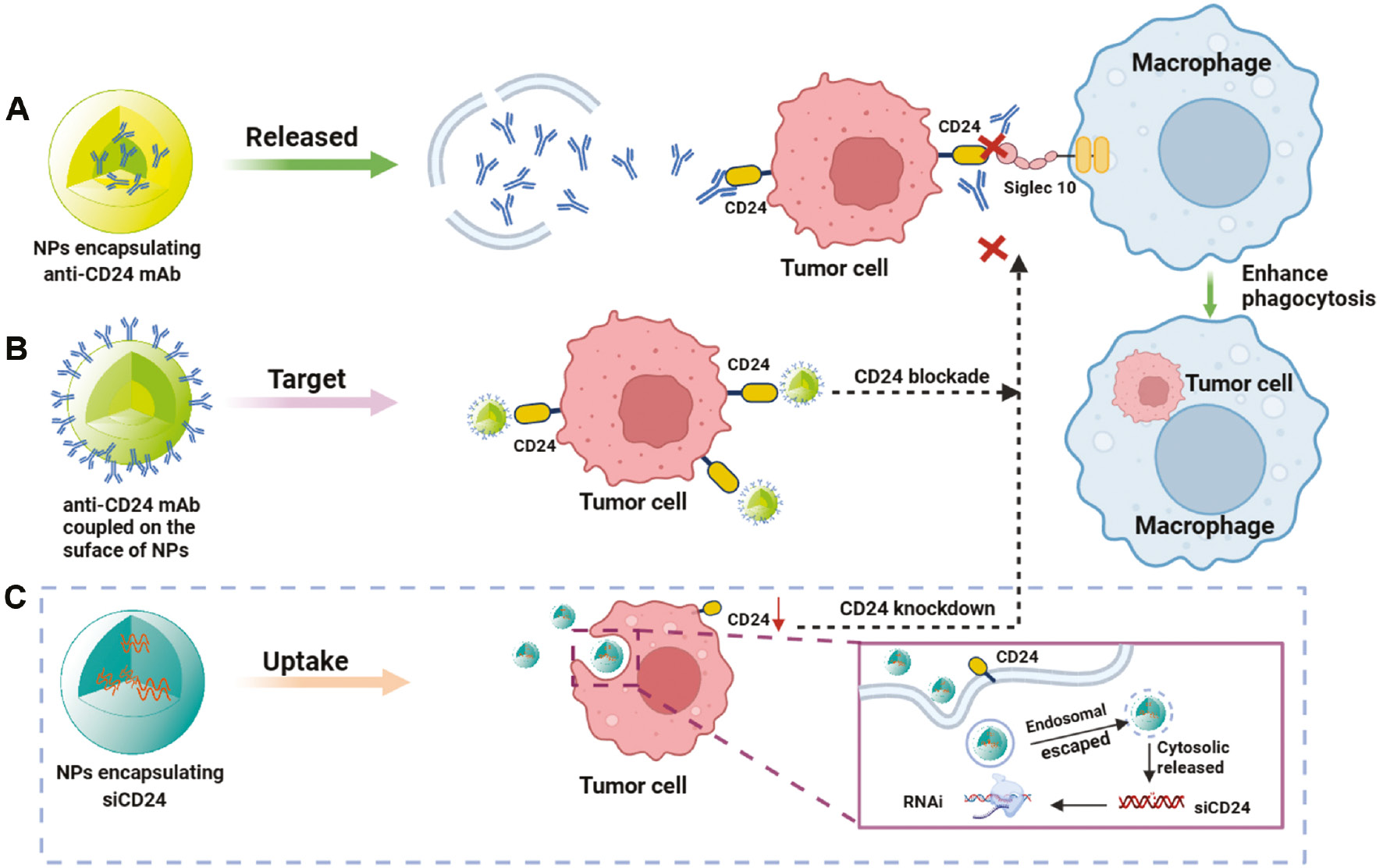
NP-mediated drug delivery can facilitate long-term drug retention in the bloodstream, primarily through the enhanced permeability and retention effect of accumulation in vascularly affected and leaky pathological sites (infarction sites, inflammation, and tumors) [90]. Among the various approaches to specifically target drug carrier systems to desired lesion sites in vivo, active and passive targeting are the two most widely studied and effective approaches (Figure 4). However, the ability to design and control passive targeting are relatively low. Relying on the attachment of specific ligands to the surface of nanocarriers to recognize and bind diseased cells is more promising than the passive accumulation of NPs at lesion sites with damaged blood vessels. Research has focused on using active targeting molecules to improve tumor specificity [91]. Active targeting involves chemically or biologically modifying the surfaces of NPs to specifically bind receptors or other cytokines that are highly expressed in target organs or tumor tissues [92]. Indeed, adding targeted components (such as antibodies) and/or imaging probes (such as fluorescent dyes) to these NPs further enhances the accumulation of drugs at tumor sites, while enabling real-time monitoring of biological distribution. As a membrane protein highly expressed in numerous types of cancers, CD24 is an excellent choice for targeted modification of nanocarriers (Figure 5A). A previous study has synthesized a delivery vehicle targeting CD24 antibody while carrying docetaxel for prostate cancer treatment in mice; the group treated with docetaxel-loaded PLGA-PEG NPs conjugated with anti-CD24 showed 10-fold greater accumulation in prostate tumors than observed in the non-target group [93]. A recent study has constructed a highly accurate targeted therapy NP delivery system for TNBC by coupling CD24 aptamer on the NP surface, thus enabling effective targeting of CD24-high cells in TNBC [94]. Simultaneously, the NP delivery system encapsulates ferroptosis agonists, and inhibits the NF2-YAP signal axis through FSP1 and CD24, thus enhancing ferroptosis and macrophage phagocytosis, and ultimately promoting cell death and inhibiting the growth of TNBC tumors [94]. Preclinical studies on CD24 targeting have provided a solid foundation for future clinical translation, particularly for ADC drugs used in tumor immunotherapy. Currently, the target antigens of approved ADC drugs tend to be specific proteins overexpressed by cancer cells, including targets HER2, Trop2, Nectin4, and EGFR in solid tumors, as well as CD19, CD22, CD33, CD30, BCMA, and CD79b in hematological malignancies [95]. CD24, a “don’t eat me” signal highly expressed in many tumor cells, has great potential for the preparation of CD24-ADC drugs for clinical translation.
Figure 4 Two major modes of NP-mediated drug delivery systems for cancer therapy. A, Passive targeting: non-targeted NPs aggregate at tumor sites through the enhanced permeability and retention effect. B, Active targeting: NPs with targeted components and/or imaging probes further enhance the accumulation of drugs at tumor sites.
Figure 5 Main strategy in NP-mediated CD24-Siglec10 axis targeted therapy. A. Encapsulation of anti-CD24 mAb in NPs for CD24-Siglec10 axis blockade. B. Biomimetic NPs with surface-modified anti-CD24 mAbs for CD24-Siglec10 axis blockade. C. Integration of siCD24 in NPs to knock down CD24 to block the CD24-Siglec10 axis.
NP-mediated delivery of monoclonal antibodies (for blockade) in cancer therapy
The use of mAbs is an important component of tumor targeted therapy. mAbs enhance complement activity and innate immune effector functions primarily through binding the Fc receptor on target cells, and serve as targeted therapeutic options for transplant rejection, malignancies, autoimmune and infectious diseases, and a range of new indications. However, the use of mAbs poses a risk of immune reactions, such as serum sickness, acute allergic reactions, and the development of anti-antibodies [96]. Because of the instability of mAbs in vivo, efficient and highly stable drug delivery is another challenge. To overcome these limitations, and achieve more efficient in vivo delivery of antibodies, NP-mediated delivery systems for carrying biopharmaceuticals have been developed [97–99]. Regarding NP-mediated delivery of CD24 mAbs, some preclinical studies and potential strategies have been examined in recent years and achieved good results (Figure 5B). Ming’s team has designed engineered NPs (P-aCD24/CEL+P/shMFN1) to deliver anti-CD24 mAb (aCD24) for synergistic cancer cell-targeted therapy and TAM-targeted immunomodulation [100]. On the basis of the dual response of the vector to pH and MMP2 in the TME, P-aCD24/CEL achieves release of aCD24, reactivates macrophage phagocytosis of tumor cells, and ultimately improves macrophage-based immunotherapy [100]. This combined immunotherapy strategy shows great potential in the treatment of TNBC. Recently, a novel study has successfully coupled purified high-concentration CD24 antibody to nanospheres (nanosphere anti-CD24) through cross-linking; these particles can be selectively internalized by HCC cells with high expression of CD24; subsequently, the CD24 protein on the cell membrane is transported to lysosomes for degradation. The degradation of CD24 weakens macrophage immune suppression, as regulated by the CD24-Siglec10 axis signaling pathway. This novel platform not only inhibits tumor growth in xenograft mouse models but also has no detectable toxicity to normal tissues [69]. Interestingly, a recent study has indicated that substantia nigra lectin, a sialic acid binding lectin, blocks the terminal sialic acid of CD24, thereby inhibiting the interaction between CD24 and Siglec10. Simultaneously, introduction of lectin coupled with photothermal NPs to block CD24 significantly enhances tumor cell phagocytosis by macrophages [101].
Notably, in recent years, exosomes have attracted attention as a natural NP drug delivery carrier, which can be used to deliver nucleic acids, proteins, and small-molecule drugs [102]. The combination of the CD24 target and exosomes for disease treatment is highly innovative. EXO-CD24, comprising CD24 protein bound to extracellular vesicles, regulates cytokine storms by delivering CD24 drugs in vivo through the secretion of vesicles. EXO-CD24 was originally a targeted anti-cancer drug but is currently used primarily in patients with severe pneumonia (e.g., in COVID-19) and has shown substantial clinical effects [103, 104]. Approved clinical trials are currently underway (Table 2). However, even if EXO-CD24 has good effects against severe infection and infection-associated chronic fibrosis, its adverse effects in patients with tumor or autoimmunity disease must be evaluated. The risks and benefits should be comprehensively considered through case-summary analysis. Simultaneously, we expect EXO-CD24 to play a critical role in tumor immunotherapy.
NP-mediated delivery of nucleic acid drugs (siRNA) for cancer therapy
Current nucleic acid drugs primarily include siRNA, miRNA, mRNA, and DNA. In the past decade, nucleic acid drugs have shown great potential in tumor immunotherapy, owing to their ability to specifically target genes [105–107]. Because naked siRNAs are readily degraded by nucleases and are easily captured by the reticuloendothelial system in the bloodstream, they tend not to bind target sequences effectively [108]. Determining how to safely and effectively deliver therapeutic siRNA to target cells will be key to the successful use of siRNA nucleic acid drugs for disease treatment. A very promising siRNA delivery strategy uses NP-mediated delivery systems to protect siRNA from degradation and promote intracellular uptake. For example, a previous study has indicated that dual silencing with co-delivered CD47 siRNA and PD-L1 siRNA via EpCAM-targeted cationic liposomes is a platform with low toxicity and significantly enhanced in anti-tumor activity [109]. Zhang’s team has delivered both CD47 siRNA and mitoxantrone hydrochloride via PLGA NPs [110]. The CD47 gene was silenced to suppress “self” signaling, whereas MTO induced calreticulin surface exposure to provide an “eat-me” signal. This strategy has been found to synergistically increase phagocytosis of tumor cells by macrophages and significantly increase antitumor activity in two animal models of aggressive tumors: melanoma and colon cancer [110]. Similarly, a recent study has encapsulated R848 and siCD47 in amphiphilic PEG-PLGA NPs to safely and effectively deliver siCD47 and R848 to tumor tissues; this treatment (CD47 blockade) activated dendritic cells to kill effector T-cells, enhanced antigen presentation, regulated the TME, and ultimately resulted in anti-tumor immunotherapy [111]. NPs are critical delivery vehicles for the systemic delivery of relevant immune checkpoint small-nucleic-acid drugs (e.g., siRNA). However, more preclinical studies on the CD24-Siglec10 axis have focused on the delivery of the CD24 or Siglec10 siRNA by lentivirus or plasmids transfection to achieve knockdown [6, 74, 76, 112–114], whereas the use of novel nanocarriers for CD24 or Siglec10 siRNA delivery has not been extensively reported. With newer iterations of nanomaterials, we believe that NP-mediated CD24 nucleic acid drug therapy will be a promising delivery strategy in the future (Figure 5C).
Conclusion and outlook
On the basis of numerous preclinical and clinical studies, the CD24-Siglec10 axis has attracted substantial attention as a common marker of cancer or cancer stem cells. However, the signaling mechanisms upstream and downstream of the CD24-Siglec10 axis are not fully understood. Studies are necessary to investigate the structure and function of the CD24-Siglec10 axis, and to elucidate its roles in different tumor signaling pathways, anti-tumor immunity, and the TME. Given that the CD24-Siglec10 signaling axis enables cancer cells to avoid macrophage-mediated phagocytosis, targeting the CD24-Siglec10 axis for cancer immunotherapy may be a very promising therapeutic strategy. Multiple CD24-targeting drugs have entered clinical trials, and targeting the CD24-Siglec10 axis is likely to be the most promising ICB therapy after PD1/PDL1 and CD47. However, this therapeutic approach is associated with varying degrees of adverse effects and biosafety issues, such as “off-target” effects. The rapid development of nanomedicine has opened new pathways for cancer immunotherapy. NP-mediated drug delivery systems are a powerful and beneficial tool to optimize the delivery of anti-CD24 drugs. Despite progress in targeted delivery of CD24 drugs, several challenges—such as the toxicity of the NPs themselves, and the controlled and reproducible synthesis of nanomedicines—must be addressed to accelerate clinical translation. In conclusion, we believe that, through the continuing research efforts, antitumor therapeutic research targeting the CD24-Siglec10 axis will become the most promising research area in future cancer immunotherapy.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by the Guangzhou Bureau OF Basic Science Grant 202201020576, 111 project (No. B20062), National Natural Science Foundation of China (82001822), Guangzhou Municipal Science and Technology Bureau (201704020131), Guangdong Provincial Fund for Distinguished Young Scholars (2021B1515020066), and “Three Million for Three Years” Project of the High-level Talent Special Funding Scheme of Sun Yat-Sen Memorial Hospital (132090023).
References
- Horning SJ. A new cancer ecosystem. Science (New York, N.Y.) 2017;355(6330):1103. [PMID: 28302798 DOI: 10.1126/science.aan1295]
- Bejarano L, Jordāo MJC, Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Discov 2021;11(4):933-59. [PMID: 33811125 DOI: 10.1158/2159-8290.CD-20-1808]
- Goliwas KF, Deshane JS, Elmets CA, Athar M. Moving immune therapy forward targeting TME. Physiol Rev 2021;101(2):417-25. [PMID: 32790578 DOI: 10.1152/physrev.00008.2020]
- Christofides A, Strauss L, Yeo A, Cao C, Charest A, Boussiotis VA. The complex role of tumor-infiltrating macrophages. Nat Immunol 2022;23(8):1148-56. [PMID: 35879449 DOI: 10.1038/s41590-022-01267-2]
- Barclay AN, Van den Berg TK. The interaction between signal regulatory protein alpha (SIRPα) and CD47: structure, function, and therapeutic target. Annu Rev Immunol 2014;32:25-50. [PMID: 24215318 DOI: 10.1146/annurev-immunol-032713-120142]
- Barkal AA, Brewer RE, Markovic M, Kowarsky M, Barkal SA, et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 2019;572(7769):392-6. [PMID: 31367043 DOI: 10.1038/s41586-019-1456-0]
- Wenger RH, Ayane M, Bose R, Köhler G, Nielsen PJ. The genes for a mouse hematopoietic differentiation marker called the heat-stable antigen. Eur J Immunol 1991;21(4):1039-46. [PMID: 2019286 DOI: 10.1002/eji.1830210427]
- Ozawa Y, Harutani Y, Oyanagi J, Akamatsu H, Murakami E, et al. CD24, not CD47, negatively impacts upon response to PD-1/L1 inhibitors in non-small-cell lung cancer with PD-L1 tumor proportion score < 50. Cancer Sci 2021;112(1):72-80. [PMID: 33084148 DOI: 10.1111/cas.14705]
- Hwang WC, Song D, Lee H, Oh C, Lim SH, et al. Inhibition of phospholipase D1 induces immunogenic cell death and potentiates cancer immunotherapy in colorectal cancer. Exp Mol Med 2022;54(9):1563-76. [PMID: 36131027 DOI: 10.1038/s12276-022-00853-6]
- Ni YH, Zhao X, Wang W. CD24, a review of its role in tumor diagnosis, progression and therapy. Curr Gene Ther 2020;20(2):109-26. [PMID: 32576128 DOI: 10.2174/1566523220666200623170738]
- Panagiotou E, Syrigos NK, Charpidou A, Kotteas E, Vathiotis IA. CD24: a novel target for cancer immunotherapy. J Pers Med 2022;12(8):1235. [PMID: 36013184 DOI: 10.3390/jpm12081235]
- Wu X, Srinivasan P, Basu M, Zimmerman T, Li S, et al. CD24-Fc suppression of immune related adverse events in a therapeutic cancer vaccine model of murine neuroblastoma. Front Immunol 2023;14:1176370. [PMID: 37346042 DOI: 10.3389/fimmu.2023.1176370]
- Han X, Li H, Zhou D, Chen Z, Gu Z. Local and targeted delivery of immune checkpoint blockade therapeutics. Acc Chem Res 2020;53(11):2521-33. [PMID: 33073988 DOI: 10.1021/acs.accounts.0c00339]
- Jin Q, Zhu W, Zhu J, Zhu J, Shen J, et al. Nanoparticle-mediated delivery of inhaled immunotherapeutics for treating lung metastasis. Adv Mater (Deerfield Beach, Fla.) 2021;33(7):e2007557. [PMID: 33448035 DOI: 10.1002/adma.202007557]
- Meir R, Shamalov K, Sadan T, Motiei M, Yaari G, et al. Fast image-guided stratification using anti-programmed death ligand 1 gold nanoparticles for cancer immunotherapy. ACS nano 2017;11(11):11127-34. [PMID: 29028305 DOI: 10.1021/acsnano.7b05299]
- Choi B, Jung H, Yu B, Choi H, Lee J, et al. Sequential MR image-guided local immune checkpoint blockade cancer immunotherapy using ferumoxytol capped ultralarge pore mesoporous silica carriers after standard chemotherapy. Small (Weinheim an der Bergstrasse, Germany) 2019;15(52):e1904378. [PMID: 31697036 DOI: 10.1002/smll.201904378]
- Springer T, Galfrè G, Secher DS, Milstein C. Monoclonal xenogeneic antibodies to murine cell surface antigens: identification of novel leukocyte differentiation antigens. Eur J Immunol 1978;8(8):539-51. [PMID: 81133 DOI: 10.1002/eji.1830080802]
- Kay R, Rosten PM, Humphries RK. CD24, a signal transducer modulating B cell activation responses, is a very short peptide with a glycosyl phosphatidylinositol membrane anchor. J Immunol 1991;147(4):1412-6. [PMID: 1831224 DOI: 10.4049/jimmunol.147.4.1412]
- Kay R, Takei F, Humphries RK. Expression cloning of a cDNA encoding M1/69-J11d heat-stable antigens. J Immunol 1990;145(6):1952-9. [PMID: 2118158 DOI: 10.4049/jimmunol.145.6.1952]
- Lim SC. CD24 and human carcinoma: tumor biological aspects. Biomed Pharmacother 2005;59 Suppl 2:S351-4. [PMID: 16507407 DOI: 10.1016/s0753-3322(05)80076-9]
- Bleckmann C, Geyer H, Reinhold V, Lieberoth A, Schachner M, et al. Glycomic analysis of N-linked carbohydrate epitopes from CD24 of mouse brain. J Proteome Res 2009;8(2):567-82. [PMID: 19053835 DOI: 10.1021/pr800729r]
- Tan Y, Zhao M, Xiang B, Chang C, Lu Q. CD24: from a hematopoietic differentiation antigen to a genetic risk factor for multiple autoimmune diseases. Clin Rev Allergy Immunol 2016;50(1):70-83. [PMID: 25666875 DOI: 10.1007/s12016-015-8470-2]
- Bretz NP, Salnikov AV, Perne C, Keller S, Wang X, et al. CD24 controls Src/STAT3 activity in human tumors. Cell Mol Life Sci 2012;69(22):3863-79. [PMID: 22760497 DOI: 10.1007/s00018-012-1055-9]
- Ahmad F, Dina K, Faina B, Eli B, Chen V. CD24 induces the activation of β-catenin in intestinal tumorigenesis. J Cancer Sci Ther 2016;08(5):135-42. [DOI: 10.4172/1948-5956.1000405]
- Su N, Peng L, Xia B, Zhao Y, Xu A, et al. Lyn is involved in CD24-induced ERK1/2 activation in colorectal cancer. Mol Cancer 2012;11:43. [PMID: 22731636 DOI: 10.1186/1476-4598-11-43]
- Ghuwalewala S, Ghatak D, Das S, Roy S, Das P, et al. MiRNA-146a/AKT/β-catenin activation regulates cancer stem cell phenotype in oral squamous cell carcinoma by targeting CD24. Front Oncol 2021;11:651692. [PMID: 34712602 DOI: 10.3389/fonc.2021.651692]
- Wei X, Zhao L, Ren R, Ji F, Xue S, et al. MiR-125b loss activated HIF1α/pAKT loop, leading to transarterial chemoembolization resistance in hepatocellular carcinoma. Hepatology (Baltimore, Md.) 2021;73(4):1381-98. [PMID: 32609900 DOI: 10.1002/hep.31448]
- Muppala S, Mudduluru G, Leupold JH, Buergy D, Sleeman JP, et al. CD24 induces expression of the oncomir miR-21 via Src, and CD24 and Src are both post-transcriptionally downregulated by the tumor suppressor miR-34a. PLoS One 2013;8(3):e59563. [PMID: 23533633 DOI: 10.1371/journal.pone.0059563]
- Wang TW, Chern E, Hsu CW, Tseng KC, Chao HM. SIRT1-mediated expression of CD24 and epigenetic suppression of novel tumor suppressor miR-1185-1 increases colorectal cancer stemness. Cancer Res 2020;80(23):5257-69. [PMID: 33046442 DOI: 10.1158/0008-5472.CAN-19-3188]
- Zhang P, Ha M, Li L, Huang X, Liu C. MicroRNA-3064-5p sponged by MALAT1 suppresses angiogenesis in human hepatocellular carcinoma by targeting the FOXA1/CD24/Src pathway. FASEB J 2020;34(1):66-81. [PMID: 31914639 DOI: 10.1096/fj.201901834R]
- Munday J, Kerr S, Ni J, Cornish AL, Zhang JQ, et al. Identification, characterization and leucocyte expression of Siglec-10, a novel human sialic acid-binding receptor. Biochem J 2001;355(Pt 2):489-97. [PMID: 11284738 DOI: 10.1042/0264-6021:3550489]
- Escalona Z, Álvarez B, Uenishi H, Toki D, Yuste M, et al. Molecular characterization of porcine Siglec-10 and analysis of its expression in blood and tissues. Dev Comp Immunol 2015;48(1):116-23. [PMID: 25280627 DOI: 10.1016/j.dci.2014.09.011]
- Pao LI, Lam KP, Henderson JM, Kutok JL, Alimzhanov M, et al. B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity 2007;27(1):35-48. [PMID: 17600736 DOI: 10.1016/j.immuni.2007.04.016]
- Shathili AM, Bandala-Sanchez E, John A, Goddard-Borger ED, Thaysen-Andersen M, et al. Specific sialoforms required for the immune suppressive activity of human soluble CD52. Front Immunol 2019;10:1967. [PMID: 31507595 DOI: 10.3389/fimmu.2019.01967]
- Ju B, Wang J, Mo L, Huang J, Hao Z, et al. Elevated CD19(+)Siglec-10(+) B cell levels are correlated with systemic lupus erythematosus disease activity. Int Immunopharmacol 2022;102:108403. [PMID: 34857478 DOI: 10.1016/j.intimp.2021.108403]
- Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science (New York, N.Y.) 2009;323(5922):1722-5. [PMID: 19264983 DOI: 10.1126/science.1168988]
- Williams LA, Hock BD, Hart DN. Human T lymphocytes and hematopoietic cell lines express CD24-associated carbohydrate epitopes in the absence of CD24 mRNA or protein. Blood 1996;88(8):3048-55. [PMID: 8874203 DOI: 10.1182/blood.V88.8.3048.bloodjournal8883048]
- De Bruijn ML, Peterson PA, Jackson MR. Induction of heat-stable antigen expression by phagocytosis is involved in in vitro activation of unprimed CTL by macrophages. J Immunol (Baltimore, Md: 1950) 1996;156(8):2686-92. [PMID: 8609384 DOI: 10.4049/jimmunol.156.8.2686]
- Raife TJ, Lager DJ, Kemp JD, Dick FR. Expression of CD24 (BA-1) predicts monocytic lineage in acute myeloid leukemia. Am J Clin Pathol 1994;101(3):296-9. [PMID: 7510927 DOI: 10.1093/ajcp/101.3.296]
- Redondo P, García-Foncillas J, Okroujnov I, de Felipe I, Quintanilla E. CD24 expression on human keratinocytes. Exp Dermatol 1998;7(4):175-8. [PMID: 9758414 DOI: 10.1111/j.1600-0625.1998.tb00320.x]
- Chen GY, Brown NK, Zheng P, Liu Y. Siglec-G/10 in self-nonself discrimination of innate and adaptive immunity. Glycobiology 2014;24(9):800-6. [PMID: 24996822 DOI: 10.1093/glycob/cwu068]
- Schabath H, Runz S, Joumaa S, Altevogt P. CD24 affects CXCR4 function in pre-B lymphocytes and breast carcinoma cells. J Cell Sci 2006;119(Pt 2):314-25. [PMID: 16390867 DOI: 10.1242/jcs.02741]
- Davidson B. CD24 is highly useful in differentiating high-grade serous carcinoma from benign and malignant mesothelial cells. Hum Pathol 2016;58:123-7. [PMID: 27589896 DOI: 10.1016/j.humpath.2016.08.005]
- Jia ZF, Wang LZ, Cao XY, Wang C, Cao DH, et al. CD24 genetic variants contribute to overall survival in patients with gastric cancer. World J Gastroenterol 2016;22(7):2373-82. [PMID: 26900300 DOI: 10.3748/wjg.v22.i7.2373]
- Weichert W, Denkert C, Burkhardt M, Gansukh T, Bellach J, et al. Cytoplasmic CD24 expression in colorectal cancer independently correlates with shortened patient survival. Clin Cancer Res 2005;11(18):6574-81. [PMID: 16166435 DOI: 10.1158/1078-0432.CCR-05-0606]
- Kim M, Min YK, Jang J, Park H, Lee S, et al. Single-cell RNA sequencing reveals distinct cellular factors for response to immunotherapy targeting CD73 and PD-1 in colorectal cancer. J Immunother Cancer 2021;9(7):e002503. [PMID: 34253638 DOI: 10.1136/jitc-2021-002503]
- Myung JH, Gajjar KA, Pearson RM, Launiere CA, Eddington DT, et al. Direct measurements on CD24-mediated rolling of human breast cancer MCF-7 cells on E-selectin. Anal Chem 2011;83(3):1078-83. [PMID: 21207944 DOI: 10.1021/ac102901e]
- Aigner S, Ramos CL, Hafezi-Moghadam A, Lawrence MB, Friederichs J, et al. CD24 mediates rolling of breast carcinoma cells on P-selectin. FASEB J 1998;12(12):1241-51. [PMID: 9737727 DOI: 10.1096/fasebj.12.12.1241]
- Carroll MJ, Fogg KC, Patel HA, Krause HB, Mancha AS, et al. Alternatively-activated macrophages upregulate mesothelial expression of P-selectin to enhance adhesion of ovarian cancer cells. Cancer Res 2018;78(13):3560-73. [PMID: 29739756 DOI: 10.1158/0008-5472.CAN-17-3341]
- Baumann P, Cremers N, Kroese F, Orend G, Chiquet-Ehrismann R, et al. CD24 expression causes the acquisition of multiple cellular properties associated with tumor growth and metastasis. Cancer Res 2005;65(23):10783-93. [PMID: 16322224 DOI: 10.1158/0008-5472.CAN-05-0619]
- Wang W, Wang X, Peng L, Deng Q, Liang Y, et al. CD24-dependent MAPK pathway activation is required for colorectal cancer cell proliferation. Cancer Sci 2010;101(1):112-9. [PMID: 19860845 DOI: 10.1111/j.1349-7006.2009.01370.x]
- Chen Z, Wang T, Tu X, Xie W, He H, et al. Antibody-based targeting of CD24 enhances antitumor effect of cetuximab via attenuating phosphorylation of Src/STAT3. Biomed Pharmacother 2017;90:427-36. [PMID: 28391164 DOI: 10.1016/j.biopha.2017.03.094]
- Altevogt P, Sammar M, Hüser L, Kristiansen G. Novel insights into the function of CD24: a driving force in cancer. Int J Cancer 2021;148(3):546-59. [PMID: 32790899 DOI: 10.1002/ijc.33249]
- Guo Y, Ke S, Xie F, Chen J, Liu X, et al. SIGLEC10(+) macrophages drive gastric cancer progression by suppressing CD8(+) T cell function. Cancer Immunol Immunother 2023;72(10):3229-42. [PMID: 37432407 DOI: 10.1007/s00262-023-03488-2]
- Yin SS, Gao FH. Molecular mechanism of tumor cell immune escape mediated by CD24/Siglec-10. Front Immunol 2020;11:1324. [PMID: 32765491 DOI: 10.3389/fimmu.2020.01324]
- Zhang P, Lu X, Tao K, Shi L, Li W, et al. Siglec-10 is associated with survival and natural killer cell dysfunction in hepatocellular carcinoma. J Surg Res 2015;194(1):107-13. [PMID: 25450598 DOI: 10.1016/j.jss.2014.09.035]
- Toubai T, Hou G, Mathewson N, Liu C, Wang Y, et al. Siglec-G-CD24 axis controls the severity of graft-versus-host disease in mice. Blood 2014;123(22):3512-23. [PMID: 24695850 DOI: 10.1182/blood-2013-12-545335]
- Chen GY, Chen X, King S, Cavassani KA, Cheng J, et al. Amelioration of sepsis by inhibiting sialidase-mediated disruption of the CD24-SiglecG interaction. Nat Biotechnol 2011;29(5):428-35. [PMID: 21478876 DOI: 10.1038/nbt.1846]
- Li D, Zheng L, Jin L, Zhou Y, Li H, et al. CD24 polymorphisms affect risk and progression of chronic hepatitis B virus infection. Hepatology 2009;50(3):735-42. [PMID: 19610054 DOI: 10.1002/hep.23047]
- Li Y, Zhou J, Zhuo Q, Zhang J, Xie J, et al. Malignant ascite-derived extracellular vesicles inhibit T cell activity by upregulating Siglec-10 expression. Cancer Manag Res 2019;11:7123-34. [PMID: 31534365 DOI: 10.2147/CMAR.S210568]
- Suzuki T, Kiyokawa N, Taguchi T, Sekino T, Katagiri YU, et al. CD24 induces apoptosis in human B cells via the glycolipid-enriched membrane domains/rafts-mediated signaling system. J Immunol 2001;166(9):5567-77. [PMID: 11313396 DOI: 10.4049/jimmunol.166.9.5567]
- Overdevest JB, Thomas S, Kristiansen G, Hansel DE, Smith SC, et al. CD24 offers a therapeutic target for control of bladder cancer metastasis based on a requirement for lung colonization. Cancer Res 2011;71(11):3802-11. [PMID: 21482678 DOI: 10.1158/0008-5472.CAN-11-0519]
- Chan SH, Tsai KW, Chiu SY, Kuo WH, Chen HY, et al. Identification of the novel role of CD24 as an oncogenesis regulator and therapeutic target for triple-negative breast cancer. Mol Cancer Ther 2019;18(1):147-61. [PMID: 30381446 DOI: 10.1158/1535-7163.MCT-18-0292]
- Salnikov AV, Bretz NP, Perne C, Hazin J, Keller S, et al. Antibody targeting of CD24 efficiently retards growth and influences cytokine milieu in experimental carcinomas. Br J Cancer 2013;108(7):1449-59. [PMID: 23511563 DOI: 10.1038/bjc.2013.102]
- Zou KL, Lan Z, Cui H, Zhao YY, Wang WM, et al. CD24 blockade promotes anti-tumor immunity in oral squamous cell carcinoma. Oral Dis 2022. [PMID: 36056698 DOI: 10.1111/odi.14367]
- Klapdor R, Wang S, Morgan M, Dörk T, Hacker U, et al. Characterization of a novel third-generation anti-CD24-CAR against ovarian cancer. Int J Mol Sci 2009;20(3):660. [PMID: 30717444 DOI: 10.3390/ijms20030660]
- Sun F, Wang Y, Luo X, Ma Z, Xu Y, et al. Anti-CD24 antibody-nitric oxide conjugate selectively and potently suppresses hepatic carcinoma. Cancer Res 2019;79(13):3395-405. [PMID: 30918001 DOI: 10.1158/0008-5472.CAN-18-2839]
- Lu RM, Hwang YC, Liu IJ, Lee CC, Tsai HZ, et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci 2020;27(1):1. [PMID: 31894001 DOI: 10.1186/s12929-019-0592-z]
- Wang K, Yu A, Liu K, Feng C, Hou Y, et al. Nano-LYTACs for degradation of membrane proteins and inhibition of CD24/Siglec-10 signaling pathway. Adv Sci 2023;10(13):e2300288. [PMID: 36866919 DOI: 10.1002/advs.202300288 ]
- Xiao N, Zhu X, Li K, Chen Y, Liu X, et al. Blocking siglec-10(hi) tumor-associated macrophages improves anti-tumor immunity and enhances immunotherapy for hepatocellular carcinoma. Exp Hematol Oncol 2021;10(1):36. [PMID: 34112250 DOI: 10.1186/s40164-021-00230-5]
- Chen P, Deng M, Liu Y, Luo J, Guo R, et al. 482 ATG-031, a first-in-class anti-CD24 antibody, showed potent preclinical anti-tumor efficacy by blocking “don’t-eat-me” signal. BMJ 2022;10(Suppl 2):A503. [DOI: 10.1136/jitc-2022-sitc2022.0482]
- Li S, Chen D, Guo H, Yang Y, Liu D, et al. IMM47, a humanized monoclonal antibody that targets CD24, exhibits exceptional anti-tumor efficacy by blocking the CD24/Siglec-10 interaction and can be used as monotherapy or in combination with anti-PD1 antibodies for cancer immunotherapy. Antib Ther 2023;6(4):240-52. [PMID: 37846296 DOI: 10.1093/abt/tbad020]
- Wang T, Sun F, Wang Y, Jiang J, Pan M, et al. NKG2D immunoligand rG7S-MICA enhances NK cell-mediated immunosurveillance in colorectal carcinoma. J Immunother 2018;41(3):109-17. [PMID: 29528990 DOI: 10.1097/CJI.0000000000000215]
- Ma ZL, Chen YP, Song JL, Wang YQ. Knockdown of CD24 inhibits proliferation, invasion and sensitizes breast cancer MCF-7 cells to tamoxifen in vitro. Eur Rev Med Pharmacol Sci 2015;19(13):2394-9.
- Su D, Deng H, Zhao X, Zhang X, Chen L, et al. Targeting CD24 for treatment of ovarian cancer by short hairpin RNA. Cytotherapy 2009;11(5):642-52. [PMID: 19593703 DOI: 10.1080/14653240902878308]
- Okabe H, Aoki K, Yogosawa S, Saito M, Marumo K, et al. Downregulation of CD24 suppresses bone metastasis of lung cancer. Cancer Sci 2018;109(1):112-20. [PMID: 29095550 DOI: 10.1111/cas.13435]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12(4):252-64. [PMID: 22437870 DOI: 10.1038/nrc3239]
- Li W, Wang F, Guo R, Bian Z, Song Y. Targeting macrophages in hematological malignancies: recent advances and future directions. J Hematol Oncol 2022;15(1):110. [PMID: 35978372 DOI: 10.1186/s13045-022-01328-x]
- Nanamori H, Sawada Y. Epigenetic modification of PD-1/PD-L1-mediated cancer immunotherapy against melanoma. Int J Mol Sci 2022;23(3):119. [PMID: 35163049 DOI: 10.3390/ijms23031119]
- Cader FZ, Hu X, Goh WL, Wienand K, Ouyang J, et al. A peripheral immune signature of responsiveness to PD-1 blockade in patients with classical Hodgkin lymphoma. Nat Med 2020;26(9):1468-79. [PMID: 32778827 DOI: 10.1038/s41591-020-1006-1]
- Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet. Oncol 2021;22(2):198-211. [PMID: 33476593 DOI: 10.1016/S1470-2045(20)30641-0]
- Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, et al. Nivolumab plus Cabozantinib versus Sunitinib for advanced renal-cell carcinoma. N Engl J Med 2021;384(9):829-41. [PMID: 33657295 DOI: 10.1056/NEJMoa2026982]
- Pan Y, Lu F, Fei Q, Yu X, Xiong P, et al. Single-cell RNA sequencing reveals compartmental remodeling of tumor-infiltrating immune cells induced by anti-CD47 targeting in pancreatic cancer. J Hematol Oncol 2019;12(1):124. [PMID: 31771616 DOI: 10.1186/s13045-019-0822-6]
- Asrir A, Tardiveau C, Coudert J, Laffont R, Blanchard L, et al. Tumor-associated high endothelial venules mediate lymphocyte entry into tumors and predict response to PD-1 plus CTLA-4 combination immunotherapy. Cancer Cell 2022;40(3):318-34.e9. [PMID: 35120598 DOI: 10.1016/j.ccell.2022.01.002]
- Wu H, Liu J, Wang Z, Yuan W, Chen L. Prospects of antibodies targeting CD47 or CD24 in the treatment of glioblastoma. CNS Neurosci Ther 2021;27(10):1105-17. [PMID: 34363319 DOI: 10.1111/cns.13714]
- Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017;545(7655):495-9. [PMID: 28514441 DOI: 10.1038/nature22396]
- Liu Y, Wang Y, Yang Y, Weng L, Wu Q, et al. Emerging phagocytosis checkpoints in cancer immunotherapy. Signal Transduct Target Ther 2023;8(1):104. [PMID: 36882399 DOI: 10.1038/s41392-023-01365-z]
- Fischer A, Blanche S, Le Bidois J, Bordigoni P, Garnier JL, et al. Anti-B-cell monoclonal antibodies in the treatment of severe B-cell lymphoproliferative syndrome following bone marrow and organ transplantation. N Engl J Med 1991;324(21):1451-6. [PMID: 2023604 DOI: 10.1056/NEJM199105233242102]
- Song NJ, Allen C, Vilgelm AE, Riesenberg BP, Weller KP, et al. Treatment with soluble CD24 attenuates COVID-19-associated systemic immunopathology. J Hematol Oncol 2022;15(1):5. [PMID: 35012610 DOI: 10.1186/s13045-021-01222-y]
- Attia MF, Anton N, Wallyn J, Omran Z, Vandamme TF. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J Pharm Pharmacol 2019;71(8):1185-98. [PMID: 31049986 DOI: 10.1111/jphp.13098]
- Fang RH, Gao W, Zhang L. Targeting drugs to tumours using cell membrane-coated nanoparticles. Nat Rev Clin Oncol 2023;20(1):33-48. [DOI: 10.1038/s41571-022-00699-x]
- Menon I, Zaroudi M, Zhang Y, Aisenbrey E, Hui L. Fabrication of active targeting lipid nanoparticles: challenges and perspectives. Mater Today Adv 2022;16:100299. [DOI: 10.1016/j.mtadv.2022.100299]
- Bharali DJ, Sudha T, Cui H, Mian BM, Mousa SA. Anti-CD24 nano-targeted delivery of docetaxel for the treatment of prostate cancer. Nanomedicine 2017;13(1):263-73. [PMID: 27565690 DOI: 10.1016/j.nano.2016.08.017]
- Hou L, Pu L, Chen Y, Bai Y, Zhou Y, et al. Targeted intervention of NF2-YAP signaling axis in CD24-overexpressing cells contributes to encouraging therapeutic effects in TNBC. ACS Nano 2022;16(4):5807-19. [PMID: 35420780 DOI: 10.1021/acsnano.1c10921]
- Fu Z, Li S, Han S, Shi C, Zhang Y. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct Target Ther 2022;7(1):93. [PMID: 35318309 DOI: 10.1038/s41392-022-00947-7]
- Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov 2010;9(4):325-38. [PMID: 20305665 DOI: 10.1038/nrd3003]
- Sousa F, Castro P, Fonte P, Kennedy PJ, Neves-Petersen MT, et al. Nanoparticles for the delivery of therapeutic antibodies: dogma or promising strategy? Expert Opin Drug Deliv 2017;14(10):1163-76. [PMID: 28005451 DOI: 10.1080/17425247.2017.1273345]
- Huckaby JT, Parker CL, Jacobs TM, Schaefer A, Wadsworth D, et al. Engineering polymer-binding bispecific antibodies for enhanced pretargeted delivery of nanoparticles to mucus-covered epithelium. Angew Chem Int Ed Engl 2019;58(17):5604-8. [PMID: 30811861 DOI: 10.1002/anie.201814665]
- Deng YQ, Zhang NN, Zhang YF, Zhong X, Xu S, et al. Lipid nanoparticle-encapsulated mRNA antibody provides long-term protection against SARS-CoV-2 in mice and hamsters. Cell Res 2022;32(4):375-82. [DOI: 10.1038/s41422-022-00630-0]
- Zhao M, Li J, Chen F, Han Y, Chen D, et al. Engineering nanoparticles boost TNBC therapy by CD24 blockade and mitochondrial dynamics regulation. J Control Release 2023;355:211-27. [PMID: 36736908 DOI: 10.1016/j.jconrel.2023.01.075]
- Choi Y, Son W, Han Y, Chae J, Yang CS, et al. Glycan targeting nanoparticle for photodynamic immunotherapy of melanoma. Acta Pharm B 2023;13(5):1903-18. [PMID: 37250157 DOI: 10.1016/j.apsb.2022.08.009]
- Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science 2020;367(6478):eaau6977. [PMID: 32029601 DOI: 10.1126/science.aau6977]
- Shapira S, Ben Shimon M, Hay-Levi M, Shenberg G, Choshen G, et al. A novel platform for attenuating immune hyperactivity using EXO-CD24 in COVID-19 and beyond. EMBO Mol Med 2022;14(9):e15997. [PMID: 35776000 DOI: 10.15252/emmm.202215997]
- Araujo-Abad S, Saceda M, de Juan Romero C. Biomedical application of small extracellular vesicles in cancer treatment. Adv Drug Deliv Rev 2022;182:114117. [PMID: 35065142 DOI: 10.1016/j.addr.2022.114117]
- Lu ZR, Laney VEA, Hall R, Ayat N. Environment-responsive lipid/siRNA nanoparticles for cancer therapy. Adv Healthc Mater 2021;10(5):e2001294. [PMID: 33615743 DOI: 10.1002/adhm.202001294]
- Hager S, Fittler FJ, Wagner E, Bros M. Nucleic acid-based approaches for tumor therapy. Cells 2020;9(9):2061. [PMID: 32917034 DOI: 10.3390/cells9092061]
- Jin JO, Kim G, Hwang J, Han KH, Kwak M, et al. Nucleic acid nanotechnology for cancer treatment. Biochim Biophys Acta Rev Cancer 2020;1874(1):188377. [PMID: 32418899 DOI: 10.1016/j.bbcan.2020.188377]
- Xu C, Li D, Cao Z, Xiong M, Yang X, et al. Facile hydrophobization of siRNA with anticancer drug for non-cationic nanocarrier-mediated systemic delivery. Nano Lett 2019;19(4):2688-93. [DOI: 10.1021/acs.nanolett.9b00657]
- Lian S, Xie R, Ye Y, Xie X, Li S, et al. Simultaneous blocking of CD47 and PD-L1 increases innate and adaptive cancer immune responses and cytokine release. EBioMedicine 2019;42:281-95. [PMID: 30878596 DOI: 10.1016/j.ebiom.2019.03.018]
- Zhang Y, Zhang Z, Li S, Zhao L, Li D, et al. A siRNA-assisted assembly strategy to simultaneously suppress “Self” and upregulate “Eat-Me” signals for nanoenabled chemo-immunotherapy. ACS Nano 2021;15(10):16030-42. [PMID: 34544242 DOI: 10.1021/acsnano.1c04458]
- Li S, Chen Y, Ma R, Du Y, Han B. Cationic lipid-assisted nanoparticles for simultaneous delivery of CD47 siRNA and R848 to promote antitumor immune responses. Front Pharmacol 2023;14:1142374. [PMID: 37063284 DOI: 10.3389/fphar.2023.1142374]
- Nersisyan S, Ahlers AK, Lange T, Wicklein D, Galatenko A, et al. Low expression of CD24 is associated with poor survival in colorectal cancer. Biochimie 2022;192:91-101. [PMID: 34637894 DOI: 10.1016/j.biochi.2021.10.004]
- Chen XX, Tao T, Gao S, Wang H, Zhou XM, et al. Knock-down of CD24 in astrocytes aggravates oxyhemoglobin-induced hippocampal neuron impairment. Neurochem Res 2022;47(3):590-600. [PMID: 34665391 DOI: 10.1007/s11064-021-03468-x]
- Suyama K, Onishi H, Imaizumi A, Shinkai K, Umebayashi M, et al. CD24 suppresses malignant phenotype by downregulation of SHH transcription through STAT1 inhibition in breast cancer cells. Cancer Lett 2016;374(1):44-53. [PMID: 26797459 DOI: 10.1016/j.canlet.2015.12.013]