Peptide Assemblies as Promising Tumor Vaccines: Current Platforms and Progress
1Department of Chemistry, School of Science, Westlake University, Institute of Natural Sciences, Westlake Institute for Advanced Study, No. 600 Yungu Road, Hangzhou 310030, Zhejiang Province, China
*Correspondence to: Huaimin Wang, E-mail: wanghuaimin@westlake.edu.cn
Published Online: June 22 2023
Cite this paper:
Wu B, Wang H. Peptide Assemblies as Promising Tumor Vaccines: Current Platforms and Progress. BIO Integration 2023; 4(2): 45–51.
DOI: 10.15212/bioi-2023-0005. Available at: https://bio-integration.org/
Download citation
© 2023 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Introduction
Peptide-based tumor vaccines usually contain epitope peptides from tumor-specific antigens that can be internalized by antigen-presenting cells (APCs) and presented on the surface to stimulate T cell responses against the tumor; however, the development of peptide-based vaccines has been challenged by imprecise antigen display and the use of immune adjuvants, the underlying mechanism of which has not been well-characterized. The self-assembled peptides mimic the higher-order protein structures for presenting peptide antigens. Thus, the self-assembled peptides have attracted increasing attention in recent years. In addition, the self-assembled peptides also have several advantages, including biocompatibility and biodegradability, multivalency, ease of design and functionality, and controlled formation of nanometric blocks, which enable scientists to generate immunogenic self-adjuvant peptide assemblies to elicit strong immune responses and develop tumor vaccines for immunotherapy [1–4].
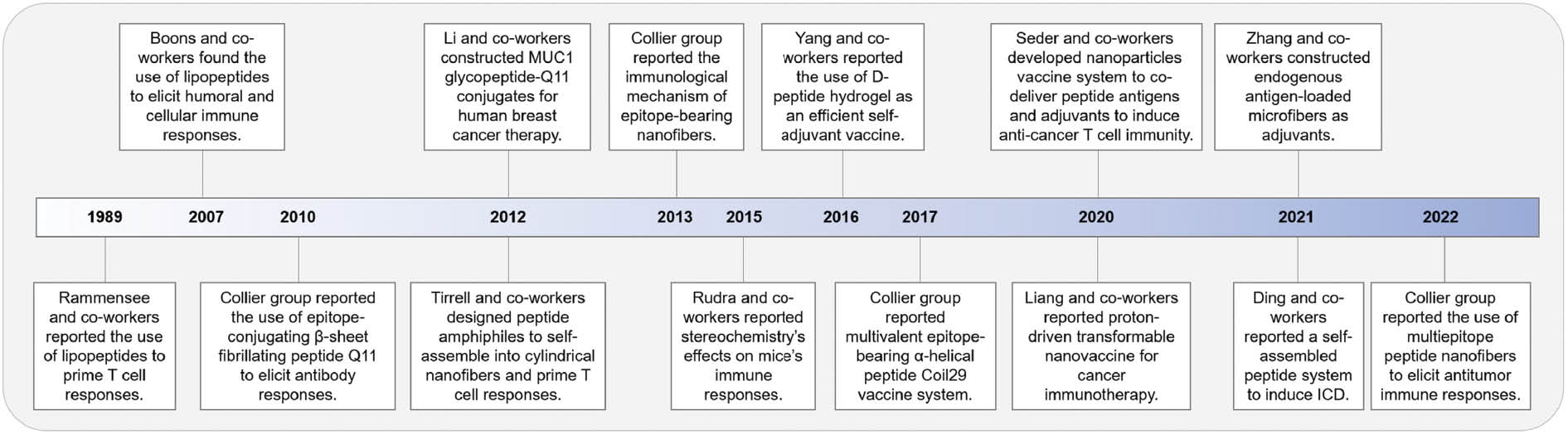
This work describes current platforms and present efforts in developing self-assembled peptides as promising tumor vaccines. We provide a timeline to outline the major developments of the self-assembled peptide-based vaccines from our point of view (Figure 1). The pros and cons of three approaches (lipopeptides, self-assembled epitope-bearing peptides, and peptide-based hydrogels) are discussed. We also explore recent advances in the development of peptide-based assemblies for in situ tumor vaccines. Finally, we propose our perspectives on the major challenges to clinical application and developing strategies.
Figure 1 Timeline of the major developments in the field of self-assembled peptide-based tumor vaccines.
Self-assembled peptide
Self-assembly is a spontaneous process by which components are self-organized to form well-ordered structures. In the first stage of the self-assembly process, the monomeric components are soluble. When a trigger is applied to change the solution conditions, the monomeric components become less soluble and are able to associate with each other. The self-assembly peptides can form diverse morphologies (e.g., nanoparticles, nanofibers, nanobundles, and nanotubes) driven by non-covalent interactions, such as hydrogen bonding, hydrophobic interactions, electrostatic interactions, and aromatic π-π stacking. The individual nanofibers can then entangle to form 3D network structures, which have the ability to absorb and retain a large amount of water to form a solid-like hydrogel. The self-assembling processes of the peptides vary with the change in the external environment, including the ionic strength, solvents, enzymes, pH, light, and temperature [5–7]. These factors make it possible to achieve widespread application by controlling the peptide self-assembly processes [8].
Self-adjuvant peptide vaccine
The strategies for using self-assembly peptides to prepare anti-cancer vaccines for active immunization include lipopeptides that contain long-chain lipids and self-assembled epitope-bearing peptides containing adopted β-sheets and α-helical structures with nanoparticle and nanofiber morphologies [9, 10].
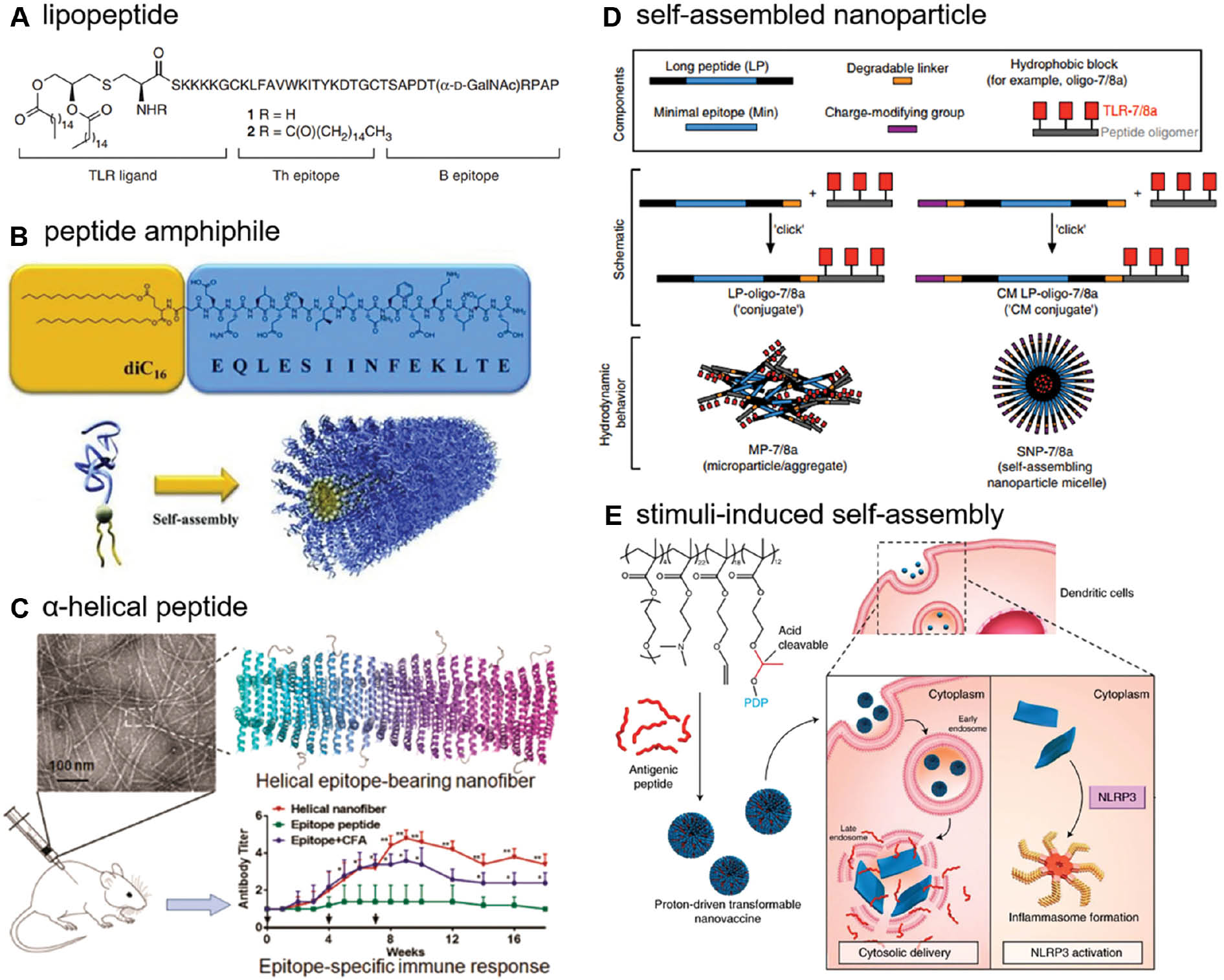
In 1989 Rammensee and co-workers [11] reported for the first time that synthetic viral lipopeptides covalently linked to tripalmitoyl-S-glycerylcysteinyl-seryl-serine (P3CSS) could prime cytotoxic T lymphocytes efficiently in vivo. This concept led to the development of lipopeptides containing glycopeptides, which are highly overexpressed in cancer cells, to circumvent the immunosuppressive evasion of the tumor. In 2007, Boons and co-workers [12] showed that a three-component vaccine composed of an immunoadjuvant lipid, a T-helper epitope peptide, and a tumor-associated mucin 1 (MUC1) glycopeptide could elicit high humoral and cellular immune responses in mice (Figure 2A). After that, Tirrell and co-workers [13] designed peptide amphiphile with a synthetic lipid tail that does not stimulate toll-like receptors (TLRs). This system self-assembled into cylindrical nanofibers, inducing a cytotoxic T cell response in mice (Figure 2B) [13]. These studies illustrate that self-assembled peptides are emerging as effective biomaterials for developing self-adjuvant vaccine systems [14].
Figure 2 Schematic of modular components of self-assembled peptide-based vaccines. A, The lipopeptide structure. The vaccine composed of a tumor-associated epitope derived from MUC1, a Th epitope peptide, and a TLR ligand Pam2-CysSK4. Reproduced with permission from reference 12 (copyright 2007, Nature Publishing Group). B, The peptide amphiphile and cylindrical nanofiber structures. A dipalmitic acid tail (yellow) is attached to the N-terminus of the epitope peptide (blue) from OVA protein. Reproduced with permission from reference 13 (copyright 2012, Wiley). C, The α-helical epitope-bearing nanofiber structure. Epitopes include the CD4+ T-cell epitope, PADRE, the CD8+ T-cell epitope, SIINFEKL, and the B-cell epitope from the epidermal growth factor receptor class III variant. Reproduced with permission from reference 18 (copyright 2017, American Chemical Society). D, The self-assembled nanoparticle components. The antigen epitope-bearing long peptide is conjugated to a charge-modifying group and a hydrophobic block at the N and C termini of the peptide through degradable linkers, respectively. Reproduced with permission from reference 21 (copyright 2020, Nature Publishing Group). E, The proton-driven transformable tumor vaccine. The pyrene-conjugated D-peptide can be cleaved in an acidic environment and re-assemble into nanosheets. Reproduced with permission from reference 23 (copyright 2020, Nature Publishing Group).
Over the past few decades, vaccine design using peptides that form β-sheets has been proven to display high densities of antigens on their surface and elicit potent responses without the need for additional adjuvants. In 2010, the Collier group [15] observed strong antibody responses by non-covalently assembling ovalbumin (OVA) epitope peptide into nanofibers using a short β-sheet fibrillating peptide Q11 (Ac-QQKFQFQFEQQ-Am), suggesting that self-assembled peptides can serve as efficient and chemically-defined adjuvants. Soon thereafter, the Collier group [16] showed that immunization with epitope-bearing β-sheet-rich nanofibers activated dendritic cells, elicited antigen-specific differentiation of T cells into T follicular helper cells, and produced high-titer and high-affinity immunoglobulin G. Li and co-workers [17] also applied this strategy when constructing MUC1 glycopeptide-Q11 conjugates for cancer therapy. The synthetic vaccine was molecularly defined and elicited a strong antibody response against a human breast tumor [17]; however, the application of this system was limited by the imprecise mechanism of the epitope position, the lengths of the β-sheet fibrillating peptide, and lateral interactions between nanofibers. Thus, the Collier group [18] applied an α-helical peptide to construct a vaccine system and in 2017 reported an α-helical peptide vaccine containing Coil29 (QARILEADAEILRAYARILEAHAEILRAQ) and different epitopes (Figure 2C). APCs readily internalize the nanofibers and elicit strong T cell responses. This work represents the first demonstration of a self-adjuvant vaccine delivery platform based on α-helical peptide nanofibers. Recently, multiepitope-bearing Coil29 peptides against the tumor were developed. This system generates strong anti-cancer effects in mice and has elucidated the potential clinical benefits for cancer therapy [19].
The effectiveness of self-adjuvant tumor vaccines encourages researchers to design other vaccine candidates to present peptide epitopes on the surface of nanostructures [20]. Seder and co-workers [21] developed a vaccine system that self-assembled into nanoparticles (∼20 nm) to co-deliver peptide antigens and adjuvants to induce anti-cancer T cell immunity. The formation of nanoparticles improves the solubility of hydrophobic peptide antigens, enhances the accumulation of nanoparticles in lymph nodes, and further increases the uptake by APCs. Unlike other vaccines using self-assembled nanofibers, Seder and co-workers [21] demonstrated successful intravenous vaccination and overcame the formulation limitations of current platforms (Figure 2D); however, the endosomal trapping of tumor antigens limits the efficiency of vaccination strategies. In these cases, the stimuli-induced self-assembly system holds great promise for improving the efficacy of vaccines [22]. For example, Liang and co-workers [23] presented a proton-driven nanotransformer-based vaccine that mechanically disrupts the endosomal membrane and delivers the peptide to the cytoplasm directly by forming assembled nanosheets (Figure 2E).
Short peptide-based materials that contain 3D networks of nanofibers are being explored as novel and promising immunostimulants for improving the biostability and bioactivity of antigen peptides. Such approaches include the D-amino acid self-assembling peptides and antigen-bearing hydrogels. Initially, the replacement of L-amino acids with D-enantiomers was applied to protect the peptide against enzyme digestion in vivo and enhance the stability of peptide therapeutics. Rudra and co-workers [24] reported stereochemistry effects on murine immune responses. The model peptide antigen, OVA, was linked to the KFE8 peptide, which is composed entirely of D-amino acids. The results demonstrated that the D-amino acid peptide nanofibers enhanced the antibody response and response persistence compared to L-amino acid nanofibers.
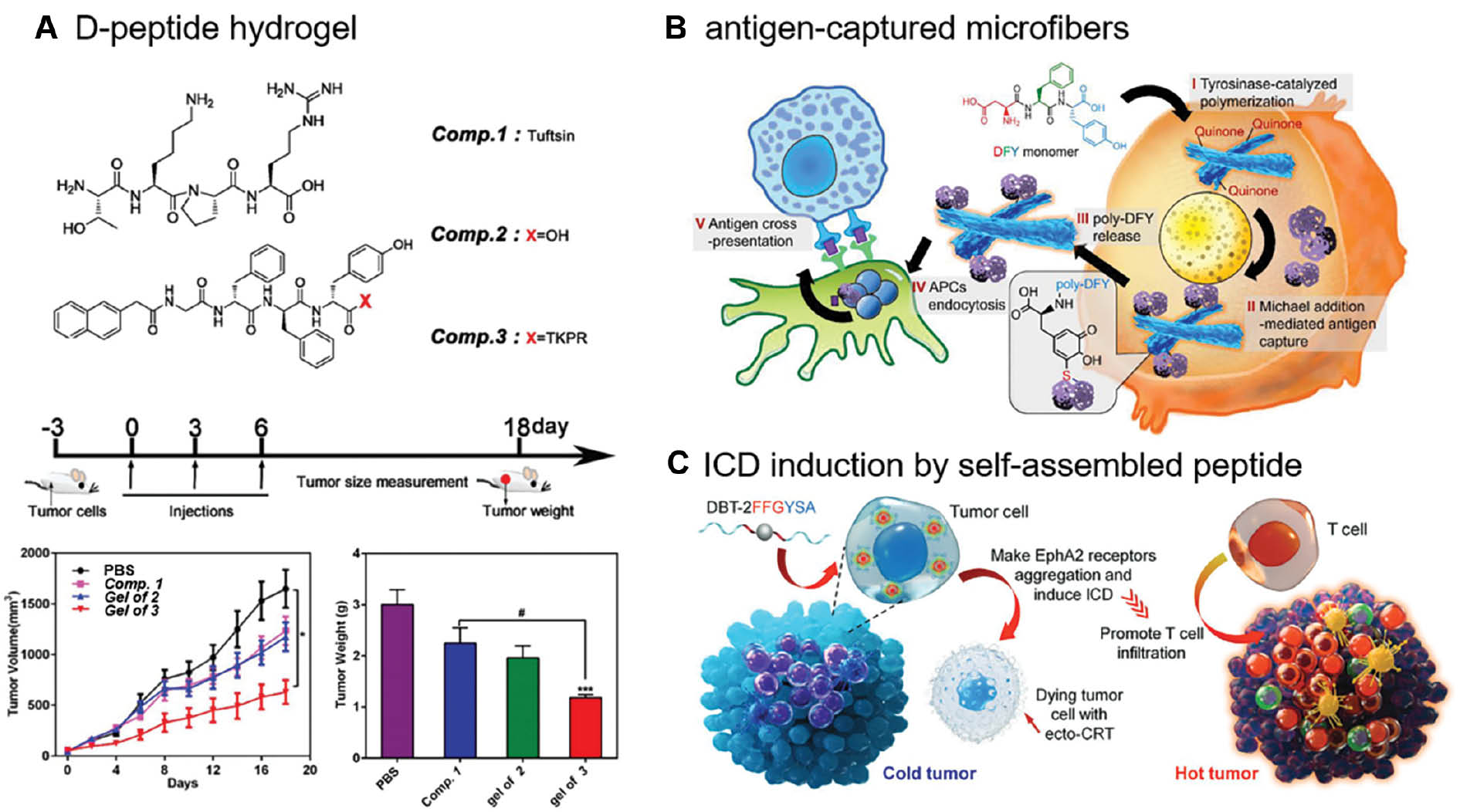
Considering the drug-carrying ability and shear-thinning property of hydrogels, researchers have designed several vaccine systems based on peptide-based hydrogels. In 2016 Yang and co-workers [25] reported the first example of a D-peptide hydrogel as an efficient self-adjuvant vaccine, providing an alternative strategy for vaccine development. The Nap-GDFDFDpY-OMe peptide co-assembled with OVA peptide to form a hydrogel upon alkaline phosphatase (ALP) hydrolysis, which could strongly induce antibody production and cytokines secretion [25]. Yang and co-workers [26] further concluded that the Nap-GDFDFDY hydrogel evoked both humoral and cellular immune responses. Moreover, the Nap-GDFDFDYTKPR hydrogel discovered on this basis combined tuftsin (TKPR) and Nap-GDFDFDY, which showed an excellent anti-tumor efficacy by stimulating a powerful CD8+ T-cell immune response, thus enhancing the phagocytic activity of macrophages and promoting the maturation of DCs (Figure 3A) [27]; however, the effects of the amino acid sequence, the nanostructure, and the properties of hydrogels on the immunostimulatory potency have not been fully elucidated. By substituting and changing the sequence of the Nap-GDFDFDY peptide, Yang and co-workers [28] demonstrated that the number of hydrophobic groups and the position of the amino acid were indispensable, which provided insight into the rational design of peptide hydrogel adjuvants.
Figure 3 Recently developed self-assembled peptide-based tumor vaccines. A, Chemical structures and anti-tumor effect of Nap-GDFDFDYTKPR peptide. Reproduced with permission from reference 27 (copyright 2012, Royal Society of Chemistry). B, The immunomodulatory mechanism of DFY peptide. The DFY peptide transforms from soluble monomers into the antigen-captured microfibers. Reproduced with permission from reference 30 (copyright 2021, American Chemical Society). C, The schematic illustration of transformation of tumor immune microenvironment mediated by a self-assembled peptide. Reproduced with permission from reference 31 (copyright 2021, Wiley).
Recently, numerous attempts have been made to discover peptide-based assemblies to serve as tumor vaccines and activate a T cell response without the aid of external antigens and other adjuvants. Yan and co-workers [29] described a self-assembled peptide fiber formed by Fmoc-FF and poly-L-lysine (PLL) in 2017 that self-assembles to form a hydrogel containing helical nanofibers through electrostatic interactions and formation of disulfide bonds. The hydrogel suppresses tumor growth by activating T cell responses [29]. Zhang and co-workers [30] constructed endogenous antigen-loaded microfibers as adjuvants and antigen vehicles based on a DFY tripeptide. This system successfully captures tumor antigens and delivers the tumor antigens to immune cells through tyrosinase-catalyzed polymerization and Michael addition cross-linking (Figure 3B) [30]. Furthermore, Ding and co-workers [31] reported a self-assembled peptide system containing a fluorophore, a self-assembling sequence (FF), and two tyrosine kinase Eph receptor A2 (EphA2) binding arms. The self-assembly of peptides promotes receptor aggregation and induces immunogenic cell death (ICD), which serves as a natural adjuvant, thus converting immunologically-cold tumors to hot tumors (Figure 3C) [31].
Summary
Despite many preclinical studies and advantages of the current strategies, some issues are in need of resolution (Table 1). A successful vaccine system must drain to the lymph nodes, capture immune cells, and induce the following immune responses [32, 33]. For self-assembled nanomaterials, it is necessary to understand the fate of self-assembled nanomaterials in vivo and the interactions between assemblies and immune cells. Moreover, current vaccine platforms focus mainly on antigenic epitopes with known amino acid sequences [34]. More effort should be devoted to developing tumor vaccines in situ because of the tumor heterogeneity and the need for personalized tumor vaccines.
Table 1 Summary of Self-Assembled Peptide-based Tumor Vaccines
| Strategies | Features | Nanostructures | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Lipopeptides | Immunoadjuvant lipid and epitope peptide | Cylindrical nanofibers | Accurate structure | The need for additional adjuvants | [11–13] |
| Epitope-bearing peptides | Self-assembled β-sheet peptide | Nanofibers | Self-adjuvant and multivalency | Imprecise mechanism of the epitope position, the lengths of the peptide, and the lateral interactions between nanofibers | [15–17] |
| Self-assembled α-helical peptide | Nanofibers | Self-adjuvant and multivalency | The known protein antigen sequence | [18, 19] | |
| Self-assembled long peptide | Nanoparticles | Intravenous vaccination, accumulation at the lymph node, and increased uptake by APCs | The need for adjuvants, the known protein antigen sequence | [21] | |
| Stimuli-induced self-assembly system | Nanoparticles | Cytoplasm delivery of antigens | The need for carriers | [23] | |
| Peptide-based hydrogels | D-amino acid self-assembled peptides | Nanofibers | Enhanced immune response and persistence | Local treatment, the known protein antigen sequence | [24–28] |
| Novel self-assembled peptide-based vaccines | Functional self-assembled peptides | Nanofibers, microfibers | Without the aid of external antigens and other adjuvants, and generate tumor vaccines in situ | Local treatment | [29–31] |
Currently, no self-assembly peptide-based vaccines are commercially available. The clinical translation remains a challenge owing to the complexity of these building blocks, large-scale manufacturing, and uncontrollable modulation of the immune system. Moreover, the adverse effects and pharmacokinetics (e.g., the timing and dosing of treatment) of self-assembled peptide vaccines need to be carefully examined before advanced clinical trials are launched. We propose several potential developing strategies for future investigation. New methods of prediction and screening of neoantigens, such as next-generation sequencing and data science, will provide a path for successful personalized tumor vaccines. In addition, innovation in vaccine delivery system enables self-assembled peptide vaccines to be spatiotemporally controlled in complex physical environments, thus allowing targeted delivery and reduced adverse effects. Because the chemo- or radio-therapy can cause ICD and enhance the immunogenicity of tumors, the combination of traditional therapy with self-assembled peptide vaccines is expected to augment the immunotherapy efficacy and produce better clinical outcomes. Given the high efficacy of self-assembly peptides and the non-cytotoxic property, we envision that multivalent self-assembled tumor vaccines will be clinically available in the near future.
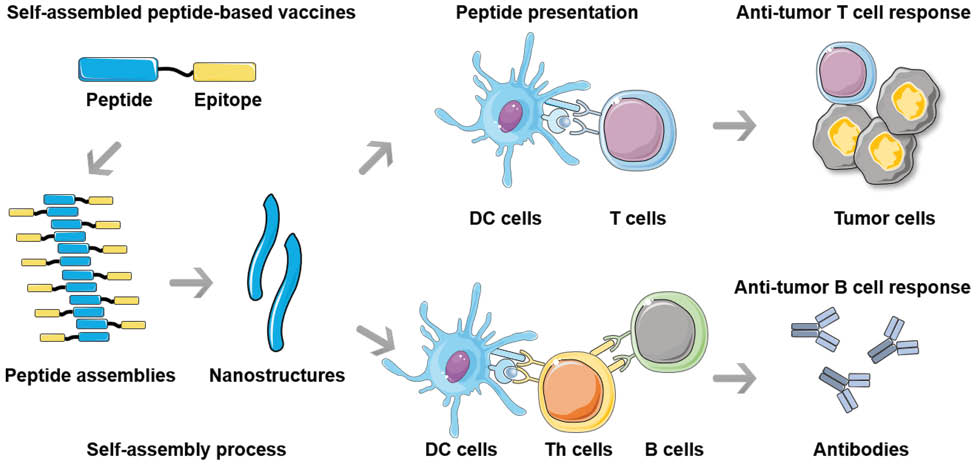
Graphical abstract
Self-assembled peptide-based vaccines, consisting of epitope-bearing peptides, can form diverse nanostructural morphologies, which could be endocytosed and presented by DCs to prime anti-tumor T cells, resulting in the induction of cellular and humoral immunity. Meanwhile, the platform can also be presented to Th cells, leading to subsequent T cell-dependent anti-tumor B cell response.
Conflict of Interest
Huaiming Wang is the Editorial Board Member of BIO Integration. He was not involved in the peer review or handling of the manuscript. The other author has no competeting interest to declare.
References
- Eskandari S, Guerin T, Toth I, Stephenson RJ. Recent advances in self-assembled peptides: implications for targeted drug delivery and vaccine engineering. Adv Drug Deliv Rev 2017;110-111:169-87. [PMID: 27356149 DOI: 10.1016/j.addr.2016.06.013]
- Abdullah T, Bhatt K, Eggermont LJ, O’Hare N, Memic A, et al. Supramolecular self-assembled peptide-based vaccines: current state and future perspectives. Front Chem 2020;8:598160. [PMID: 33195107 DOI: 10.3389/fchem.2020.598160]
- Hamley IW. Peptides for vaccine development. ACS Appl Bio Mater 2022;5(3):905-44. [PMID: 35195008 DOI: 10.1021/acsabm.1c01238]
- Li W, Su J, Li Y. Rational design of T-cell- and B-cell-based therapeutic cancer vaccines. Acc Chem Res 2022;55(18):2660-71. [PMID: 36048514 DOI: 10.1021/acs.accounts.2c00360]
- Wang J, Liu K, Xing R, Yan X. Peptide self-assembly: thermodynamics and kinetics. Chem Soc Rev 2016;45(20):5589-604. [PMID: 27487936 DOI: 10.1039/c6cs00176a]
- Yuan C, Ji W, Xing R, Li J, Gazit E, et al. Hierarchically oriented organization in supramolecular peptide crystals. Nat Rev Chem 2019;3(10):567-88. [DOI: 10.1038/s41570-019-0129-8]
- Fichman G, Gazit E. Self-assembly of short peptides to form hydrogels: design of building blocks, physical properties and technological applications. Acta Biomater 2014;10(4):1671-82. [PMID: 23958781 DOI: 10.1016/j.actbio.2013.08.013]
- Stephanopoulos N, Ortony JH, Stupp SI. Self-assembly for the synthesis of functional biomaterials. Acta Mater 2013;61(3):912-30. [PMID: 23457423 DOI: 10.1016/j.actamat.2012.10.046]
- Malonis RJ, Lai JR, Vergnolle O. Peptide-based vaccines: current progress and future challenges. Chem Rev 2020;120(6):3210-29. [PMID: 31804810 DOI: 10.1021/acs.chemrev.9b00472]
- Ma X, Xing R, Yuan C, Ogino K, Yan X. Tumor therapy based on self-assembling peptides nanotechnology. View 2020;1(4):20200020. [DOI: 10.1002/viw.20200020]
- Deres K, Schild H, Wiesmuller KH, Jung G, Rammensee HG. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature 1989;342(6249):561-4. [PMID: 2586628 DOI: 10.1038/342561a0]
- Ingale S, Wolfert MA, Gaekwad J, Buskas T, Boons GJ. Robust immune responses elicited by a fully synthetic three-component vaccine. Nat Chem Biol 2007;3(10):663-7. [PMID: 17767155 DOI: 10.1038/nchembio.2007.25]
- Black M, Trent A, Kostenko Y, Lee JS, Olive C, et al. Self-assembled peptide amphiphile micelles containing a cytotoxic T-cell epitope promote a protective immune response in vivo. Adv Mater 2012;24(28):3845-9. [PMID: 22550019 DOI: 10.1002/adma.201200209]
- Cai H, Chen M, Sun Z, Zhao Y, Kunz H, et al. Self-adjuvanting synthetic antitumor vaccines from MUC1 glycopeptides conjugated to T-cell epitopes from tetanus toxoid. Angew Chem Int Ed Engl 2013;52(23):6106-10. [PMID: 23616304 DOI: 10.1002/anie.201300390]
- Rudra JS, Tian YF, Jung JP, Collier JH. A self-assembling peptide acting as an immune adjuvant. Proc Natl Acad Sci U S A 2010;107(2):622-7. [PMID: 20080728 DOI: 10.1073/pnas.0912124107]
- Chen J, Pompano RR, Santiago FW, Maillat L, Sciammas R, et al. The use of self-adjuvanting nanofiber vaccines to elicit high-affinity B cell responses to peptide antigens without inflammation. Biomaterials 2013;34(34):8776-85. [PMID: 23953841 DOI: 10.1016/j.biomaterials.2013.07.063]
- Huang Z, Shi L, Ma J, Sun Z, Cai H, et al. A totally synthetic, self-assembling, adjuvant-free MUC1 glycopeptide vaccine for cancer therapy. J Am Chem Soc 2012;134(21):8730-3. [PMID: 22587010 DOI: 10.1021/ja211725s]
- Wu Y, Norberg PK, Reap EA, Congdon KL, Fries CN, et al. A supramolecular vaccine platform based on alpha-helical peptide nanofibers. ACS Biomater Sci Eng 2017;3(12):3128-32. [PMID: 30740520 DOI: 10.1021/acsbiomaterials.7b00561]
- Wu Y, Wen H, Bernstein ZJ, Hainline KM, Blakney TS, et al. Multiepitope supramolecular peptide nanofibers eliciting coordinated humoral and cellular antitumor immune responses. Sci Adv 2022;8(29):eabm7833. [PMID: 35857833 DOI: 10.1126/sciadv.abm7833]
- Negahdaripour M, Golkar N, Hajighahramani N, Kianpour S, Nezafat N, et al. Harnessing self-assembled peptide nanoparticles in epitope vaccine design. Biotechnol Adv 2017;35(5):575-96. [PMID: 28522213 DOI: 10.1016/j.biotechadv.2017.05.002]
- Lynn GM, Sedlik C, Baharom F, Zhu Y, Ramirez-Valdez RA, et al. Peptide-TLR-7/8a conjugate vaccines chemically programmed for nanoparticle self-assembly enhance CD8 T-cell immunity to tumor antigens. Nat Biotechnol 2020;38(3):320-32. [PMID: 31932728 DOI: 10.1038/s41587-019-0390-x]
- Grzelczak M, Liz-Marzan LM, Klajn R. Stimuli-responsive self-assembly of nanoparticles. Chem Soc Rev 2019;48(5):1342-61. [PMID: 30688963 DOI: 10.1039/c8cs00787j]
- Gong N, Zhang Y, Teng X, Wang Y, Huo S, et al. Proton-driven transformable nanovaccine for cancer immunotherapy. Nat Nanotechnol 2020;15(12):1053-64. [PMID: 33106640 DOI: 10.1038/s41565-020-00782-3]
- Appavu R, Chesson CB, Koyfman AY, Snook JD, Kohlhapp FJ, et al. Enhancing the magnitude of antibody responses through biomaterial stereochemistry. ACS Biomater Sci Eng 2015;1(7):601-9. [PMID: 33434976 DOI: 10.1021/acsbiomaterials.5b00139]
- Wang H, Luo Z, Wang Y, He T, Yang C, et al. Enzyme-catalyzed formation of supramolecular hydrogels as promising vaccine adjuvants. Adv Funct 2016;26(11):1822-9. [DOI: 10.1002/adfm.201505188]
- Luo Z, Wu Q, Yang C, Wang H, He T, et al. A powerful CD8(+) T-cell stimulating D-tetra-peptide hydrogel as a very promising vaccine adjuvant. Adv Mater 2017;29(5):1601776. [PMID: 27859662 DOI: 10.1002/adma.201601776]
- Li X, Wang Y, Wang S, Liang C, Pu G, et al. A strong CD8(+) T cell-stimulating supramolecular hydrogel. Nanoscale 2020;12(3):2111-7. [PMID: 31913398 DOI: 10.1039/c9nr08916k]
- Zhang Y, Hu Z, Li X, Ding Y, Zhang Z, et al. Amino acid sequence determines the adjuvant potency of a D-tetra-peptide hydrogel. Biomater Sci 2022;10(12):3092-8. [PMID: 35522938 DOI: 10.1039/d2bm00263a]
- Xing R, Li S, Zhang N, Shen G, Mohwald H, et al. Self-assembled injectable peptide hydrogels capable of triggering antitumor immune response. Biomacromolecules 2017;18(11):3514-23. [PMID: 28721731 DOI: 10.1021/acs.biomac.7b00787]
- Zhang Q, Zheng D, Dong X, Pan P, Zeng S, et al. A strategy based on the enzyme-catalyzed polymerization reaction of Asp-Phe-Tyr tripeptide for cancer immunotherapy. J Am Chem Soc 2021;143(13):5127-40. [PMID: 33764762 DOI: 10.1021/jacs.1c00945]
- Li J, Fang Y, Zhang Y, Wang H, Yang Z, et al. Supramolecular self-assembly-facilitated aggregation of tumor-specific transmembrane receptors for signaling activation and converting immunologically cold to hot tumors. Adv Mater 2021;33(16):e2008518. [PMID: 33734518 DOI: 10.1002/adma.202008518]
- Roth GA, Picece V, Ou BS, Luo W, Pulendran B, et al. Designing spatial and temporal control of vaccine responses. Nat Rev Mater 2022;7(3):174-95. [PMID: 34603749 DOI: 10.1038/s41578-021-00372-2]
- Vincent MP, Navidzadeh JO, Bobbala S, Scott EA. Leveraging self-assembled nanobiomaterials for improved cancer immunotherapy. Cancer Cell 2022;40(3):255-76. [PMID: 35148814 DOI: 10.1016/j.ccell.2022.01.006]
- Saxena M, van der Burg SH, Melief CJM, Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer 2021;21(6):360-78. [PMID: 33907315 DOI: 10.1038/s41568-021-00346-0]