Zebrafish: An Emerging Model for Studying Macrophage Functions in Cancer
1Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, China
*Correspondence: Linjia Jiang, E-mail: jianglj7@mail.sysu.edu.cn
Received: July 21 2022; Revised: September 30 2022; Accepted: October 18 2022; Published Online: November 1 2022
Cite this paper:
Xiuting Guo and Linjia Jiang. Zebrafish: An Emerging Model for Studying Macrophage Functions in Cancer. BIO Integration 2023; 4(1): 38–44.
DOI: 10.15212/bioi-2022-0023. Available at: https://bio-integration.org/
Download citation
© 2023 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
Zebrafish provide a convenient and unique model for studying human cancers, owing to the high similarity between zebrafish and human genomes, the availability of genetic manipulation technologies, and the availability of large numbers and transparency of zebrafish embryos. Many researchers have recently used zebrafish cancer models to examine the functions of macrophages in tumorigenesis, tumor growth and metastasis. Here, we present evidence that zebrafish cancer cells produce signals that are conserved with respect to those in humans and lead to the recruitment of heterogeneously activated macrophages in response to specific tumor types and tumorigenic stages, thereby promoting cancer initiation and progression. We also summarize how cancer cells interact with macrophages, emphasizing live imaging studies for visualization of dynamic material interchange.
Keywords
zebrafish, cancer, macrophage, recruitment, activation
Introduction
Macrophages are mononuclear phagocytes that reside in tissues and are critical for tissue development, homeostasis, innate immune responses against pathogens, tissue regeneration and autoimmune diseases [1]. Increasing evidence indicates that macrophages promote tumorigenesis, metastasis and therapeutic resistance. Macrophages that infiltrate solid tumor tissues are termed tumor-associated macrophages (TAMs), and their density in tumors is usually negatively associated with clinical outcomes [2]. The macrophage development and signaling pathways driving tumorigenesis are conserved between humans and zebrafish, thus making the zebrafish cancer model an ideal system to study the functions of macrophages in cancer.
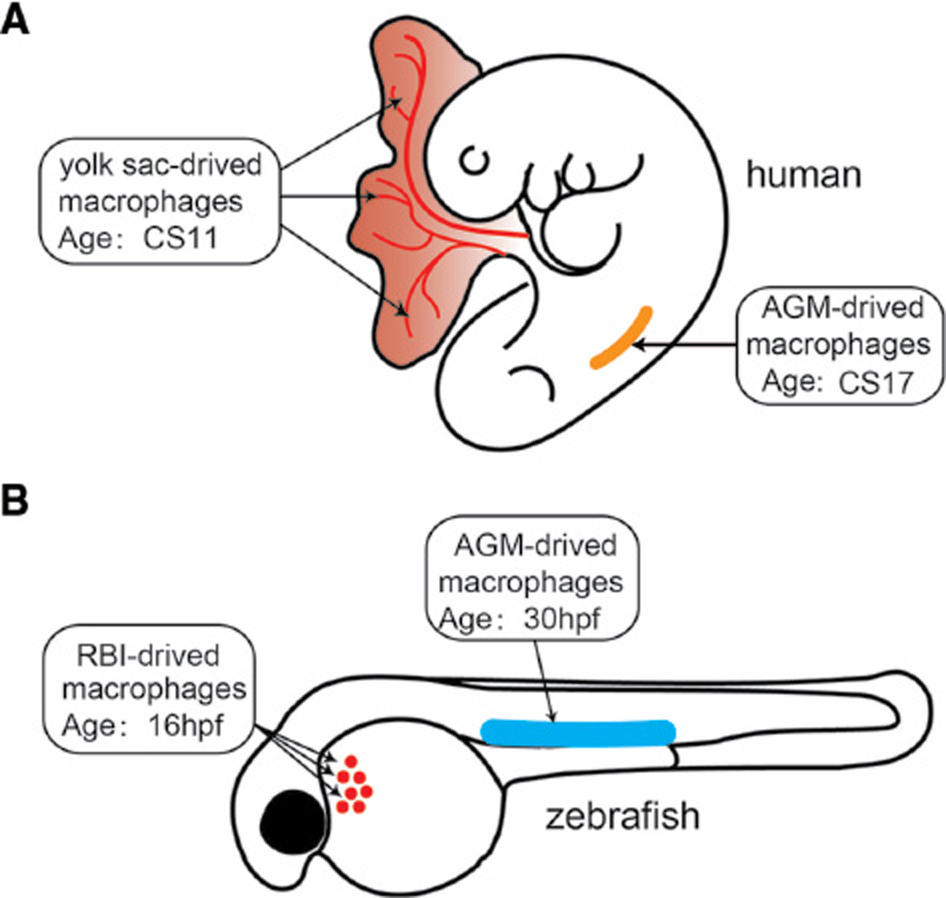
The embryonic development of macrophages between humans and zebrafish is also highly conserved (Figure 1). Human monocyte-derived macrophages originate from the yolk sac and the aorta-gonad-mesonephros, where primitive and definitive hematopoiesis occurs. These macrophages then enter the blood circulation and take up residence in various tissues, such as the brain, skin and hematopoietic tissue. Similarly, zebrafish macrophages originate from the primitive hematopoiesis in the rostral blood island and definitive hematopoiesis in the aorta-gonad-mesonephros [3, 4].
Figure 1 Macrophage development in humans and zebrafish. (A) Human macrophages first originate from the yolk sac at Carnegie stage 11 (CS11) when primitive hematopoiesis occurs. Human macrophages are then derived from the aorta-gonad-mesonephros (AGM) at CS17 when definitive hematopoiesis occurs. Similarly, zebrafish macrophages originate from the primitive hematopoiesis in the rostral blood island (RBI) at 16 hours post-fertilization (hpf), then from definitive hematopoiesis in the AGM at 30 hpf.
Zebrafish cancer models have greatly aided in understanding of the etiology and treatment of human malignancies. Overexpression of human oncogenes or mutation of tumor suppressors elicits similar tumor phenotypes in zebrafish [5, 6]. Zebrafish cancer models have provided new opportunities for studying the functions of macrophages in tumorigenesis, metastasis and therapeutic resistance. Below, we summarize recent advances, highlighting the mechanisms of macrophage recruitment into tumor tissues, the heterogeneous activation responses, and the dynamic interactions between tumors and macrophages.
The zebrafish tumor model
The signaling pathways that drive tumor progression and the pathological features of tumor tissue are substantially conserved between humans and zebrafish. Numerous unique advantages make zebrafish a powerful model system for studying cancer biology. Each pair of fertile zebrafish can regularly provide tens to hundreds of eggs every week, an amount sufficient for statistical analysis of regular experiments or even large-scale experiments. The classical transgenesis and xenograft technologies are easily accessible to create tumor models [7, 8]. The cluster regulatory interspaced short palindrome repeats (CRISPR)/Cas9 system, which cleaves double-stranded DNA at targeted gene loci, has recently been successfully used to generate knock-out and knock-in alleles in zebrafish [9]. Inhibition of pigment development in zebrafish embryos with 1-phenyl-2-thiourea [10] and the development of highly transparent Casper adult fish [11] have enabled live imaging of the dynamic interactions between tumor cells and macrophages at the single-cell level in vivo.
The development of meganuclease- and transposon-mediated transgenesis has greatly increased the efficiency of incorporating external sequences into the zebrafish genome to create transgenic models [7]. Most zebrafish cancer models are transgenic fish in which a tissue-specific promoter drives oncogene overexpression. For example, the overexpression of human oncogenic Hras driven by the Kita promoter in melanocytes shows a hyper-pigmentation phenotype at 3 days post-fertilization (dpf) and progresses to transformed melanocyte masses in the tail at 1–3 months of age [12]. Zebrafish melanomas recapitulate the histological and molecular characteristics of human melanoma and can be transplanted into other recipient fish. Activating mutations in the Kras oncogene account for approximately 7% of all human liver cancer cases. Transgenic zebrafish overexpressing oncogenic Kras (G12V) with the liver-specific fabp10 promoter show liver hyperplasia that progresses to hepatocellular carcinoma (HCC) [13, 14]. Zebrafish liver tumorigenesis is also dependent on the Ras pathway, because liver tumor size decreases when the downstream effectors of Ras are targeted in the Raf-MEK-ERK pathway. Therefore, the conservation between zebrafish and human tumorigenesis is evident in the histological and molecular hallmarks of zebrafish tumors, all of which recapitulate the human phenotype.
Inactivation of tumor suppressors in zebrafish has also been used to generate tumor models. Zebrafish with tp53 deficiency develop malignant peripheral nerve sheath tumors, sarcomas and other malignancies [15]. Tumor cells can be transplanted into syngeneic zebrafish to visualize invasion, metastasis and angiogenesis. Deleting another tumor suppressor gene, atrx, with CRISPR/Cas9, in addition to p53 deficiency, promotes the development of sarcomas and other malignancies [16].
The adaptive immune system is not fully developed and functional in zebrafish until 3 weeks post-fertilization. Therefore, immunodeficient zebrafish embryos are ideal for human cancer cell transplantation without requiring immunosuppression [17]. The xenograft cancer model is created by transplanting cultured cell lines or patient-derived primary tumor cells into zebrafish embryos at 2 dpf. Cancer cells are usually labeled with fluorescent markers such as CM-DiI before being injected into various locations of the zebrafish embryos, such as the yolk sac, perivitelline space and posterior vein, for cancer cell growth. In addition, glioblastoma cells have been transplanted into the hindbrain to create an orthotopic tumor model [18]. The xenograft zebrafish tumor model is a valuable tool for studying tumor growth, metastasis and angiogenesis [8]. Some xenografted tumor cells proliferate in the seeded region, whereas other cells migrate into the vessel and disseminate to distal areas. The Tg(flk:EGFP) zebrafish line with GFP-labeled vasculature has been used to monitor angiogenesis in tumor tissue. By using 3D imaging and time-lapse microscopy, researchers can visualize the intravasation process (from tumor cells into blood vessels) and the extravasation process (from blood vessels into target tissues) in vivo [19].
The function of macrophages in cancer
Substantial research effort has been devoted to tumor immune surveillance to develop new therapeutic strategies for treating cancer. Compared with T or B lymphocytes, innate immune cells, particularly macrophages, are the major component of the tumor immune microenvironment [20]. Tumor cells release signals that recruit tissue-resident macrophages and monocyte-derived macrophages. Macrophages exhibit either anti-tumor or pro-tumor characteristics depending on three factors: (i) tumor type, (ii) stage of tumor development and (iii) therapeutic regimen [5, 21]. However, the detailed mechanisms of macrophage involvement in tumorigenesis, metastasis and therapeutic resistance remain unclear. The innate immune systems in zebrafish and humans are developmentally and functionally conserved, thus making zebrafish a powerful tool to study the interactions between cancer cells and macrophages [22, 23].
Macrophage recruitment
A high density of infiltrated macrophages is usually associated with poor clinical outcomes for many solid tumors, although both positive and negative associations have been reported for tumors in the lungs, stomach, prostate and bone [24]. These conflicting data on the relationship between macrophage infiltration and tumor outcomes may be associated with the type/stage of cancer evaluated. In agreement with human clinical research, zebrafish studies have indicated that the recruitment of macrophages into tumor tissues is a crucial step in tumorigenesis.
Non-alcoholic fatty liver disease (NAFLD) is a clinical problem associated with the progression of HCC. Macrophage-induced inflammation plays an essential role in the advancement of NAFLD to HCC. Several studies have suggested that NAFLD escalates abnormal lipid accumulation and oxidative stress in hepatocytes, thus triggering liver inflammation and the progression to HCC [25]. Nevertheless, a proper in vivo model is required to uncover the specific mechanisms that regulate lipid metabolism and HCC pathogenesis. A zebrafish HCC model has been established from transgenic Tg(fabp10a:pt-β-cat) zebrafish expressing activated β-catenin driven by the hepatocyte-specific promoter fabp10a. Researchers have fed zebrafish larvae a high-fat diet (HFD) from 2 to 12 dpf to investigate the relationship between lipid accumulation and HCC. The HFD induces macrophage infiltration and pro-inflammatory polarization, thus enhancing HCC progression, and increasing the numbers of leukocytes and T cells in the liver. Metformin, a drug commonly used to treat diabetes, markedly decreases HFD-enhanced macrophage recruitment and HCC progression [26].
Clinical data indicate that HCC occurs more frequently and aggressively in men than women [27], and macrophage infiltration has been suggested to explain this sex-specific difference in liver tumorigenesis. In the zebrafish HCC model with inducible expression of oncogenic Kras (G12V), hepatocytes secrete tumor growth factor β1 (TGfβ1), thereby recruiting macrophages [28]. Interestingly, cortisol, an adrenal hormone produced predominantly in males, increases TGfβ1 production. Subsequently, TGfβ1 recruits more macrophages into the liver, thereby promoting hepatocyte carcinogenesis in male fish; this finding may explain the sex differences in HCC development in humans [29].
In very early stages of melanoma tumorigenesis, Hras-transformed pre-neoplastic cells produce hydrogen peroxide, which recruits macrophages. Blocking hydrogen peroxide synthesis, either pharmacologically or through morpholino-mediated knockdown of Duox, the enzyme responsible for hydrogen peroxide synthesis, has been found to decrease melanoma cell growth [30]. A glioblastoma zebrafish model has been created through neural cell overexpression of Akt, a commonly up-regulated gene in human glioblastoma. Live imaging has revealed that peripheral macrophages in the circulation are recruited into the brain parenchyma, where they become microglia [31]. Cxcl12 released from oncogenic Akt-transformed neural cells interacts with Cxcr4 in macrophages. The mutation of Cxcr4 completely abrogates macrophage recruitment and pre-neoplastic cell proliferation. Chia et al. (2019) have used live imaging with a calcium transgenic reporter to discover that the glioblastoma cells activate calcium signaling and release ATP, which recruits macrophages by interacting with the P2y12 receptor [32]. Therefore, Cxcl12-Cxcr4 and ATP-P2y12 signaling direct macrophage infiltration into glioblastoma cells and promote oncogenic proliferation.
Macrophage infiltration also participates in breast cancer resistance against estrogen-receptor-targeting therapies. However, how estrogen-receptor-targeting reagents, such as the partial agonist tamoxifen and the pure antagonist fulvestrant, affect macrophage infiltration remains to be elucidated. . Treatment with 17β-estradiol (E2 estrogen) in a breast cancer mouse model has increased extracellular Ccl2 and Ccl5, which recruit macrophages to promote cancer growth [33]. In the zebrafish xenograft model, fulvestrant, but not tamoxifen, decreases macrophage infiltration in breast cancer and thus inhibits the dissemination of breast cancer into the peripheral tissues [34].
Macrophage activation
Generally, macrophages can be classified into two polarization states according to their roles in inflammation: the classically activated M1 macrophages are pro-inflammatory, whereas the alternatively activated M2 macrophages are anti-inflammatory [35]. During tumorigenesis, tumor-associated macrophages usually initially elicit a pro-inflammatory response, then progressively become more anti-inflammatory in later stages [36, 37]. This tumor initiation process partially mimics the wound-healing response, in which the dynamic behaviors of macrophages have been revealed in zebrafish. Nguyen-Chi et al. (2015) have created Tg(mpeg1:mCherry;tnfa:GFP) zebrafish to label tumor necrosis factor-alpha (Tnfα)-expressing macrophages as mCherry+GFP+ and Tnfα negative macrophages as mCherry+GFP– [38]. The purified Tnfα-positive macrophages up-regulate M1-like markers, such as Tnfα, Il1b and Il6, whereas Tnfα-negative macrophages up-regulate M2-like markers, such as Tgfβ1, Ccr2 and Cxcr4b. This finding confirms that Tnfα-positive macrophages are inflammatory, whereas Tnfα-negative macrophages are anti-inflammatory. Fate tracing of macrophages has demonstrated that wound and bacterial infection initially trigger the recruitment of inflammatory Tnfα+ macrophages. These M1-like macrophages later convert into M2-like phenotype, thus resolving inflammation and promoting tissue repair.
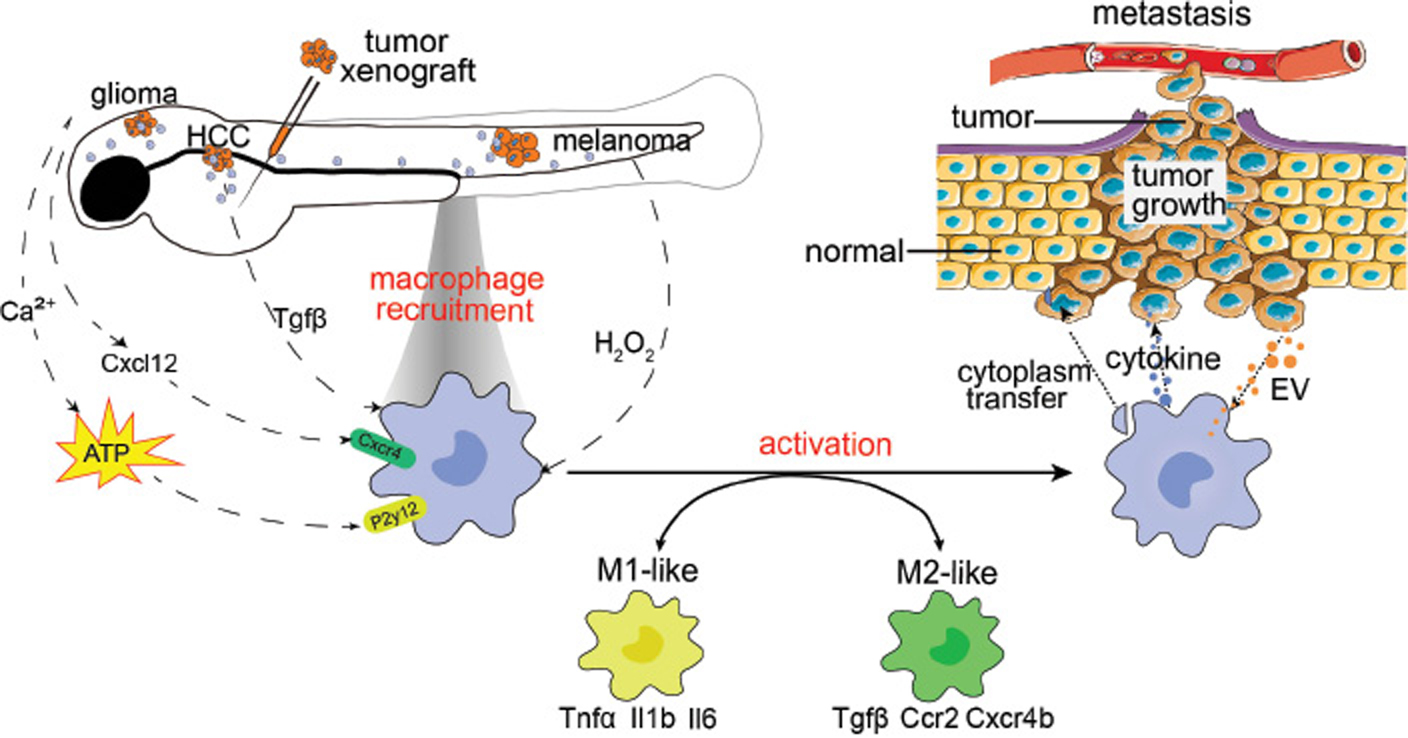
However, the balance between M1- and M2-like macrophages is disrupted in tumors, thus resulting in continual inflammation and/or immune suppression. During tumor initiation, both M1- and M2-like macrophages are involved, on the basis of studies in orthotopic zebrafish tumor models (Figure 2). In the zebrafish HCC model, recruited macrophages up-regulate inflammatory genes, including Il1b, Il6 and Csf1. Macrophage depletion decreases pre-neoplastic cell proliferation and restores the liver size to normal [29]. In an Hras-induced zebrafish melanoma model, tumor cells recruit heterogeneous macrophages exhibiting M1 and M2 markers, thus promoting melanoma growth [39].
Figure 2 Graphic model of how tumors and macrophages interact. To study macrophage function during tumorigenesis, tumor growth and metastasis, researchers have generated several zebrafish tumor models such as glioma, hepatocellular carcinoma (HCC), melanoma and tumor xenografts. Tumor cells secrete hydrogen peroxide, Cxcl12, ATP and Tgfβ, and subsequently recruit tissue-resident or peripheral macrophages into tumor tissues. Furthermore, tumor cells secrete extracellular vesicles (EV), which educate, and lead to activation of, recruited macrophages. Depending on the tumor type and tumorigenic stage, macrophages differentiate into M1-like inflammatory or M2-like anti-inflammatory phenotypes. Macrophages transfer cytoplasm and secrete cytokines that support tumor cell growth and dissemination, promote angiogenesis and suppress immune responses.
Metastasis often occurs early during tumorigenesis and is responsible for most mortality in cancer patients [40]. Macrophages at metastatic sites provide a supportive niche for metastatic tumor cells [41]. In line with human and mouse research findings, the zebrafish studies described above have revealed that M2 macrophages enhance tumor metastasis. Human tumor cells grafted into zebrafish embryos partially mimic the metastatic tumor cells disseminated into distal organs, thus allowing the zebrafish xenograft model to be used in metastatic cancer studies. Povoa et al. (2021) have shown that the engraftment efficiency of patient tumor cells into zebrafish embryos negatively correlates with the abundance of M1-like macrophages infiltrated into tumors, and positively correlates with M2-like macrophages. When transplanting primary or metastatic colorectal cancer cells from the same patient into zebrafish embryos, metastatic cancer cells, compared with primary cancer cells, have higher engraftment efficiency and lower infiltration of M1-like macrophages. Macrophage depletion by L-clodronate, which explicitly targets macrophages and induces cell death, significantly increases the engraftment efficiency of primary cancer cells [42]. In tumor engraftment, clonal evolution has been revealed by single-cell transcriptome profiling: some clones shrink, some expand, and some maintain their original size. Enrichment pathway analyses have indicated the induction of several inflammatory cytokines, such as Cx3cl1 and Cxcl1, in the shrinking clones; these cytokines might function as chemoattractants for M1-like macrophages, thus leading to clonal clearance. In contrast, Il10 signaling, the classic immunosuppression pathway, is activated in expanding clones and might be responsible for converting M1-like macrophages into M2-like macrophages that protect the tumor clones. On the basis of measurement of the number of tumor cells disseminated from the original seeding sites in the yolks of xenografted zebrafish, macrophages isolated from patients with metastatic but not non-metastatic tumors have been found to promote tumor cell dissemination [43]. In addition, metastasis is enhanced in differentiated M2 macrophages obtained from bone marrow monocytes induced by IL4, IL10 and Tgfβ. However, the same effect has not been observed in M1-like macrophages induced by IFNγ and LPS.
The influence of macrophages on tumor cells
Zebrafish tumor-associated macrophages release pro-tumor growth factors and trophic signals that promote tumor cell growth and metastasis, similarly to those found in humans and mice. The macrophages secrete inflammatory factors, such as Tnfα, which promote tumor cell survival and tumorigenesis in zebrafish liver cancer models [29, 44]. Prostaglandin E2 (PGE2) is a potent trophic signal released by macrophages that promotes pro-neoplastic cell proliferation by activating β-catenin [45, 46]. Feng et al. have identified that cyclooxygenase 2 (Cox2) is activated in macrophages, and promotes PGE2 secretion and melanoma growth. The non-steroidal anti-inflammatory drug aspirin blocks the Cox2-PGE2 pathway and inhibits melanoma growth [45].
Macrophages suppress T-cell-based immune responses against tumor growth. After the transplantation of an in vitro culture system comprising melanoma cells, macrophages and T cells into zebrafish embryos, Wu et al. have discovered that IL33-treated macrophages protect melanoma cells against T-cell-mediated killing. IL33 increases the expression of metalloprotease 9 (MMP9) in macrophages. MMP9 cleaves membrane-bound proteins in immune cells, such as NKG2D in T cells and MHCI in melanoma cells, thereby blocking T-cell-mediated anti-tumor cytotoxicity [47].
Macrophages also produce proteases that impair the extracellular matrix and stimulate angiogenesis, and consequently promote tumor progression. In addition to cleaving immune receptors, MMP-9 functions as a proteolytic enzyme that degrades the extracellular matrix [48]. By producing MMP-9, macrophages facilitate the cleavage of extracellular matrix and facilitate tumor-cell dissemination from the primary tumor site [49]. Macrophages, but not neutrophils, enhance vascularization, which is dependent on the vascular endothelial growth factor (VEGF) pathway, within xenografted tumors. Time-lapse imaging has demonstrated that infiltrated macrophages are closely associated with the growing tips of blood vessels, thus driving vessel sprouting [50]. Xenografts of VEGFA-secreting tumors, but not VEGFA-low tumors, require macrophages for vascularization, thus indicating that macrophages enhance VEGF-driven angiogenesis. The M1-like macrophages, which promote blood vessel formation and angiogenesis during embryonic development and wound healing, may be involved in tumor angiogenesis [51, 52]. Nevertheless, more evidence is necessary to support this hypothesis.
Dynamic interactions between tumor cells and macrophages
The transparency and genetic tractability of zebrafish embryos enable the recording of dynamic interactions between fluorophore-labeled tumor cells and macrophages. Tumor cells activate macrophages through either direct cell-cell contact or secreted extracellular vesicles (EVs). Through another route, macrophages directly transfer their cytoplasm into tumor cells and consequently promote metastasis.
Macrophages closely associate with melanoma cells for sustained periods [30]. Capturing of tumor EVs by macrophages is crucial for macrophage activation. The dynamic routes of this process have been precisely documented after transplantation of fluorophore-labeled melanoma-derived EVs into zebrafish. Scans with 3D transmission electron microscopy and time-lapse imaging have revealed the dynamic localization and internalization of EVs by patrolling macrophages at high spatiotemporal resolution. The macrophages in the blood vessel lumen extend broad protrusions that capture EVs, which are stored in the late endosome-lysosome. Tumor EVs activate macrophages, thereby inducing inflammatory cytokines and promoting melanoma metastasis [53].
After transplantation of melanoma cells into zebrafish embryos, the interaction between macrophages and tumor cells has been recorded through high-resolution live imaging. Roh-Johnson et al. have used a Cre/LoxP-mediated GFP-expressing system wherein the transfer of macrophage cytoplasm, including Cre recombinase, results in a melanoma color switch from DsRed to GFP. With the Cre/LoxP system as a readout, the observations have revealed that Tnfα-negative M2-like macrophages are in contact with tumor cells for sustained time periods and transfer cytoplasm into tumor cells. The cytoplasmic transfer between macrophages and tumor cells is essential for tumor cell dissemination to distal regions [39].
Conclusion
The zebrafish genetic and tumor-cell-xenografted cancer models combined with macrophage-specific reporter models are valuable tools for studying the roles of macrophages in tumorigenesis, tumor growth and metastasis. Zebrafish models have revealed that tumor cells secrete signals that recruit tissue-resident or peripheral macrophages into tumor tissues. Depending on the tumor type and tumorigenic stage, macrophages are activated into M1-like inflammatory or M2-like anti-inflammatory phenotypes. Activated macrophages in turn influence tumor cells by transferring cytoplasm and secreting cytokines, thereby supporting tumor growth and dissemination, promoting angiogenesis and suppressing immune responses (Table 1).
Table 1 Interactions between tumors and macrophages
| Tumor type | Tumor signal for recruiting macrophages | Macrophage signal | Effects on tumors | Zebrafish cancer model | Refs. |
|---|---|---|---|---|---|
| Liver cancer | Tgfβ1 | Tnfα | Tumorigenesis | Transgenic larvae | [28, 29, 44] |
| Melanoma | H2O2 | Cox-PEG2, MMP9 | Tumor growth, immune escape | Transgenic larvae | [30, 45–47] |
| Glioma | Cxcl12, ATP | N.A. | Tumor growth | Transgenic larvae | [31–32] |
| Breast cancer | Ccl2, Ccl5 | VEGF | Enhanced vascularization | Xenografted embryo | [33, 50] |
Future perspectives
Although several zebrafish cancer models have been established, zebrafish researchers must develop new models or optimize the existing models. For example, zebrafish leukemia models usually require a very long time (9–24 months) to manifest a phenotype, thus posing challenges in the analysis of macrophage function [54]. Manipulating novel oncogenes in zebrafish or combining several models may aid in rapid development of severe phenotypes. M1 macrophages are usually classified as anti-tumor, and M2 macrophage are usually classified as pro-tumor. However, the classification of M1 or M2 macrophages is somewhat oversimplified, because studies have shown that TAMs are a heterogeneous population. Clarifying the TAM heterogeneity at the single-cell level and integrating such information into functional studies will be necessary in future research. Furthermore, single-cell studies would help identify novel macrophage biomarkers that could be used to generate reporters for labeling novel macrophage populations and discovering their functions.
Acknowledgments
We thank Professor Phei Er Saw for critically reading and editing the manuscript.
Funding
We acknowledge the National Key R&D Program of China (2018YFA0108304), Guangdong Basic and Applied Basic Research Foundation (2021B1515020012), NSFC (31871467) for supporting this project.
References
- Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol 2014;14:392-404. [PMID: 24854589 DOI: 10.1038/nri3671]
- Komohara Y, Jinushi M, Takeya M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci 2014;105:1-8. [PMID: 24168081 DOI: 10.1111/cas.12314]
- Herbomel P, Thisse B, Thisse C. Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development 1999;126:3735-45. [PMID: 10433904 DOI: 10.1242/dev.126.17.3735]
- Xu J, Du L, Wen Z. Myelopoiesis during zebrafish early development. J Genet Genomics 2012;39:435-42. [PMID: 23021543 DOI: 10.1016/j.jgg.2012.06.005]
- Elliot A, Myllymaki H, Feng Y. Inflammatory responses during tumour initiation: from zebrafish transgenic models of cancer to evidence from mouse and man. Cells 2020;9:1018. [PMID: 32325966 DOI: 10.3390/cells9041018]
- Hason M, Bartunek P. Zebrafish msodels of cancer-new insights on modeling human cancer in a non-mammalian vertebrate. Genes (Basel) 2019;10:935. [PMID: 31731811 DOI: 10.3390/genes10110935]
- Kawakami K. Transposon tools and methods in zebrafish. Dev Dyn 2005;234:244-54. [PMID: 16110506 DOI: 10.1002/dvdy.20516]
- Chen X, Li Y, Yao T, Jia R. Benefits of zebrafish xenograft models in cancer research. Front Cell Dev Biol 2021;9:616551. [PMID: 33644052 DOI: 10.3389/fcell.2021.616551]
- Liu K, Petree C, Requena T, Varshney P, Varshney GK. Expanding the CRISPR toolbox in zebrafish for studying development and disease. Front Cell Dev Biol 2019;7:13. [PMID: 30886848 DOI: 10.3389/fcell.2019.00013]
- Chen XK, Kwan JS, Chang RC, Ma AC. 1-phenyl 2-thiourea (PTU) activates autophagy in zebrafish embryos. Autophagy 2021;17:1222-31. [PMID: 32286915 DOI: 10.1080/15548627.2020.1755119]
- White RM, Sessa A, Burke C, Bowman T, LeBlanc J, et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2008;2:183-9. [PMID: 18371439 DOI: 10.1016/j.stem.2007.11.002]
- Santoriello C, Gennaro E, Anelli V, Distel M, Kelly A, et al. Kita driven expression of oncogenic HRAS leads to early onset and highly penetrant melanoma in zebrafish. PLoS One 2010;5:e15170. [PMID: 21170325 DOI: 10.1371/journal.pone.0015170]
- Nguyen AT, Emelyanov A, Koh CH, Spitsbergen JM, Lam SH, et al. A high level of liver-specific expression of oncogenic Kras(V12) drives robust liver tumorigenesis in transgenic zebrafish. Dis Model Mech 2011;4:801-13. [PMID: 21729876 DOI: 10.1242/dmm.007831]
- Nguyen AT, Emelyanov A, Koh CH, Spitsbergen JM, Parinov S, et al. An inducible kras(V12) transgenic zebrafish model for liver tumorigenesis and chemical drug screening. Dis Model Mech 2012;5:63-72. [PMID: 21903676 DOI: 10.1242/dmm.008367]
- Ignatius MS, Hayes MN, Moore FE, Tang Q, Garcia SP, et al. tp53 deficiency causes a wide tumor spectrum and increases embryonal rhabdomyosarcoma metastasis in zebrafish. eLife 2018;7:e37202. [PMID: 30192230 DOI: 10.7554/eLife.37202]
- Oppel F, Tao T, Shi H, Ross KN, Zimmerman MW, et al. Loss of atrx cooperates with p53-deficiency to promote the development of sarcomas and other malignancies. PLoS Genet 2019;15:e1008039. [PMID: 30970016 DOI: 10.1371/journal.pgen.1008039]
- Konantz M, Balci TB, Hartwig UF, Dellaire G, Andre MC, et al. Zebrafish xenografts as a tool for in vivo studies on human cancer. Ann N Y Acad Sci 2012;1266:124-37. [PMID: 22901264 DOI: 10.1111/j.1749-6632.2012.06575.x]
- Rampazzo E, Persano L, Pistollato F, Moro E, Frasson C, et al. Wnt activation promotes neuronal differentiation of glioblastoma. Cell Death Dis 2013;4:e500. [PMID: 23429286 DOI: 10.1038/cddis.2013.32]
- Fornabaio G, Barnhill RL, Lugassy C, Bentolila LA, Cassoux N, et al. Angiotropism and extravascular migratory metastasis in cutaneous and uveal melanoma progression in a zebrafish model. Sci Rep 2018;8:10448. [PMID: 29992995 DOI: 10.1038/s41598-018-28515-6]
- Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med 2015;21:938-45. [PMID: 26193342 DOI: 10.1038/nm.3909]
- Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell 2015;27:462-72. [PMID: 25858805 DOI: 10.1016/j.ccell.2015.02.015]
- Miao KZ, Kim GY, Meara GK, Qin X, Feng H. Tipping the scales with zebrafish to understand adaptive tumor immunity. Front Cell Dev Biol 2021;9:660969. [PMID: 34095125 DOI: 10.3389/fcell.2021.660969]
- Rosowski EE. Illuminating macrophage contributions to host-pathogen interactions in vivo: the power of zebrafish. Infect Immun 2020;88:e00906-19. [PMID: 32179583 DOI: 10.1128/IAI.00906-19]
- Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One 2012;7:e50946. [PMID: 23284651 DOI: 10.1371/journal.pone.0050946]
- Meli R, Mattace Raso G, Calignano A. Role of innate immune response in non-alcoholic fatty liver disease: metabolic complications and therapeutic tools. Front Immunol 2014;5:177. [PMID: 24795720 DOI: 10.3389/fimmu.2014.00177]
- de Oliveira S, Houseright RA, Graves AL, Golenberg N, Korte BG, et al. Metformin modulates innate immune-mediated inflammation and early progression of NAFLD-associated hepatocellular carcinoma in zebrafish. J Hepatol 2019;70:710-21. [PMID: 30572006 DOI: 10.1016/j.jhep.2018.11.034]
- Yeh YT, Chang CW, Wei RJ, Wang SN. Progesterone and related compounds in hepatocellular carcinoma: basic and clinical aspects. Biomed Res Int 2013;2013:290575. [PMID: 23484104 DOI: 10.1155/2013/290575]
- Yan C, Huo X, Wang S, Feng Y, Gong Z. Stimulation of hepatocarcinogenesis by neutrophils upon induction of oncogenic kras expression in transgenic zebrafish. J Hepatol 2015;63:420-8. [PMID: 25828472 DOI: 10.1016/j.jhep.2015.03.024]
- Yan C, Yang Q, Gong Z. Tumor-associated neutrophils and macrophages promote gender disparity in hepatocellular carcinoma in zebrafish. Cancer Res 2017;77:1395-407. [PMID: 28202512 DOI: 10.1158/0008-5472.CAN-16-2200]
- Feng Y, Santoriello C, Mione M, Hurlstone A, Martin P. Live imaging of innate immune cell sensing of transformed cells in zebrafish larvae: parallels between tumor initiation and wound inflammation. PLoS Biol 2010;8:e1000562. [PMID: 21179501 DOI: 10.1371/journal.pbio.1000562]
- Chia K, Mazzolini J, Mione M, Sieger D. Tumor initiating cells induce Cxcr4-mediated infiltration of pro-tumoral macrophages into the brain. eLife 2018;7:e31918. [PMID: 29465400 DOI: 10.7554/eLife.31918]
- Chia K, Keatinge M, Mazzolini J, Sieger D. Brain tumours repurpose endogenous neuron to microglia signalling mechanisms to promote their own proliferation. eLife 2019;8:e46912. [PMID: 31313988 DOI: 10.7554/eLife.46912]
- Svensson S, Abrahamsson A, Rodriguez GV, Olsson AK, Jensen L, et al. CCL2 and CCL5 are novel therapeutic targets for estrogen-dependent breast cancer. Clin Cancer Res 2015;21:3794-805. [PMID: 25901081 DOI: 10.1158/1078-0432.CCR-15-0204]
- Abrahamsson A, Rodriguez GV, Dabrosin C. Fulvestrant-mediated attenuation of the innate immune response decreases ER(+) breast cancer growth in vivo more effectively than tamoxifen. Cancer Res 2020;80:4487-99. [PMID: 32855207 DOI: 10.1158/0008-5472.CAN-20-1705]
- Rhee I. Diverse macrophages polarization in tumor microenvironment. Arch Pharm Res 2016;39:1588-96. [PMID: 27562774 DOI: 10.1007/s12272-016-0820-y]
- Gabrilovich DI. Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012;12:253-68. [PMID: 22437938 DOI: 10.1038/nri3175]
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010;141:39-51. [PMID: 20371344 DOI: 10.1016/j.cell.2010.03.014]
- Nguyen-Chi M, Laplace-Builhe B, Travnickova J, Luz-Crawford P, Tejedor G, et al. Identification of polarized macrophage subsets in zebrafish. eLife 2015;4:e07288. [PMID: 26154973 DOI: 10.7554/eLife.07288]
- Roh-Johnson M, Shah AN, Stonick JA, Poudel KR, Kargl J, et al. Macrophage-dependent cytoplasmic transfer during melanoma invasion in vivo. Dev Cell 2017;43:549-62:e6. [PMID: 29207258 DOI: 10.1016/j.devcel.2017.11.003]
- Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell 2011;147:275-92. [PMID: 22000009 DOI: 10.1016/j.cell.2011.09.024]
- Mantovani A, Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med 2015;212:435-45. [PMID: 25753580 DOI: 10.1084/jem.20150295]
- Povoa V, Rebelo de Almeida C, Maia-Gil M, Sobral D, Domingues M, et al. Innate immune evasion revealed in a colorectal zebrafish xenograft model. Nat Commun 2021;12:1156. [PMID: 33608544 DOI: 10.1038/s41467-021-21421-y]
- Wang J, Cao Z, Zhang XM, Nakamura M, Sun M, et al. Novel mechanism of macrophage-mediated metastasis revealed in a zebrafish model of tumor development. Cancer Res 2015;75:306-15. [PMID: 25492861 DOI: 10.1158/0008-5472.CAN-14-2819]
- de Oliveira S, Houseright RA, Korte BG, Huttenlocher A. DnaJ-PKAc fusion induces liver inflammation in a zebrafish model of fibrolamellar carcinoma. Dis Model Mech 2020;13:dmm042564. [PMID: 32102783 DOI: 10.1242/dmm.042564]
- Feng Y, Renshaw S, Martin P. Live imaging of tumor initiation in zebrafish larvae reveals a trophic role for leukocyte-derived PGE(2). Curr Biol 2012;22:1253-9. [PMID: 22658594 DOI: 10.1016/j.cub.2012.05.010]
- Gala MK, Chan AT. Molecular pathways: aspirin and Wnt signaling-a molecularly targeted approach to cancer prevention and treatment. Clin Cancer Res 2015;21:1543-48. [PMID: 25501125 DOI: 10.1158/1078-0432.CCR-14-0877]
- Wu J, Chen Z, Wickstrom SL, Gao J, He X, et al. Interleukin-33 is a novel immunosuppressor that protects cancer cells from TIL killing by a macrophage-mediated shedding mechanism. Adv Sci (Weinh) 2021;8:e2101029. [PMID: 34486239 DOI: 10.1002/advs.202101029]
- Travnickova J, Tran Chau V, Julien E, Mateos-Langerak J, Gonzalez C, et al. Primitive macrophages control HSPC mobilization and definitive haematopoiesis. Nat Commun 2015;6:6227. [PMID: 25686881 DOI: 10.1038/ncomms7227]
- van Kempen LC, Coussens LM. MMP9 potentiates pulmonary metastasis formation. Cancer Cell 2002;2:251-2. [PMID: 12398887 DOI: 10.1016/s1535-6108(02)00157-5]
- Britto DD, Wyroba B, Chen W, Lockwood RA, Tran KB, et al. Macrophages enhance Vegfa-driven angiogenesis in an embryonic zebrafish tumour xenograft model. Dis Model Mech 2018;11:dmm035998. [PMID: 30396905 DOI: 10.1242/dmm.035998]
- Gerri C, Marin-Juez R, Marass M, Marks A, Maischein HM, et al. Hif-1α regulates macrophage-endothelial interactions during blood vessel development in zebrafish. Nat Commun 2017;8:15492. [PMID: 28524872 DOI: 10.1038/ncomms15492]
- Gurevich DB, Severn CE, Twomey C, Greenhough A, Cash J, et al. Live imaging of wound angiogenesis reveals macrophage orchestrated vessel sprouting and regression. EMBO J 2018;37:e97786. [PMID: 29866703 DOI: 0.15252/embj.201797786]
- Hyenne V, Ghoroghi S, Collot M, Bons J, Follain G, et al. Studying the fate of tumor extracellular vesicles at high spatiotemporal resolution using the zebrafish embryo. Dev Cell 2019;48:554-72.e7. [PMID: 30745140 DOI: 10.1016/j.devcel.2019.01.014]
- Xu M, Ye Y, Ye Z, Xu S, Liu W, et al. Human BCR/ABL1 induces chronic myeloid leukemia-like disease in zebrafish. Haematologica 2020;105:674-86. [PMID: 31289206 DOI: 10.3324/haematol.2019.215939]