Contrast-Enhanced Ultrasound Features of Primary Hepatic Lymphoepithelioma-Like Carcinoma: Comparison with Hepatocellular Carcinoma
1Department of Ultrasound, Zhongshan Hospital, Fudan University, Shanghai, China
2Department of Ultrasound, Binzhou Medical University Hospital, Binzhou, China
3Shanghai Institute of Imaging Medicine, Shanghai, China
aThe authors contribute equally to this manuscript.
*Correspondence to: Wenping Wang, Department of Ultrasound, Zhongshan Hospital, Fudan University, Shanghai Institute of Imaging Medicine, No. 180 Fenglin Road, Zhongshan Hospital, Fudan University, Shanghai, 20032, China. E-mail: puguang61@126.com
Received: November 27 2023; Revised: December 29 2023; Accepted: January 25 2024; Published Online: March 1 2024
Cite this paper:
Hong Qin, Zhengbiao Ji, Qiannan Zhao, Kun Wang, Feng Mao, Hong Han and Wenping Wang. Contrast-Enhanced Ultrasound Features of Primary Hepatic Lymphoepithelioma-Like Carcinoma: Comparison with Hepatocellular Carcinoma. BIO Integration 2024; 5: e996.
DOI: 10.15212/bioi-2023-0019. Available at: https://bio-integration.org/
Download citation
© 2024 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
Background: Primary hepatic lymphoepithelioma-like carcinoma (LELC) is a malignant tumor with a low incidence, but the number of case reports has increased in recent years. The prognosis of hepatic LELC is better than hepatocellular carcinoma (HCC). The differentiation between hepatic LELC and HCC has clinical value during follow-up treatment. The purpose of our study was to compare contrast-enhanced ultrasound (CEUS) imaging features in patients with hepatic LELC and HCC.
Methods: Twelve patients with an average age of 60.1±9.5 years and histopathologically confirmed hepatic LELC were included in the study. Forty-three patients with an average age of 57.4±9.0 years and a histopathological diagnosis of HCC were designated as the control group by means of propensity score matching (1:4). The clinical data, B-mode ultrasound (BMUS), and CEUS features were retrospectively analyzed between patients with hepatic LELC and HCC.
Results: The serum a-fetoprotein (58.1% [25/43] vs.16.7% [2/12]; p=0.017) and des-gamma-carboxy prothrombin levels (74.4% [32/43] vs.16.7% [2/12]; p=0.001) were not significantly elevated in patients with hepatic LELCs compared to HCCs. LELCs were mainly hypoechoic based on BMUS, while the echogenicity of HCCs varied (p=0.016). A halo sign was less common in patients with hepatic LELCs than HCCs (16.7% [2/12] vs. 58.1% [25/43]; p=0.011). Of hepatic LELCs, 75% (9/12) had homogeneous hyperenhancement based on CEUS, whereas 58.1% (25/43) of HCCs had heterogeneous hyperenhancement (p=0.004). Early washout was noted in 91.7% (11/12) of hepatic LELCs compared to 46.5% (20/43) of HCCs (p=0.005). Furthermore, hepatic LELCs were more likely to exhibit peripheral rim-like hyperenhancement (83.3% [10/12] vs. 11.6% [5/43]; p < 0.001).
Conclusion: BMUS and CEUS are helpful in discriminating between hepatic LELC and HCC. A hypoechoic mass, the rare halo sign, homogeneous hyperenhancement in the arterial phase, higher frequencies of early washout, and peripheral rim-like hyperenhancement are useful ultrasound features that can help differentiate hepatic LELCs from HCCs.
Keywords
Contrast-enhanced ultrasound, hepatocellular carcinoma, liver tumors, lymphoepithelioma-like carcinoma.
Introduction
Hepatocellular carcinoma (HCC), which is highly invasive, is the most common primary liver cancer, accounting for 75%-85% of cases [1]. The incidence of HCC has gradually increased in recent years.
Lymphoepithelioma-like carcinoma (LELC) is a tumor consisting of abundant lymphocytes infiltrating undifferentiated epithelial cells [2], which was initially reported in the nasopharynx and is rare in the liver, but the number of reports has markedly increased in recent years. The World Health Organization defined hepatic LELC as a specific subtype of liver cancer in 2010 [3]. Hepatic LELC is considered a unique model of the immune response to liver cancer, and for patients with hepatic LELC that cannot be treated with surgical resection, immunotherapy may have a survival benefit [4–7]. Due to the unique histopathologic features of hepatic LELC, the prognosis is relatively favorable with studies showing 5-year survival rates of 57%–94% [8–10]. In a previous study that compared 20 cases of lymphoepithelioma-like hepatocellular carcinoma with 389 cases of HCC [3], the clinical outcome of lymphoepithelioma-like hepatocellular carcinoma was significantly better than HCC (94.1% vs. 63.9%; p=0.007).
The clinical manifestations of hepatic LELC are atypical. The current diagnosis mainly relies on a pathohistologic biopsy [11, 12], but this method is invasive and may lead to complications, such as tumor dissemination. Therefore, a preoperative non-invasive diagnosis of hepatic LELC is essential. Computed tomography (CT), magnetic resonance imaging (MRI), and ultrasonography are the most common non-invasive methods for diagnosing liver lesions. However, there is radiation exposure associated with CT and patients with impaired liver and kidney function cannot tolerate the contrast agents used in enhanced CT and MRI. Ultrasonography is easy to operate, inexpensive, and can detect lesions dynamically and in real time. Moreover, ultrasonography is increasingly used in the examination of clinical liver lesions. Because hepatic LELC is rare and the imaging features of LELC have not been clearly defined, this study compared and summarized the B-mode ultrasound (BMUS) and contrast-enhanced ultrasound (CEUS) manifestations of hepatic LELC and HCC to provide an imaging basis for the differential diagnosis of hepatic LELC and HCC.
Patients and methods
Study population
This study was approved by the Institutional Review Board of our hospital (No. B2022-223R). The requirement for informed consent was waived.
Patients with histopathologically confirmed hepatic LELC who underwent hepatic CEUS from July 2015 to January 2023 in our Ultrasound Department were enrolled. The inclusion criteria were as follows: (1) hepatic LELC was confirmed by surgical pathology; and (2) CEUS examination was performed within 1 month before surgery. Patients diagnosed with HCC between June 2020 and January 2023 were included who met the following criteria: (1) surgically confirmed primary HCC; and (2) CEUS was performed within 1 month prior to surgery. Patients with diagnosed with HCC were excluded for the following reasons: (1) non-surgical treatment; (2) secondary hepatic tumors; and (3) poor image quality that affected the interpretation of the results.
Ultrasound examination
All patients underwent BMUS and CEUS examinations by experienced radiologists using a LOGIQ E20 (GE Healthcare, Milwaukee, WI, USA) or a Resona 9 ultrasound system (Mindray Medical Solutions, Shenzhen, China). The ultrasound system included convex array probes with a probe frequency of 2-5 MHz using a low mechanical index real-time harmonic contrast-enhanced ultrasound technique. First, the liver was thoroughly scanned using BMUS to identify the target lesion. Then, the best observation section of the lesion was selected for CEUS examination. After the contrast agent (SonoVue; Bracco, Milan, Italy) was prepared according to the standard protocol. Specifically, 1.5–2.0 ml of SonoVue was injected via the antecubital vein, followed by 5 ml of normal saline. The contrast agent was injected while recording the time of injection. The images and videos were saved for subsequent analysis. The CEUS process consists of the following 3 phases: arterial phase (AP), 0-30 s; portal venous phase (30-120 s); and late phase (>120 s).
Imaging analysis
Two radiologists with > 5 years of experience in the diagnosis of liver tumors were asked to independently review the images. Inconsistent analyses of the lesion image characteristics were reconciled by consensus of two physicians who were blinded to the final diagnosis. For multiple lesions, the largest lesion was selected for analysis. BMUS features that were assessed included the following: (a) location (left lobe/right lobe/caudate lobe/left-right lobe junction); (b) diameter; (c) echogenicity (hypoechoic/isoechoic/hyperechoic/mixed echoic); (d) margin (clear/ill-defined); (e) morphology (regular/irregular); (f) grayscale homogeneity (homogeneous/heterogeneous); and (g) blood flow.
Then, the optimal contrast plane was selected for CEUS. The CEUS features that were analyzed included the following: (a) AP enhancement pattern (homogeneous enhancement/heterogeneous enhancement/rim-like enhancement/dendritic enhancement); (b) the degree of enhancement of tumors in comparison to the liver parenchyma at three phases (hyperenhancement/isoenhancement/slight hypoenhancement/hypoenhancement); (c) early washout (< 60 s); (d) unenhanced area (present/absent); and (e) peripheral rim-like hyperenhancement (present/absent). The central enhancement of the lesion washed out in the portal or delayed phase and the peripheral enhancement did not wash out or wasmildly washed out.
Statistical analysis
SPSS 26.0 software (IBM, NY, USA) was used for data analysis. Normality was assessed using the Shapiro-Wilk test. Categorical variables are expressed by numbers and percentages. Continuous variables were described by the mean ± standard deviation (normal distribution) or the median and interquartile range. To minimize the influence of potential confounding factors between patients with LELC and HCC on selection bias, the propensity score matching method (LELC:HCC [1:4]) was adopted according to age and lesion size. The propensity score matching was performed using the nearest-neighbor matching method with a caliper distance of 0.2 and no replacement. The characteristics of patients with hepatic LELC and HCC were compared using the χ2 test or Fisher’s exact test for categorical variables and Student’s t-test or the Mann–Whitney U test for continuous variables. Differences in statistical significance were considered at a p < 0.05.
Results
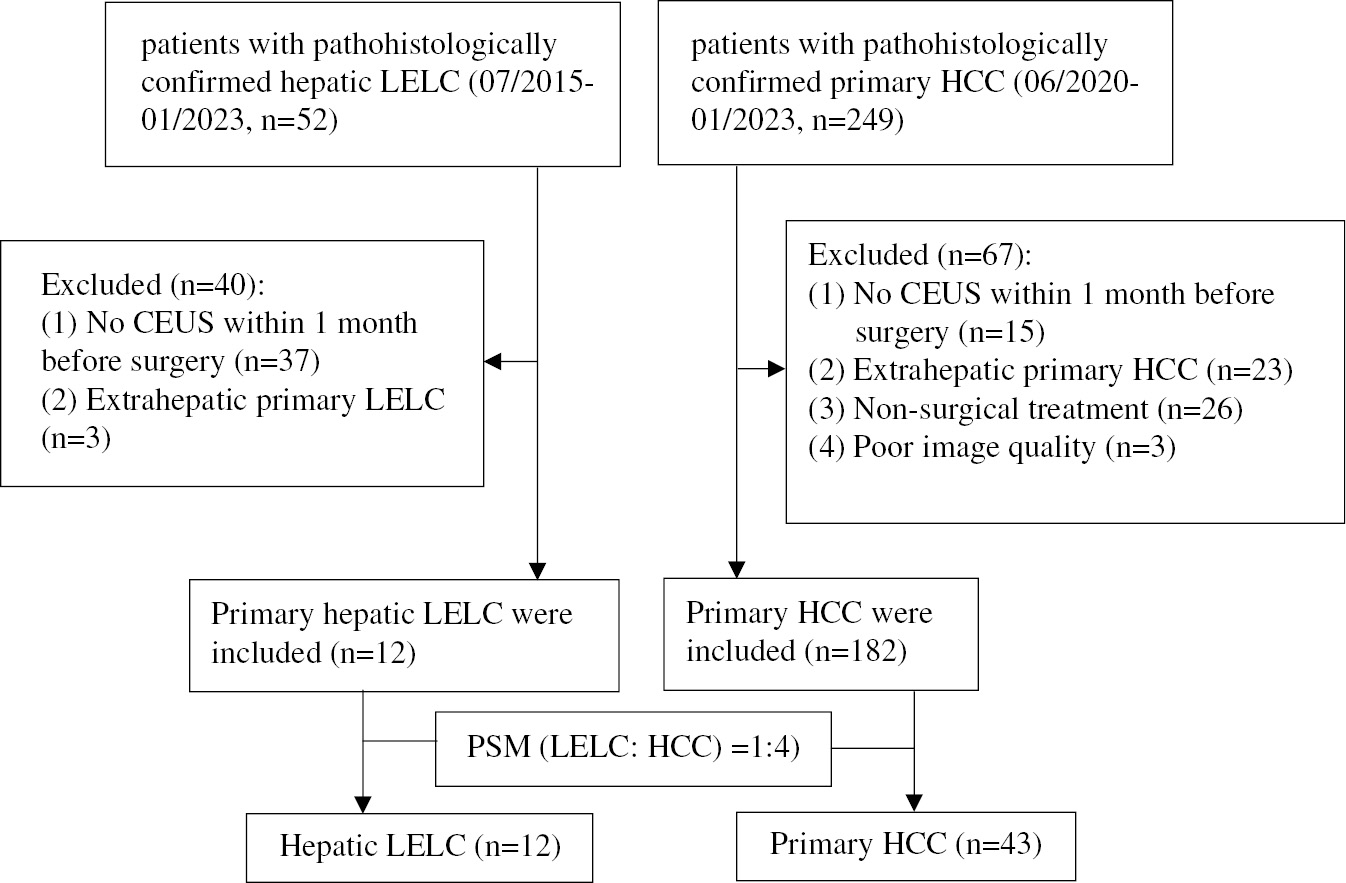
A total of 52 patients with histopathologically confirmed LELC; 37 patients who did not undergo CEUS within 1 month prior to surgery and 3 patients with extrahepatic primary LELC were excluded. Twelve patients (8 males and 4 females) with histopathologically confirmed hepatic LELC were included in the study with an average age of 60.1 ± 9.5 years (range, 50–82 years). After propensity score matching, 43 patients (30 males and 13 females) with HCC and a mean age of 57.4 ± 9.0 years (range, 42–83 years) were included. Figure 1 shows the inclusion procedure for patients with hepatic LELC and HCC inclusion.
Figure 1 Flowchart for inclusion of patients with hepatic LELC and HCC.
Clinical and laboratory data
One patient (8.3% [1/12]) with hepatic LELC complained of abdominal pain. Abdominal distension was noted in 1 (8.3% [1/12]) and 10 patients (83.3% [10/12]) by physical examination. Six patients (50% [6/12]) with hepatic LELC had cirrhosis and 11 patients (91.7% [11/12]) had chronic hepatitis B.
An elevated serum alpha-fetoprotein [AFP] (58.1% [25/43] vs. 16.7% [2/12]; p=0.011) and des-gamma-carboxy prothrombin (DCP) levels (74.4% [32/43] vs. 16.7% [2/12]; p=0.001) were uncommon in patients with hepatic LELC compared to patients with HCC. The levels of other tumor markers, such as carcinoembryonic antigen and carbohydrate antigen 19-9, did not differ between hepatic LELC and HCC patients (p > 0.05). The baseline characteristics of patients with hepatic LELC and HCC before and after propensity score matching are presented in Tables 1 and 2.
Table 1 Baseline Patient Characteristics Before Propensity Score Matching
| Variables | LELC (n=12) | HCC (n=182) | P Value |
|---|---|---|---|
| Age (years) | 60±3a | 45 (39−53)b | <0.001 |
| Gender | 0.541 | ||
| Male | 77 (42.3%) | 105 (57.7%) | |
| Female | 4 (33.3%) | 8 (66.7%) | |
| Chronic hepatitis B | 11 (91.7%) | 150 (82.4%) | 0.668 |
| Cirrhosis | 87 (47.8%) | 6 (50%) | 0.883 |
| Tumor size (mm)b | 34 (18−60) | 58 (50−65) | 0.003 |
Note: Unless otherwise indicated, data are numbers of patients with percentages in parentheses.
LELC: lymphoepithelioma-like carcinoma; HCC: hepatocellular carcinoma.
aData are the mean ± standard deviations.
bData are the median values and data in parentheses are interquartile ranges.
Table 2 Baseline Patient Characteristics After PSM
| Variables | LELC (n=12) | HCC (n=43) | P Value |
|---|---|---|---|
| Age (years)a | 60.1±9.5 | 57.4±9.0 | 0.372 |
| Gender | 0.999 | ||
| Male | 8 (66.7%) | 30 (69.8%) | |
| Female | 4 (33.3%) | 13 (30.2%) | |
| Cirrhosis | 6 (50%) | 32 (74.4%) | 0.206 |
| Chronic hepatitis B | 11 (91.7%) | 39 (90.7%) | 0.999 |
| DCP (≥ 40 AU/ml) | 2 (16.7%) | 32 (74.4%) | 0.001 |
| AFP (≥ 20 ng/ml) | 2 (16.7%) | 25 (58.1%) | 0.011 |
| CEA (≥ 5 ng/ml) | 1 (8.3%) | 6 (14%) | 0.979 |
| CA19-9 (≥ 34 U/ml) | 2 (16.7%) | 6 (14%) | 0.999 |
| CA 72-4 (≥ 10 U/ml) | 1 (11.1%) | 1 (2.9%) | 0.371 |
| CA-125 (≥ 25 U/ml) | 1 (16.7%) | 1 (2.9%) | 0.274 |
Note: Unless otherwise indicated, data are numbers of patients with percentages in parentheses.
LELC: lymphoepithelioma-like carcinoma; HCC: hepatocellular carcinoma; DCP: des-gamma-carboxy prothrombin; AFP: alpha-fetoprotein; CEA: carcinoembryonic antigen, CA19-9: carbohydrate antigen 19-9, CA724: carbohydrate antigen 72-4; CA-125: cancer antigen 125.
aData are the mean ± standard deviation.
BMUS
The hepatic LELC and HCC groups had similar lesion diameters (34 mm vs. 40 mm; p=0.521). Seven of 12 hepatic LELCs (58.3%) were located in the right liver lobe; the remaining 5 lesions were located in the left liver lobe (33.3% [4/12]) and the left-right lobe junction (8.3% [1/12]). Hypoechoic lesions were detected in 9 hepatic LELC patients (75% [9/12]), mixed echoic lesions were detected in 2 patients (16.7% [2/12]), and a hyperechoic lesion was detected in 1 patient (8.3% [1/12]). In contrast, 53.5% (23/43) of the HCC lesions were hypoechoic, 30.2% (13/43) were hyperechoic, and 16.3% (7/43) were isoechoic (p=0.016). Furthermore, lesions in the hepatic LELC group exhibited significantly fewer halo signs than the HCC group (2 [16.7%] vs. 25 [58.1%]; p=0.011; Table 3). There were no radical differences between the hepatic LELC and HCC groups with respect to lesion grayscale homogeneity, morphology, tumor margin, and CDFI manifestations (p>0.05).
Table 3 Comparison of BMUS Characteristics of Hepatic LELC and HCC
| Variables | LELC (n=12) | HCC (n=43) | P Value |
|---|---|---|---|
| Location | 0.517 | ||
| Right liver | 7 (58.3%) | 30 (69.8%) | |
| Left liver | 4 (33.3%) | 11 (25.6%) | |
| Caudate lobe | 0 | 1 (2.3%) | |
| Left-right lobe junction | 1 (8.3%) | 1 (2.3%) | |
| Echogenicity | 0.016 | ||
| Hypoechoic | 9 (75%) | 23 (53.5%) | |
| Isoechoic | 0 | 7 (16.3%) | |
| Hyperechoic | 1 (8.3%) | 13 (30.2%) | |
| Mixed echoic | 2 (16.7%) | 0 | |
| Grayscale homogeneity | 0.926 | ||
| Homogeneous | 7 (58.3%) | 28 (65.1%) | |
| Heterogeneous | 5 (41.7%) | 15 (34.9%) | |
| Morphology | 0.059 | ||
| Regular | 3 (25%) | 24 (55.8%) | |
| Irregular | 9 (75%) | 19 (44.2%) | |
| Tumor margin | 0.069 | ||
| Clear | 4 (33.3%) | 27 (62.8%) | |
| Ill-defined | 8 (66.7%) | 16 (37.2%) | |
| Halo sign | 2 (16.7%) | 25 (58.1%) | 0.011 |
| Color Doppler signal | 7 (58.3%) | 23 (53.5%) | 0.766 |
Note: Unless otherwise indicated, data are numbers of patients with percentages in parentheses.
LELC: lymphoepithelioma-like carcinoma; HCC: hepatocellular carcinoma.
CEUS
All patients underwent CEUS examinations. All hepatic LELC lesions manifested hyperenhancement in the AP on CEUS. Nine of 12 (75%) of the hepatic LELC lesions showed homogeneous enhancement in the arterial phase; the remaining 3 lesions showed heterogeneous, rim-like, and dendritic enhancement. However, 25 (58.1%) of the lesions in the HCC group exhibited heterogeneous enhancement, 13 lesions (30.2%) exhibited homogeneous enhancement, 4 lesions (9.3%) exhibited dendritic enhancement, and 1 lesion (2.3%) exhibited rim-like enhancement, which was a significantly different distribution from the hepatic LELC group (p=0.004). Early washout (onset < 60 s) was observed in 91.7% (11/12) of hepatic LELC lesions compared to 46.5% (20/43; p = 0.005) of the HCC lesions. Furthermore, 83.3% (10/12) of hepatic LELC lesions demonstrated peripheral rim-like hyperenhancement (Figure 2) compared to 11.6% (5/43) of the HCC lesions (p < 0.001; Table 4).
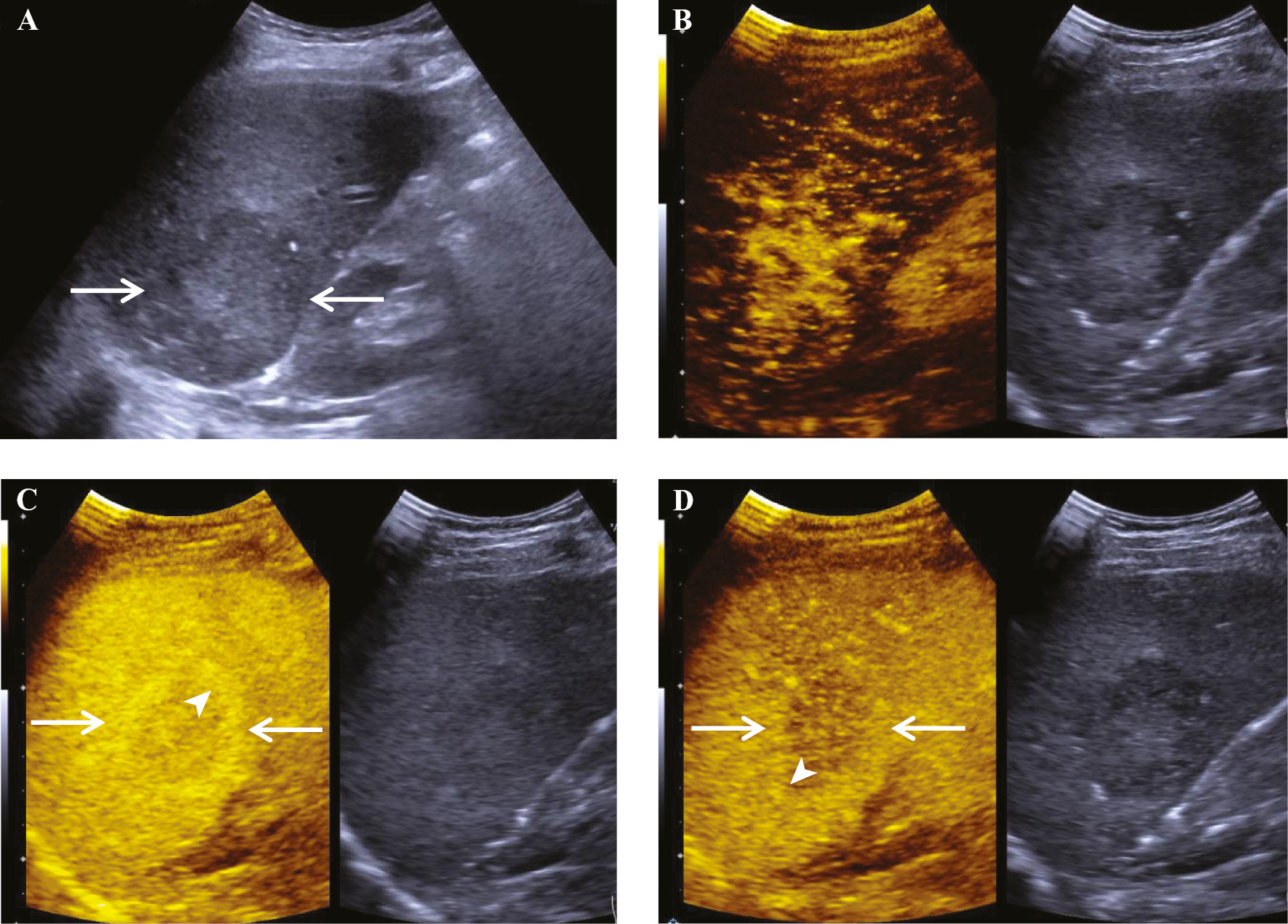
Figure 2 A 56-year-old man with primary lymphoepithelioma-like carcinoma of the liver. (A) B-mode ultrasound demonstrated a hypoechoic heterogeneous parenchymal mass in the right lobe of the liver near the lower corner that was 51×48 mm in size with ill-defined borders and an irregular morphology (arrows). (B) The lesion showed dendritic hyperenhancement in the arterial phase. (C) In the portal phase, the central enhancement of the lesion (arrows) gradually washed out, the peripheral enhancement did not wash out, and the lesion formed a peripheral rim-like hyperenhancement (arrowhead). (D) In the delayed phase the central enhancement of the lesion (arrows) further washed out and the peripheral enhancement was mildly washed out, forming a peripheral rim-like hyperenhancement (arrowhead).
Table 4 Comparison of CEUS Features of Hepatic LELC and HCC
| Variables | LELC (n=12) | HCC (n=43) | P Value |
|---|---|---|---|
| AP enhancement pattern | 0.004 | ||
| Homogeneous enhancement | 9 (75%) | 13 (30.2%) | |
| Heterogeneous enhancement | 1 (8.3%) | 25 (58.1%) | |
| Rim-like enhancement | 1 (8.3%) | 1 (2.3%) | |
| Dendritic enhancement | 1 (8.3%) | 4 (9.3%) | |
| AP enhancement degree | 0.999 | ||
| Hyperenhancement | 12 (100%) | 41 (95.3%) | |
| Isoenhancement | 0 | 1 (2.3%) | |
| Hypoenhancement | 0 | 1 (2.3%) | |
| PVP enhancement degree | 0.238 | ||
| Isoenhancement | 1 (8.3%) | 12 (27.9%) | |
| Slight hypoenhancement | 8 (66.7%) | 17 (39.5%) | |
| Hypoenhancement | 3 (25%) | 14 (32.6%) | |
| Early washout (< 60 s) | 11 (91.7%) | 20 (46.5%) | 0.005 |
| LP enhancement degree | 0.121 | ||
| Isoenhancement | 0 | 6 (14%) | |
| Slight hypoenhancement | 1 (9.1%) | 13 (30.2%) | |
| Hypoenhancement | 10 (90.9%) | 24 (55.8%) | |
| Unenhanced area | 2 (16.7%) | 13 (30.2%) | 0.571 |
| Peripheral rim-like hyperenhancement | 10 (83.3%) | 5 (11.6%) | <0.001 |
Note: Unless otherwise indicated, data are numbers of patients with percentages in parentheses.
LELC: lymphoepithelioma-like carcinoma; HCC: hepatocellular carcinoma.
Discussion
Primary hepatic LELC is a special type of liver cancer with a relatively favorable prognosis, although treatment of primary hepatic LELC may be different from highly invasive malignant tumors, such as HCC [13–17]. Thus, pretreatment identification of hepatic LELC is crucial for both therapeutic and prognostic proposes. In this study the clinical manifestations and BMUS and CEUS characteristics of hepatic LELC and HCC were retrospectively compared. It was confirmed that features, including the serum AFP and DCP levels, lesion echogenicity, halo sign, AP enhancement pattern, early washout, and peripheral rim-like hyperenhancement in the portal and delayed phases differed markedly between patients with hepatic LELC and HCC.
In the present study patients with hepatic LELC were predominantly male (66.7%) with an average age of 60.1 ± 9.5 years, which differs from previous studies in which hepatic LELC was predominantly observed in female patients [6]. This finding may be a result of the small sample size. The serum AFP (p=0.017) and DCP levels (p=0.001) were not significantly elevated in patients with hepatic LELC compared to patients with HCC. Other biochemical markers, such as carcinoembryonic antigen, carbohydrate antigen 19-9, carbohydrate antigen 72-4, and cancer antigen 125, were mostly in the normal range, which is consistent with the lack of significant elevation of tumor markers in patients with hepatic LELC in previous studies [3, 10].
A limited number of reports have described the ultrasound manifestations of hepatic LELC; most of the extant literature involves case reports [18–21]. In this study we found that all hepatic LELC lesions were isolated and most patients in previous studies also had single lesions [22]. The majority (75% [9/12]) of the hepatic LELC lesions were hypoechoic masses, which differed markedly from the diversity of echogenic manifestations among HCC lesions (hypoechoic, isoechoic, and hyperechoic; p=0.016). A study exploring the ultrasonographic manifestations of two patients with lymphoepithelioma-like cholangiocarcinoma showed [19] that the lesions exhibited hypoechoic masses on BMUS. In addition, there were fewer halo signs in hepatic LELC lesions compared to HCC lesions (p=0.011). The halo sign may be related to the different pathologic components of the lesion; however, there was no marked difference between hepatic LELC and HCC lesions with respect to lesion grayscale homogeneity, morphology, tumor margin, and color Doppler flow imaging manifestations.
A CEUS examination allows dynamic and continuous observation of lesion changes and has been widely used in the diagnosis of liver lesions. All hepatic LELC lesions and 95.3% of HCC lesions in the current study presented with hyperenhancement in the AP on CEUS. There were marked differences in the enhancement patterns between hepatic LELC and HCC lesions. Of liver LELC lesions, 75% demonstrated homogeneous enhancement and 8.3% exhibited heterogeneous, rim-like, and dendritic enhancement. Of HCC lesions, 58.1% showed heterogeneous enhancement, 30.2% exhibited homogeneous enhancement, 9.3% exhibited rim-like enhancement, and 2.3% dendritic enhancement. This difference may be related to the distribution of different pathologic tissue components [2]. Furthermore, 83.3% of hepatic LELC lesions in the current study showed peripheral rim-like hyperenhancement in the portal and delayed phases, which is in agreement with the results reported by Ling et al. [19]. In the current study the CEUS manifestations of lymphoepithelioma-like cholangiocarcinoma were analyzed, which showed that the central enhancement of the lesion gradually washed out, hypoenhancement emerged in the portal and delayed phases, and the peripheral enhancement did not wash out or mildly washed out, thus presenting as hyperenhancement. This CEUS manifestation may indicate tumor infiltration or the presence of a pseudocapsule. In addition, Yang et al. [22] found similar manifestations in contrast-enhanced CT and contrast-enhanced MRI and suggested that the peripheral rim-like hyperenhancement was a pseudocapsule consisting of fibrosis. Whether a similar enhancement characteristic in different imaging modalities (CEUS, contrast-enhanced CT, and contrast-enhanced MRI) is the result of the same pathologic component has not been established, thus further studies are warranted. The Contrast-Enhanced Ultrasound Liver Imaging Reporting and Data System (CEUS LI-RADS) [23] released by the American College of Radiology (ACR) defines in detail the diagnostic criteria for liver malignant tumors, including washout, which is divided into early and late washout with an interval of 60 s. One of the diagnostic criteria for HCC is late washout. In the current study, 91.7% of hepatic LELC lesions exhibited early washout. According to the CEUS LI-RADS washout time standard, these lesions may be classified as LR-M (probably or definitely malignant), but not necessarily HCC, which is essential in the differentiation from HCC. In addition, CEUS also detects the presence of an unenhanced area within the lesion, which suggests hemorrhage or necrosis. Indeed, 16.7% of the hepatic LELC lesions in the current study exhibited an unenhanced area.
Our study had some limitations. A small sample size was included due to the rarity of hepatic LELCs. Second, there was some selection bias, but to minimize the occurrence of such bias, propensity score matching was used.
In conclusion, this study demonstrated that BMUS and CEUS combined with clinical data are helpful in the differential diagnosis of hepatic LELC and HCC. Compared with HCC, hepatic LELC had an insignificant elevation of serum AFP and DCP levels, and was mainly manifested as a single hypoechoic mass on BMUS, with a rare halo sign around the lesion. CEUS showed that the lesion mostly had homogeneous enhancement, and early washout and peripheral rim-like hyperenhancement were more common.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 82272013).
Conflict of interest disclosure
The authors declared that there were no conflicts of interest.
Data availability statement
Data will be made available from the corresponding author on reasonable request.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71(3):209-49. [PMID: 33538338 DOI: 10.3322/caac.21660]
- Labgaa I, Stueck A, Ward SC. Lymphoepithelioma-like carcinoma in liver. Am J Pathol 2017;187(7):1438-44. [PMID: 28500863 DOI: 10.1016/j.ajpath.2017.02.022]
- Chan AW, Tong JH, Pan Y, Chan SL, Wong GL, et al. Lymphoepithelioma-like hepatocellular carcinoma: an uncommon variant of hepatocellular carcinoma with favorable outcome. Am J Surg Pathol 2015;39(3):304-12. [PMID: 25675010 DOI: 10.1097/PAS.0000000000000376]
- Tsai JH, Liau JY, Lee CH, Jeng YM. Lymphoepithelioma-like intrahepatic cholangiocarcinoma is a distinct entity with frequent pTERT/TP53 mutations and comprises 2 subgroups based on Epstein-Barr virus infection. Am J Surg Pathol 2021;45(10):1409-18. [PMID: 33859071 DOI: 10.1097/PAS.0000000000001716]
- Chan AW, Zhang Z, Chong CC, Tin EK, Chow C, et al. Genomic landscape of lymphoepithelioma-like hepatocellular carcinoma. J Pathol 2019;249(2):166-72. [PMID: 31168847 DOI: 10.1002/path.5313]
- Patel KR, Liu TC, Vaccharajani N, Chapman WC, Brunt EM. Characterization of inflammatory (lymphoepithelioma-like) hepatocellular carcinoma: a study of 8 cases. Arch Pathol Lab Med 2014;138(9):1193-202. [PMID: 25171701 DOI: 10.5858/arpa.2013-0371-OA]
- Tao L, Chen Y, Huang Y, Yin W, Yu G. SSTR2a is constantly expressed in lymphoepithelioma-like carcinoma with squamous differentiation other than that with glandular differentiation. J Clin Pathol 2021;74(11):704-8. [PMID: 33132215 DOI: 10.1136/jclinpath-2020-206903]
- Pan YJ, Liu W, Qiu QX, Miao SL, Zeng MS, et al. Prognostic value of LI-RADS category on MRI in patients with primary hepatic lymphoepithelioma-like carcinoma. Eur Radiol 2023;33(9):5993-6000. [PMID: 37014407 DOI: 10.1007/s00330-023-09598-w]
- Liu LH, Wang ML, Jiang F, Chen LL, Ji Y, et al. Distinct radiological features of lymphoepithelioma-like intrahepatic cholangiocarcinoma: comparison with classical intrahepatic cholangiocarcinoma. Abdom Radiol (NY) 2023;48(6):2038-48. [PMID: 37004556 DOI: 10.1007/s00261-023-03890-5]
- Khandakar B, Liu JR, Thung S, Li Y, Rhee H, et al. Lymphoepithelioma-like neoplasm of the biliary tract with ‘probable low malignant potential’. Histopathology 2022;80(4):720-8. [PMID: 34608670 DOI: 10.1111/his.14580]
- Liu F, Xu Q, Regmi P, Li FY, Lin YX. Case report: primary lymphoepithelioma-like intrahepatic cholangiocarcinoma. Front Oncol 2023;13:1146933. [PMID: 37197425 DOI: 10.3389/fonc.2023.1146933]
- Kim MJ, Lee S, An C. Problematic lesions in cirrhotic liver mimicking hepatocellular carcinoma. Eur Radiol 2019;29(9):5101-10. [PMID: 30788586 DOI: 10.1007/s00330-019-06030-0]
- Li R, Cheng K, Li X, Chang C, Lv W, et al. Case report: immunotherapy plus chemotherapy and stereotactic ablative radiotherapy (ICSABR): a novel treatment combination for Epstein-Barr virus-associated lymphoepithelioma-like intrahepatic cholangiocarcinoma. Front Pharmacol 2023;14:1147449. [PMID: 37614316 DOI: 10.3389/fphar.2023.1147449]
- Sapuppo E, Brunetti O, Tessitore D, Brandi G, Di Giovanni N, et al. Rare histotypes of epithelial biliary tract tumors: a literature review. Crit Rev Oncol Hematol 2023;181:103892. [PMID: 36481306 DOI: 10.1016/j.critrevonc.2022.103892]
- Zhu Y, Dang Z, Xu H, Yuan Y, Chen Y, et al. High PD-L1 level of advanced hepatic lymphoepithelioma-like carcinoma response favorably to lenvatinib plus toripalimab. Cancer Sci 2022;113(5):1880-4. [PMID: 35298067 DOI: 10.1111/cas.15339]
- Lee H, Yoon JH, Kim H, Yi NJ, Hong SK, et al. False positive diagnosis of hepatocellular carcinoma in liver resection patients. J Korean Med Sci 2017;32(2):315-20. [PMID: 28049244 DOI: 10.3346/jkms.2017.32.2.315]
- Calderaro J, Rousseau B, Amaddeo G, Mercey M, Charpy C, et al. Programmed death ligand 1 expression in hepatocellular carcinoma: relationship with clinical and pathological features. Hepatology 2016;64(6):2038-46. [PMID: 27359084 DOI: 10.1002/hep.28710]
- Liao TC, Liu CA, Chiu NC, Yeh YC, Chiou YY. Lymphoepithelioma-like cholangiocarcinoma: a mimic of hepatocellular carcinoma on imaging features. World J Gastroenterol 2015;21(13):4089-95. [PMID: 25852298 DOI: 10.3748/wjg.v21.i13.4089]
- Ling W, Lu C, Huang H, Qiu T, Lu Q, et al. Ultrasonographic findings of intrahepatic lymphoepithelioma-like cholangiocarcinoma associated with Epstein-Barr virus: two cases report. Medicine (Baltimore) 2019;98(3):e14206. [PMID: 30653176 DOI: 10.1097/MD.0000000000014206]
- Jeng YM, Chen CL, Hsu HC. Lymphoepithelioma-like cholangiocarcinoma: an Epstein-Barr virus-associated tumor. Am J Surg Pathol 2001;25(4):516-20. [PMID: 11257627 DOI: 10.1097/00000478-200104000-00012]
- Chen TC, Ng KF, Kuo T. Intrahepatic cholangiocarcinoma with lymphoepithelioma-like component. Mod Pathol 2001;14(5):527-32. [PMID: 11353065 DOI: 10.1038/modpathol.3880342]
- Yang Q, Cai Q, Wen H, Mao Y, Ban X, et al. The CT and MRI features of primary intrahepatic lymphoepithelioma-like cholangiocarcinoma. AJR Am J Roentgenol 2021;216(2):393-402. [PMID: 33325732 DOI: 10.2214/AJR.20.22937]
- Choi HH, Rodgers SK, Fetzer DT, Wasnik AP, Millet JD, et al. Ultrasound liver imaging reporting and data system (US LI-RADS): an overview with technical and practical applications. Acad Radiol 2021;28(10):1464-76. [PMID: 32718745 DOI: 10.1016/j.acra.2020.06.004]