Roles of Tenascin-XB in the Glioma Immune Microenvironment
1Department of Neurosurgery, Nanfang Hospital, Southern Medical University, Guangzhou Dadao Bei Street 1838, Guangzhou 510515, Guangdong, People’s Republic of China
2Department of Neurosurgery, Brain Injury Center, Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, People’s Republic of China
3Department of Obstetrics and Gynecology, Baiyun Branch, Nanfang Hospital, Southern Medical University, Guangzhou 123123, People’s Republic of China
4Department of stomatology, the Seventh Affiliated Hospital of Sun Yat-sen University, Shenzhen 518107, China
aThese authors contributed equally to this study.
*Correspondence to: Shanqiang Qu, MD, PhD, E-mail: qushq3@163.com, Tel: 86-20-61641806, Fax: 86-20-61641806, https://orcid.org/0000-0002-2709-0101
Received: April 20 2022; Revised: June 9 2022; Accepted: June 21 2022; Published Online: July 7 2022
Cite this paper:
Chaofu Ma, Ouwen Qiu, Chengying Huang, Jing Huang and Shanqiang Qu. Roles of Tenascin-XB in the Glioma Immune Microenvironment. BIO Integration 2023; 4(1): 18–29.
DOI: 10.15212/bioi-2022-0014. Available at: https://bio-integration.org/
Download citation
© 2023 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
Background: Previous studies have reported the critical roles of tumor cells and the tumor microenvironment in tumor prognosis and immunotherapeutic response. However, how Tenascin-XB (TNXB) expression relates to glioma prognosis and to the levels of tumor-infiltrating immune cells in various cancers has remained elusive. Therefore, this work aimed to investigate the expression, prognostic value, biological function and correlation between TNXB expression and the levels of tumor-infiltrating immune cells in glioma tissues.
Methods: First, we explored TNXB expression in glioma tissues by using online biological databases. Second, we assessed the clinical importance of TNXB expression with chi-squared tests, Cox regression and Kaplan-Meier curve analyses. Third, we examined the relationship between TNXB expression and the levels of tumor-infiltrating immune cells in glioma tissues in an online database. Additionally, we assessed the associations of TNXB expression with genetic markers of immune cells and common immune-checkpoint molecules.
Results: Elevated TNXB expression in glioma tissues correlated with tumor grade, according to several databases. Elevated TNXB expression was significantly associated with negative clinicopathological manifestations and poorer prognosis, on the basis of TCGA (n=510) data. Furthermore, univariate and multivariate Cox regression indicated that TNXB was an independent indicator of glioma prognosis. Pathway enrichment analyses suggested that TNXB participates in the immune response, humoral immune response and interferon-gamma-mediated signaling pathways. Importantly, TNXB expression was significantly associated with higher levels of tumor-infiltrating immune cells in diverse cancers. Furthermore, TNXB expression was strongly associated with genetic markers of immune cells and common immune-checkpoint molecules (e.g., PD-1, PD-L1, CTLA4, TIM-3, LAG3, PDCD1LG2, TIGIT and Siglec-15).
Conclusions: TNXB expression correlates with poorer prognosis and higher levels of tumor-infiltrating immune cells in several cancers. In addition, TNXB expression is likely to contribute to the regulation of dendritic cells, exhausted T cells, regulatory T cells and tumor-associated macrophages in gliomas. Consequently, TNXB may serve as an important prognostic marker and may play an immunomodulatory role in tumors.
Keywords
Immune checkpoint molecules, Prognostic value, Tenascin-XB expression, Tumor microenvironments.
Introduction
Glioma is the most prevalent malignant primary cancer of the brain. Despite the various therapies available for glioma, this cancer remains lethal with poor prognosis. The median survival after glioma diagnosis is 5 years [1]. Although radiation therapy and chemotherapy with temozolomide (TMZ) lengthen survival and improve quality of life, the survival advantages remain palliative [2]. Therefore, more effective therapeutic strategies are urgently needed to prolong overall survival in patients with glioma.
Immunotherapy has recently become a major focus among researchers worldwide [3] and has achieved great advances since 2015 [4]. Programmed cell death protein 1 (PD-1) blockade has become an important therapy for Hodgkin’s lymphoma [5]. However, the response rates of immunotherapy are modest in solid tumors (e.g., brain glioma) [6]. Many patients do not respond to immunosuppressive drugs [7]. Thus, elucidating the detailed molecular mechanism underlying tumor-cell sensitivity to T cells should have enormous clinical value. Recently, numerous studies have suggested that the immune response to immunosuppressive drugs is strongly associated with the tumor microenvironment (TME) [8–11]. The TME contains a variety of secretory proteins that act on immune checkpoints of T cells and inhibit natural-killer T cells [12]. Immunoediting has indicated a dynamic interaction between rapidly developing malignant cells and the immune system, characterized by an ability to provoke highly specific and clonal responses against tumor cells [13]. Wu et al. have found that tumor-associated macrophages (TAMs) induce the invasive and metastatic ability of bladder cancer cells through CXCL814. Furthermore, the infiltration of TAMs in the TME increases levels of CXCL8, thereby stimulating the secretion of matrix metalloproteinase-9 and E-cadherin in bladder cancer cells [14]. In the TME, the mechanism of tumor immune escape is highly complicated, and genomic instability contributes to the evasion of targeted therapy in cancer cell metastasis [15]. Thus, further study of the TME is essential to discover potential new targets and to identify specific molecules for screening patient sensitivity to immunotherapy.
The Tenascin family in mammals comprises five subtypes: Tenascin-C, Tenascin-R, Tenascin-X, Tenascin-XB (TNXB) and Tenascin-N [11]. TNXB facilitates the association between cells and the extracellular matrix, thus increasing epithelial tumor progression [16]. Importantly, increasing evidence suggests that TNXB participates in the malignant biological progress of tumors and may be used as an independent prognostic marker [17, 18]. However, the role of TNXB in the TME of gliomas has remained elusive.
In the present study, we first explored the association between TNXB expression and patients’ clinicopathological manifestations through large-sample datasets and further examined the clinical prognostic value of TNXB in patients with glioma. More importantly, we assessed the relationship between TNXB expression and the levels of tumor-infiltrating immune cells and common immune-checkpoint molecules in a variety of tumor types.
Materials and methods
Gene-expression data
To identify the role of TNXB, we compared TNXB expression between glioma tissues and normal brain tissues. The mRNA-sequencing profiles of TNXB were obtained from the Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/gds/), Oncomine (http://www.oncomine.org) and The Cancer Genome Atlas (TCGA; https://tcga-data.nci.nih.gov/tcga/) databases. The analysis of TCGA data was conducted through UALCAN (http://ualcan.path.uab.edu/index.html).
Patients
To clarify the clinicopathological importance of TNXB in gliomas, we collected patients’ clinical data from the CGGA database (http://cgga.org.cn/, Dataset ID: mRNAseq_325). Data for 220 patients with glioma who completed follow-up, with a mean age of 44 years, were analyzed. The demographic characteristics are summarized in Table S1. This work was approved by the Ethics Committee of Southern Hospital. Informed consent was not required, because the data analyzed were all from public online databases.
Validation cohort
To determine the prognostic role of TNXB, we used another independent cohort of 510 patients with low-grade glioma (LGG) from TCGA database as a validation cohort. In this cohort, the patients’ mean age was 42.9 years, and 55.3% (282) were men.
Biological functions of TNXB in patients with LGG
To better understand the biological functions of TNXB in patients with LGG, we performed Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses in R programming language. To identify interactions among proteins and among differentially expressed genes, we used the Search Tool for the Retrieval of Interacting Genes (STRING) database (https://www.string-db.org/) [19], an authoritative database for evaluating protein interactions. To detect similarities among protein domains of TNXB, we used the GeneMANIA database (https://genemania.org/) [20], a web interface for generating hypotheses regarding gene function, providing information on interactions among protein and genes, pathways, co-expression and co-localization.
TIMER database analysis
To systematically explore the association between TNXB expression and immune cells or immune molecules, we used the Tumor Immune Estimation Resource (TIMER; https://cistrome.shinyapps.io/timer/) [21] database for analysis, in which six previously released statistical modules were applied to explore the association between gene expression and the infiltration of immune cells in tumors.
Statistical analysis
Statistical analysis was performed in SPSS version 22.0 (IBM, USA), GraphPad version 7.3 (GraphPad Software Inc., USA) and R 3.6.1 programming language. Chi-square tests were used to compare categorical variables, and Student’s t-tests were used to compare continuous variables; otherwise, Mann-Whitney U tests were used. Correlation analysis was performed with Spearman’s rank correlation coefficient. Overall survival of patients was analyzed with Kaplan-Meier survival curves. Prognostic value was analyzed with univariate and multivariate Cox regression. The value of diagnostic biomarkers was analyzed with receiver operating characteristic curves. A P-value <0.05 was considered statistically significant (*P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001).
Results
TNXB expression is upregulated in glioma tissues
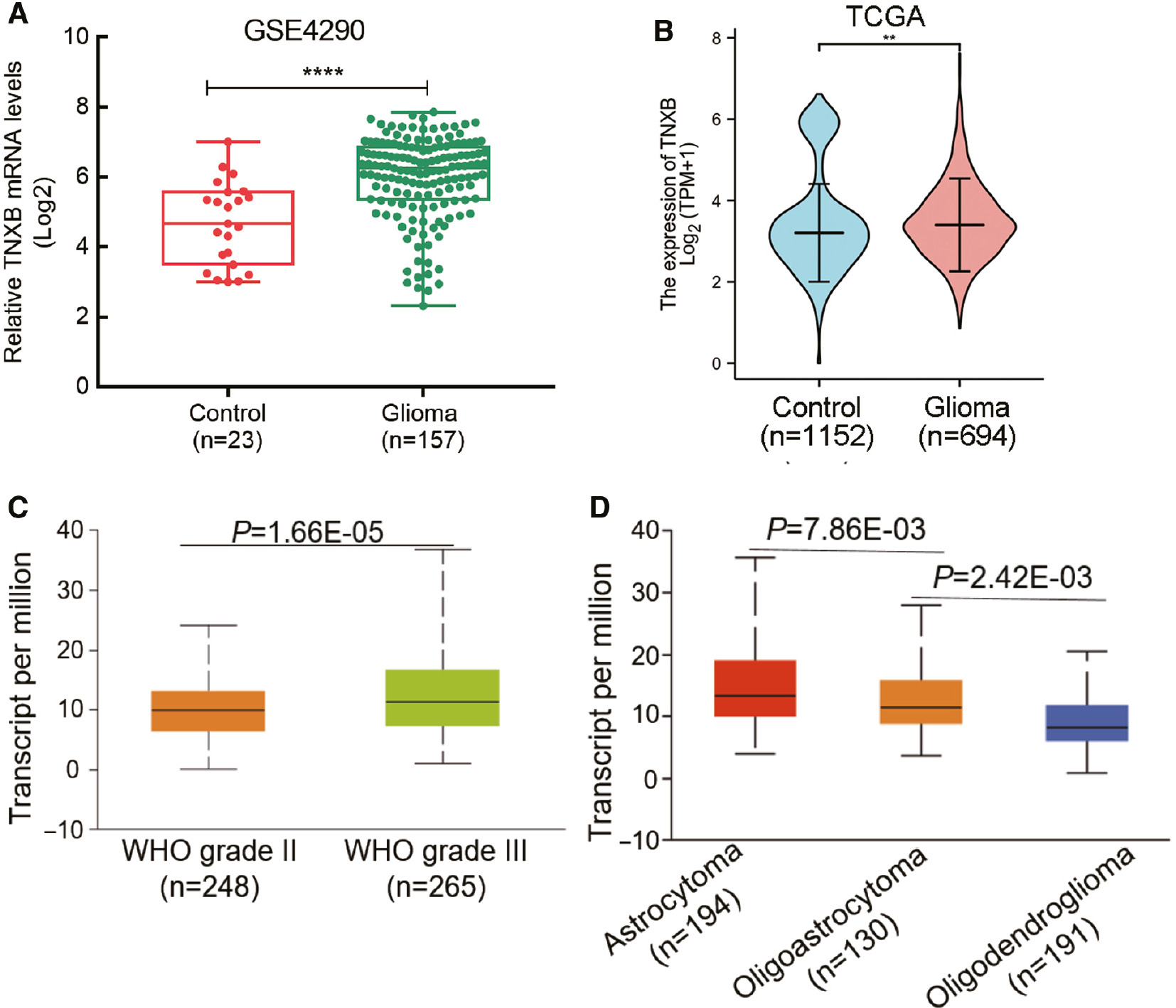
First, we compared TNXB expression between normal tissues and glioma tissues through analyzing RNA-Seq data. In comparison to normal tissues, in glioma tissues, the TNXB expression was markedly upregulated in the GEO dataset (ID: GSE4290, Figure 1A). As expected, we observed comparable results between the TCGA and Oncomine databases (Figure 1B and Table S2, respectively). TNXB expression was significantly higher in World Health Organization (WHO) grade III glioma than in WHO grade II glioma (Figure 1C). Moreover, TNXB expression differed among tissues with various stages of malignant pathology (Figure 1D). Together, these findings indicated significantly elevated TNXB expression in glioma tissues, which was markedly associated with the WHO histologic grade of gliomas.
Figure 1 TNXB mRNA is markedly overexpressed in gliomas. (A, B) TNXB is overexpressed in glioma tissues compared with normal tissues in the GEO and TCGA datasets. (C, D) TNXB mRNA levels correlate with glioma WHO grade and histopathology.
TNXB overexpression is associated with malignant behaviors of glioma
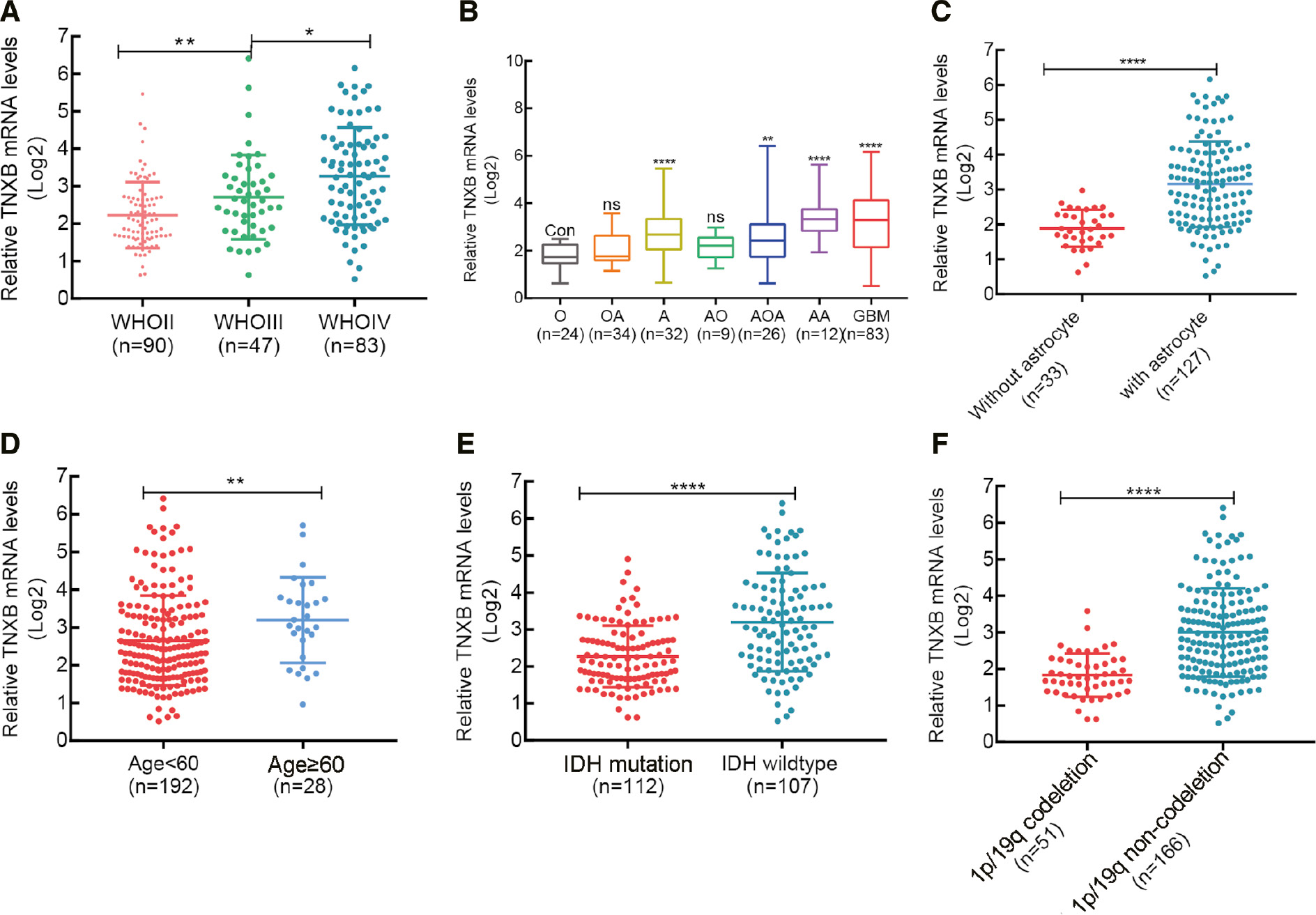
To further confirm the above results, we divided 220 patients with glioma into two groups according to WHO histologic grade, histopathology, astrocytes, patient age, 1p/19q codeletion status and isocitrate dehydrogenase (IDH) mutational status. We observed that TNXB expression directly correlated with tumor grade (Figure 2A) and astrocytes (Figure 2B, C), and was associated with older age, wild-type IDH and 1p/19q non-codeletion in patients with glioma (Figure 2D–F). Additionally, we assessed differences in patients’ clinicopathological features between two groups with differing TNXB expression. Chi-square tests revealed significant associations between TNXB expression and age, WHO grade, histopathology, IDH mutational status and 1p/19q status, but not sex, radiotherapy and chemotherapy (Table 1). Furthermore, TNXB expression was closely associated with malignant glioma progression.
Figure 2 Correlation between TNXB expression and patients’ clinicopathological manifestations in a CGGA glioma cohort (n=220). (A–F) TNXB expression correlates with WHO grade, histopathology, astrocytic components of glioma, patient age, and IDH and 1p/19q status.
Table 1 Association between TNXB Expression and Clinical Characteristics in Gliomas
| Features | TNXB Expression | P-value | |
|---|---|---|---|
| Low | High | ||
| Age | |||
| ≥60 | 7 | 21 | 0.005 |
| <60 | 103 | 89 | |
| Sex | |||
| Male | 69 | 67 | 0.781 |
| Female | 41 | 43 | |
| WHO grade | |||
| WHO II | 60 | 30 | <0.001 |
| WHO III | 23 | 24 | |
| WHO IV | 27 | 56 | |
| Histopathology | |||
| O | 23 | 1 | <0.001 |
| OA | 24 | 10 | |
| A | 12 | 19 | |
| AO | 7 | 2 | |
| AOA | 12 | 12 | |
| AA | 2 | 10 | |
| GBM | 27 | 56 | |
| IDH | |||
| Mutation | 70 | 42 | <0.001 |
| Wild type | 39 | 68 | |
| 1p/19q | |||
| Codeletion | 45 | 6 | <0.001 |
| Non-codeletion | 63 | 103 | |
| Radiotherapy | |||
| Yes | 99 | 94 | 0.445 |
| No | 8 | 11 | |
| Chemotherapy | |||
| Yes | 53 | 65 | 0.128 |
| No | 50 | 40 | |
O: oligodendroglioma; OA: oligoastrocytoma; A: astrocytoma; AO: anaplastic oligodendroglioma; AOA: anaplastic oligoastrocytoma; AA: anaplastic astrocytoma; GBM: glioblastoma.
TNXB overexpression influences the prognosis of patients with glioma
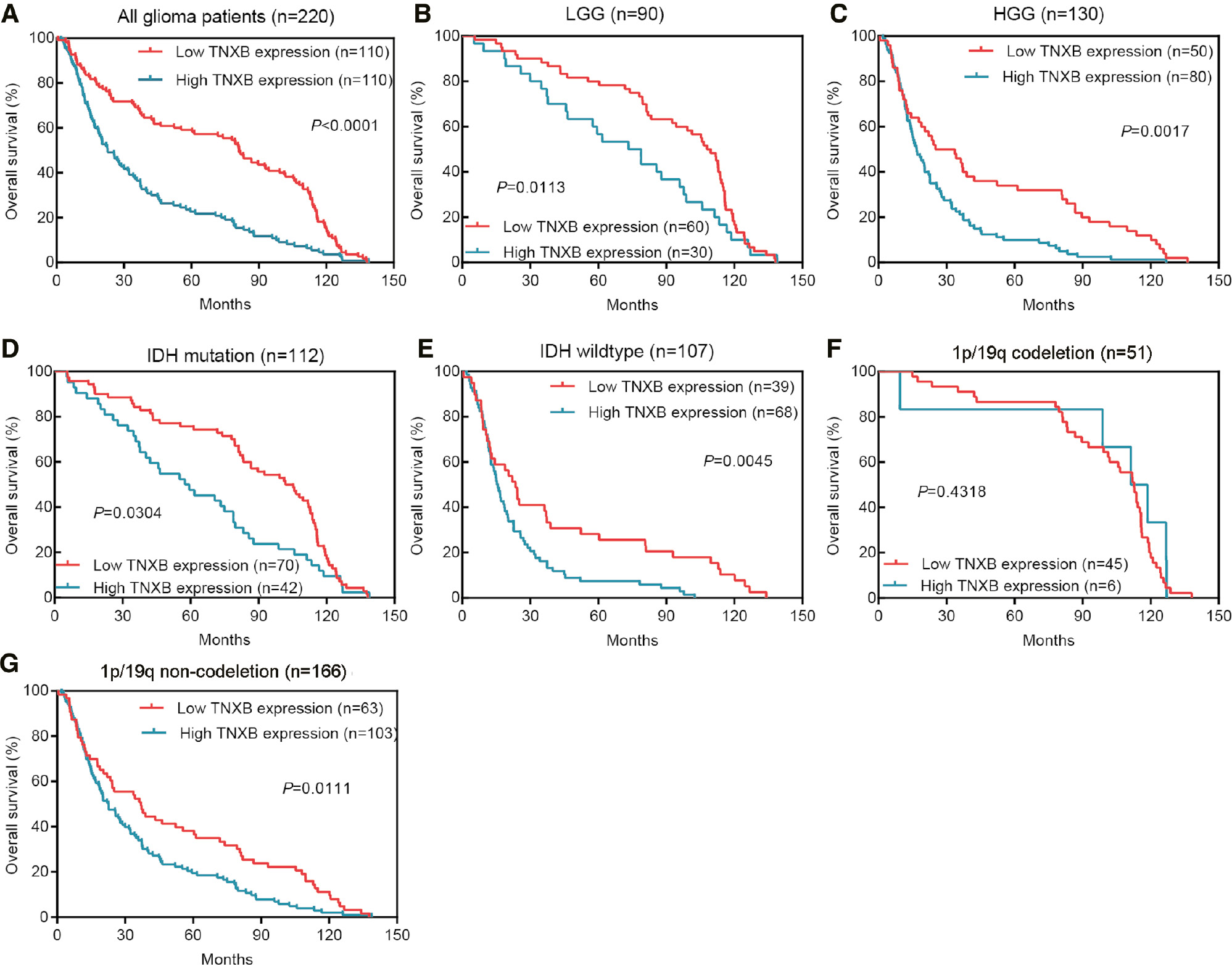
We further explored whether TNXB overexpression might be associated with poor prognosis. Kaplan-Meier survival curves suggested that patients with poorer prognosis tended to be in the group with high TNXB expression (Figure 3A). Furthermore, we stratified patients according to WHO histologic grade, mutation status of IDH and 1p/19q status, then performed survival analysis on each group. As shown in Figure 3B–G, we observed equivalent results between groups. No significant difference was found in 1p/19q codeletion status because of the small sample size.
Figure 3 Poor patient prognosis is associated with elevated TNXB expression in the CGGA cohort. (A) Kaplan-Meier survival curves indicating an association between poorer prognosis and high TNXB expression. (B–G) Subgroup analysis, stratified by WHO grade, IDH mutational status and 1p/19q status. Kaplan-Meier survival curves show similar trends in the stratified groups except for the 1p/19q codeletion group (n=51).
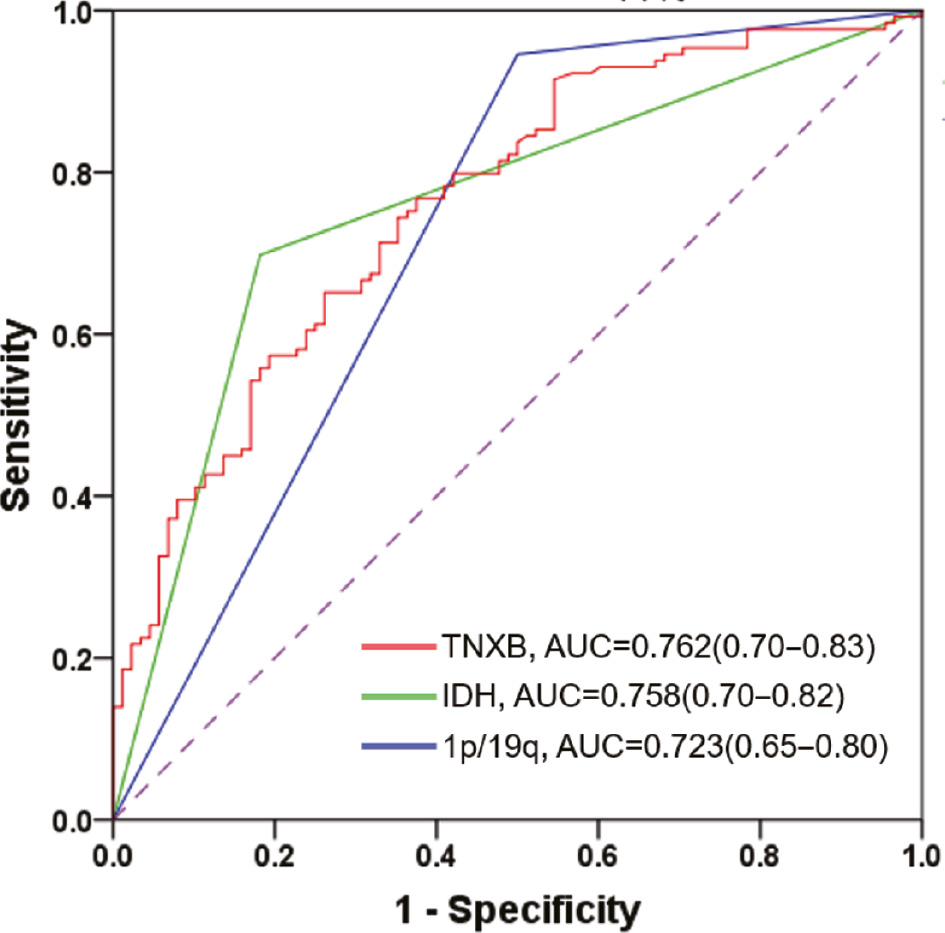
To explore whether TNXB might be an independent predictor of prognosis, we performed univariate and multivariate Cox regression analyses. The results suggested that TNXB may serve as an independent prognostic biomarker for patients with glioma (Table 2). Receiver operating characteristic curves indicated that the predictive performance of TNXB was significantly superior to that of common clinical indicators, including IDH mutation and 1p/19q codeletion (Figure 4). Together, our findings suggested that TNXB expression is markedly elevated in patients with glioma and might serve as a potential prognostic biomarker for these patients.
Table 2 Univariate and Multivariate Analysis of Overall Survival in 220 Patients with Glioma
| Characteristic | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P-value | HR | 95%CI | P-value | |
| Age | 2.79 | 1.86–4.19 | <0.001 | 1.63 | 1.06–2.53 | 0.028 |
| Sex | 1.01 | 0.76–1.32 | 0.973 | |||
| WHO grade | 2.14 | 1.81–2.53 | <0.001 | 1.06 | 0.53–2.14 | 0.863 |
| Histopathology | 1.38 | 1.29–1.48 | <0.001 | 1.23 | 0.96–1.75 | 0.095 |
| IDH | 0.34 | 0.26–0.45 | <0.001 | 0.66 | 0.44–0.99 | 0.042 |
| 1p/19q | 0.38 | 0.27–0.52 | <0.001 | 0.86 | 0.52–1.43 | 0.561 |
| Radiotherapy | 0.67 | 0.42–1.08 | 0.103 | |||
| Chemotherapy | 1.54 | 1.16–2.04 | 0.003 | 0.70 | 0.49–0.99 | 0.049 |
| TNXB expression | 1.99 | 1.51–2.62 | <0.001 | 1.42 | 1.04–1.95 | 0.028 |
HR: hazard ratio; CI: confidence interval.
Figure 4 The predictive value of TNXB protein is superior to the status of IDH mutation or 1p/19q codeletion.
Validation of the prognostic value of TNXB in a TCGA cohort
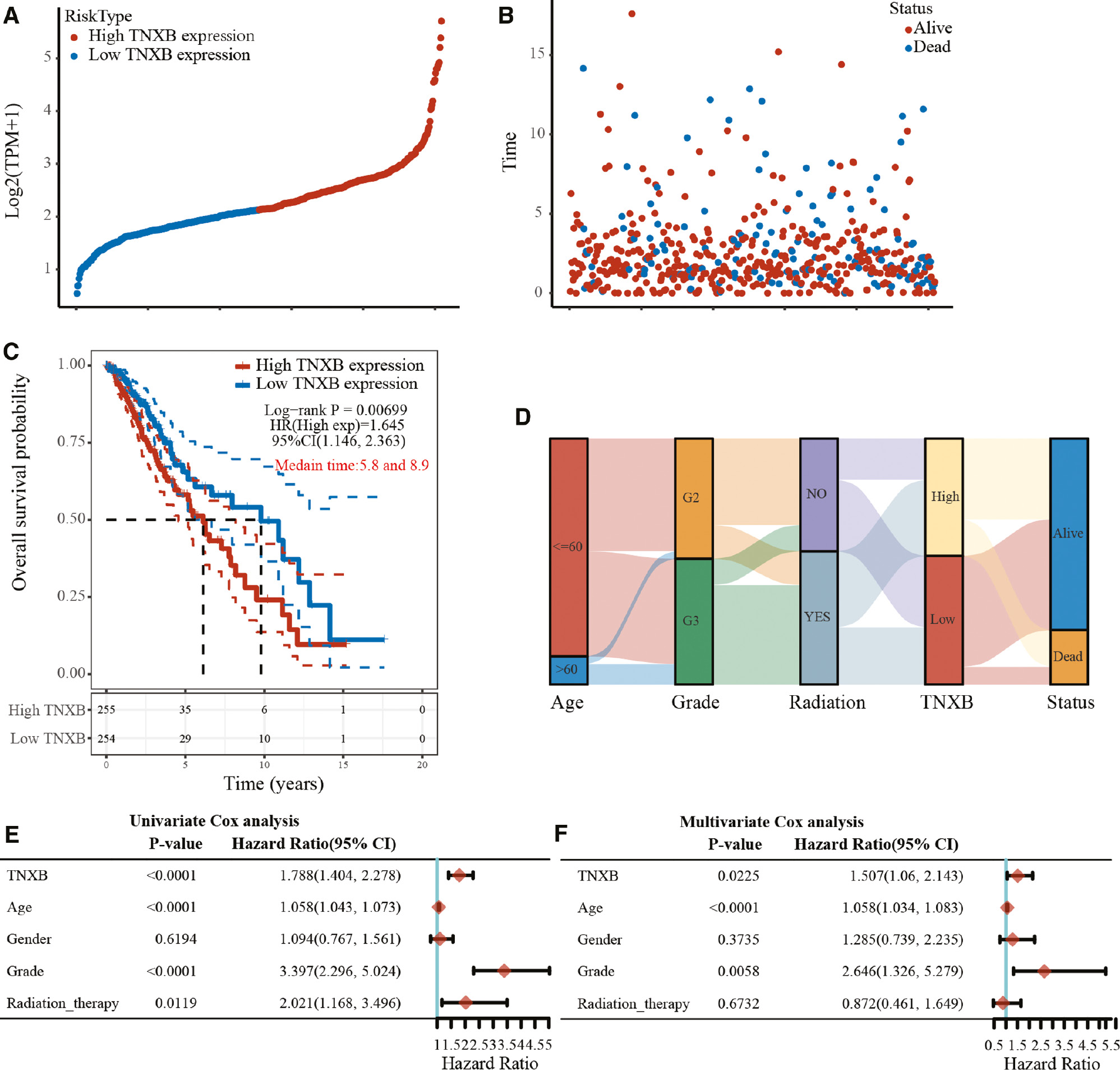
We further used data 510 patients with LGG from TCGA as a validation cohort to verify the prognostic value of TNXB in patients with glioma. As illustrated in Figure 5A, B, when the gene expression was ranked from low to high (Figure 5A), the corresponding survival time markedly decreased, and numerous patients who had high TNXB expression died (Figure 5B). Consistently, the Kaplan-Meier survival curve also indicated that patients in the group with elevated TNXB expression in a TCGA cohort had shorter survival times (P=0.00699, Figure 5C). The median survival times were 5.8 and 8.9 years in the high- and low-TNXB-expression groups, respectively. As shown in Figure 5D, elevated TNXB expression was associated with older age and WHO grade III glioma. These findings were further confirmed in univariate and multivariate Cox regression (Figure 5E, F).
Figure 5 Validation of the prognostic value of TNXB expression in the validation cohort from TCGA (LGG, n=510). (A, B) TNXB expression and survival time/status for each patient. (C) Kaplan-Meier survival curve indicating an association between poorer prognosis and elevated TNXB expression. (D) Sankey diagram indicating correlations between TNXB expression and patient age, WHO grade, radiotherapy and survival status. (E, F) Univariate and multivariate Cox regression analyses of TNXB expression and patients’ clinicopathological features in a TCGA cohort.
Biological functions and signaling pathways associated with TNXB protein
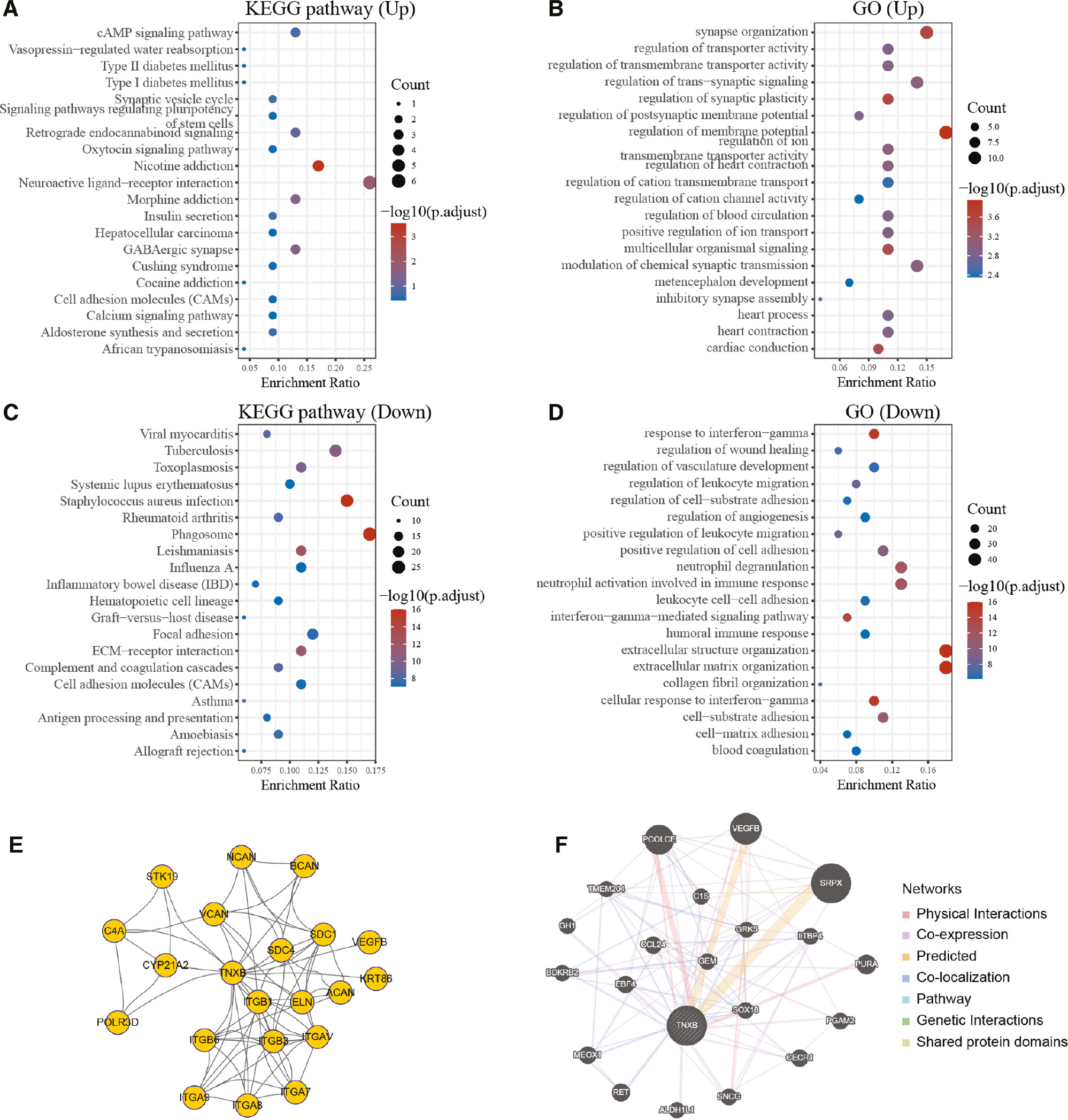
To gain a detailed understanding of the biological functions and signaling pathways associated with TNXB protein, we performed KEGG pathway enrichment analysis and GO function annotation. We identified the up-regulated genes (n=79) and down-regulated genes (n=283) co-expressed with the TNXB gene in a TCGA cohort (fold change >2, P<0.01; Figure S1). GO and KEGG pathway enrichment analyses of co-expressed genes were conducted to identify potential molecular functions of TNXB (Figure 6A–D). Importantly, TNXB was found to be involved in the immune response, humoral immune response and interferon-gamma-mediated signaling pathway. The PPI network for TNXB was constructed with the STRING database. Additionally, another PPI network including the proteins interacting with TNXB, sharing common pathways with TNXB, co-expressed with TNXB, co-localizing with TNXB and with similar protein domains to TNXB was also constructed with the GeneMANIA database. The above findings suggested that TNXB expression correlated with the levels of tumor-infiltrating immune cells in LGG.
Figure 6 Protein functional annotation and protein-protein-interaction network analysis of the TNXB gene in a TCGA cohort. (A–D) GO and KEGG pathway enrichment analyses of upregulated and downregulated differentially expressed genes in the various TNXB expression groups. (E) Protein-protein interaction networks of TNXB, established with the STRING database. (F) Functional network of TNXB, constructed by GeneMANIA.
TNXB expression is associated with the tumor immune microenvironment in glioma tissues
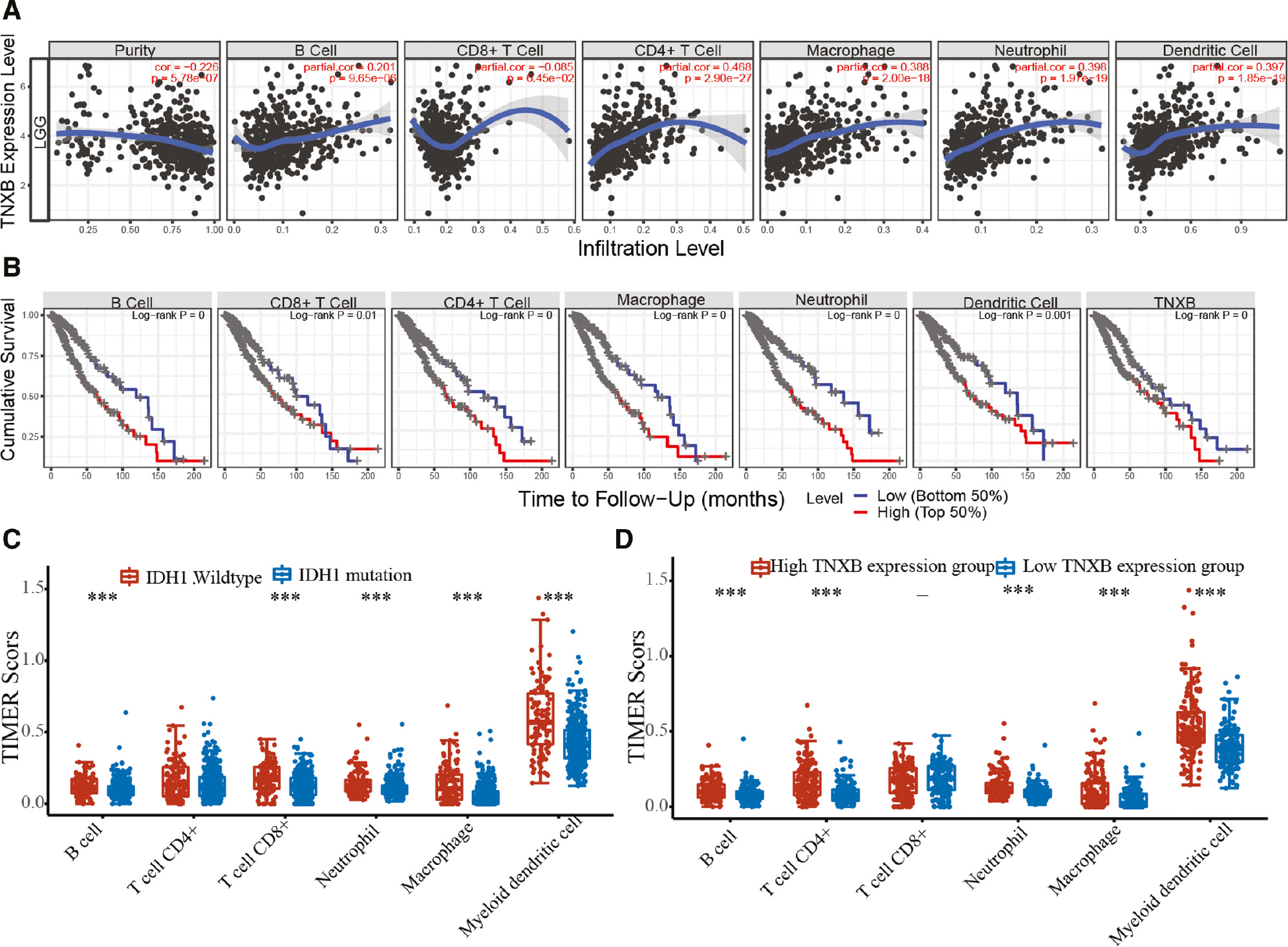
After determining that TNXB may be involved in the immune response and inflammatory signaling pathways, we attempted to clarify whether TNXB expression might correlate with the levels of tumor-infiltrating immune cells in LGG and whether it might be used as a biomarker to evaluate the TME. In LGG, TNXB expression was positively associated with the levels of tumor-infiltrating B cells (r=0.201, P=9.65e-06), CD4+ T cells (r=0.468, P=2.90e-27), macrophages (r=0.388, P=2.00e-18), neutrophils (r=0.398, P=1.97e-19) and dendritic cells (r=0.397, P=1.85e-19), but negatively associated with tumor purity (r=-0.225, P=5.78e-07; Figure 7A). We additionally assessed the correlation between TNXB expression and the genetic markers of diverse immune cells or functional T cells in LGG by using the TIMER database. As shown in Table S3, in LGG, TNXB expression was associated with several genetic markers of diverse immune cells, for instance, tumor-infiltrating B cells, TAMs, M2 macrophages, dendritic cells and Th2 cells, thus indicating that TNXB might regulate macrophage polarization in LGG. Moreover, TNXB expression correlated with TAM-associated genes and biomarkers [e.g., CCL2, TGFβ1, interleukin 10 (IL-10) and CD68], thus highlighting a strong association between TNXB and TAM infiltration (Table S4). Similarly, TNXB expression was significantly associated with markers of dendritic cells, regulatory T (Treg) cells and exhausted T cells (Table S3), thus further confirming TNXB’s correlation with immune escape in the LGG.
Figure 7 Relationship between TNXB expression and immune infiltration levels in patients with LGG (n=510). (A) TNXB expression is positively associated with glioma purity, the abundances of B cells, CD4+ T cells, macrophages, neutrophils and dendritic cells, but is negatively associated with CD8+ T cell infiltration. (B) Effects of immune-cell infiltration and TNXB expression on the survival and prognosis of patients with glioma. (C, D) Elevated immune-cell infiltration is positively correlated with wild-type IDH1 and high TNXB expression in patients with LGG.
Kaplan-Meier survival curve analysis indicated that patients with elevated tumor-infiltrating B cells, CD8+ T cells, CD4+ T cells, macrophages, neutrophils and dendritic cells, or high TNXB expression had poorer prognosis (Figure 7B). Consistently, multivariate Cox regression analysis indicated that patient age and TNXB expression were the independent risk factors for patient prognosis with gliomas (Table S5). In addition, tumor-infiltrating B cells, CD8+ T cells, neutrophils, macrophages and dendritic cells were significantly higher in patients with wild-type IDH-1 than those with IDH-1 mutation (Figure 7C). These findings illustrated that TNXB expression is associated with levels of tumor-infiltrating immune cells in LGG, particularly LGG with IDH wild-type. We further compared the levels of tumor-infiltrating B cells, CD4+ T cells, CD8+ T cells, neutrophils, macrophages and dendritic cells between the groups with low and high TNXB expression (Figure 7D). The results of this analysis were consistent with those illustrated in Figure 7A.
Furthermore, we attempted to determine whether the association between TNXB expression and tumor-infiltrating immune cells might be applicable to other types of cancer. On the basis of our findings, the correlation was also present in other cancers (Figures S2–3).
Relationship between TNXB expression and immunological checkpoint molecules
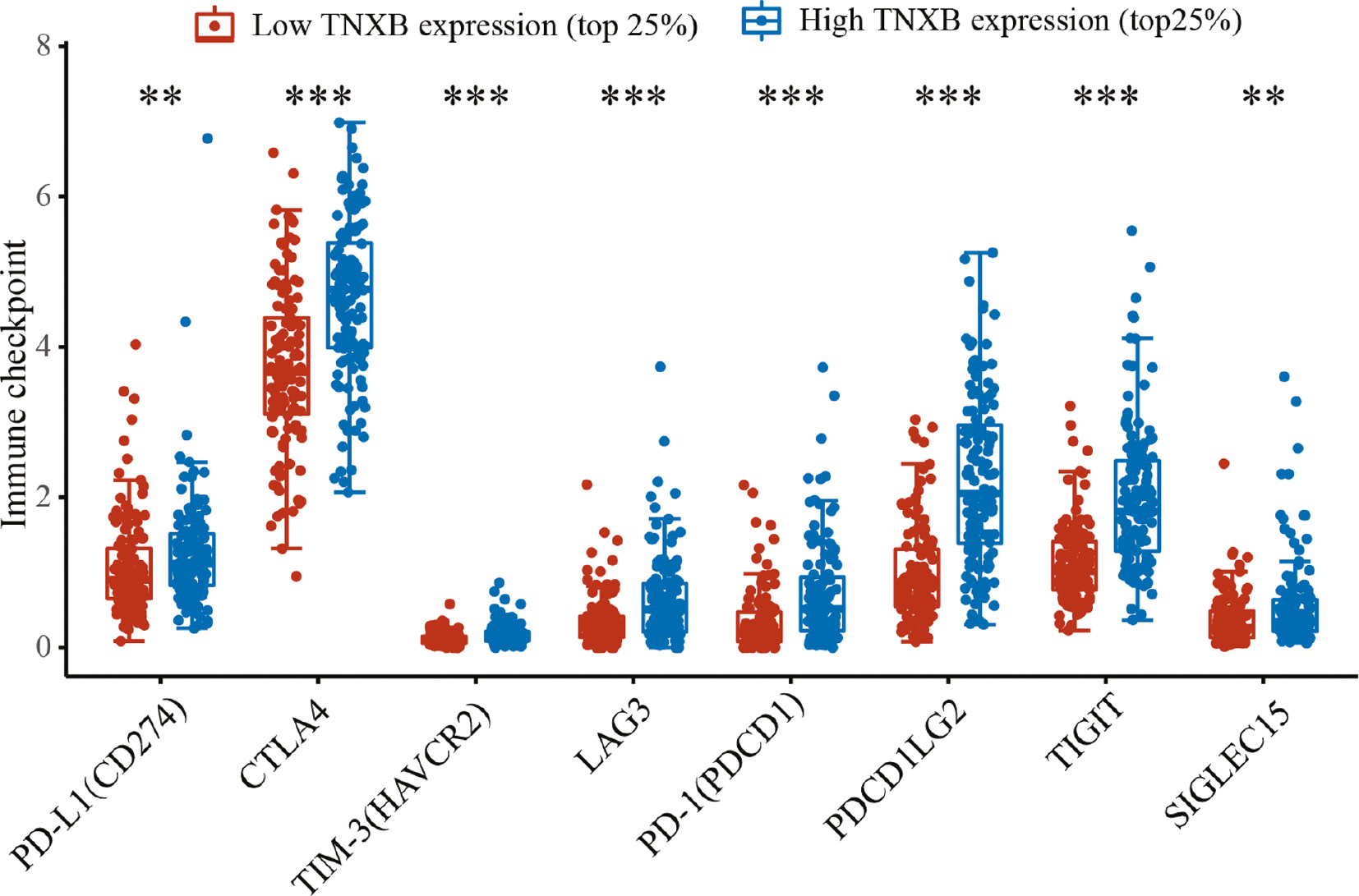
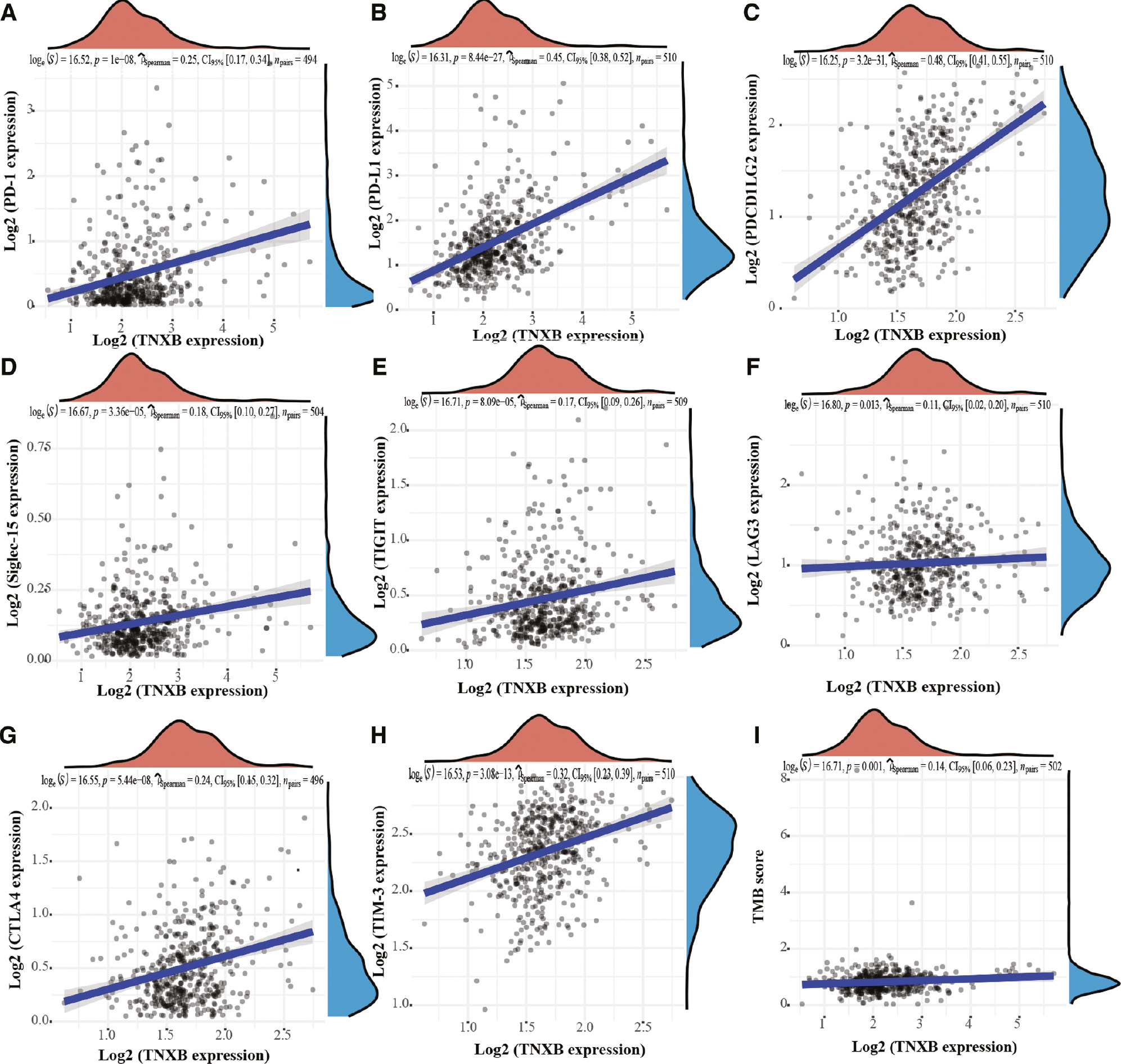
To evaluate the efficacy of TNXB in predicting the response to checkpoint molecules in patients with tumors, we determined the mRNA levels of eight immune-checkpoint molecules—PD-L1 (CD274), CTLA4, TIM-3 (HAVCR2), LAG3, PD-1 (PDCD1), PDCDILG2, TIGIT and Siglec-15—in LGG samples. As shown in Figure 8, the mRNA levels of the eight immune-checkpoint molecules significantly differed between the groups with low and high TNXB expression. Furthermore, scatter plots indicated that TNXB expression was markedly associated with the expression levels of PD-1, PD-L1, PDCD1 LG2, Siglec-15, TIGIT, LAG3, CTLA4 and TIM-3 (r=0.25, P=1e-08; r=0.45, P=8.44e-27; r=0.48, P=3.2e-31; r=0.18, P=3.36e-05; r=0.17, P=8.09e-05; r=0.11, P=0.013; r=0.24, P=5.44e-8; and r=0.32, P=3.08e-13, respectively, Figure 9A–H). These results confirmed the close correlation of TNXB expression with the glioma immune microenvironment.
Figure 8 Immune-checkpoint-associated genes are differentially expressed in the low-TNXB-expression group (the top quartile of patients with the lowest TNXB expression) and high-TNXB-expression group (the top quartile of patients with the highest TNXB expression).
Figure 9 Association between TNXB expression and common immune-checkpoint molecules and TMB scores in patients with LGG. (A–H) TNXB expression is associated with levels of PD-1, PD-L1, PDCD1LG2, Siglec-15, TIGIT, LAG3, CTLA4 and TIM-3. (I) TNXB expression is positively associated with the TMB score of glioma tissues.
Tumor mutational burden (TMB) has been proposed to be a potent indicator of immunotherapy response [22]. Elevated TMB levels would be expected to produce large numbers of neo-antigens; therefore, patients would be expected to benefit from immunotherapy [23]. Thus, we assessed the correlation between TNXB expression and the TMB of gliomas. TTNXB expression was weakly associated with the TMB score of gliomas (r=0.14, P=0.001; Figure 9I). The above outcomes demonstrated that TNXB may serve as a promising indicator to validate the response to immunotherapy in tumor patients.
A lollipop plot was constructed to display the distribution of mutations and protein domains for the TNXB gene among cancer hotspot mutations recurring at the same genomic site. The somatic mutation rate of the TNXB gene is 1.57%, and the transcript name is NM_019105 (Figure S4A). Moreover, the Oncoplot showed the somatic landscape for different TNXB expression groups in 510 patients with LGG from TCGA cohort (Figure S4B). Genes were ordered according to their mutational frequency, and more than half the mutations were missense mutations and frameshifting deletions in LGG.
Discussion
Studies are increasingly demonstrating that the sensitivity of cancer patients to immunotherapy significantly varies among age groups [24]. Screening patients who are sensitive to immunotherapy would have enormous clinical value. Previous studies have shown that the immune infiltration status of the TME in patients with cancer plays a substantial role in the response to tumor immunotherapy [25]. However, few studies have predicted the immune infiltration status of patients with cancer by detecting single molecules. Therefore, we used patient demographic and clinicopathological data to analyze the expression level, prognostic value, immune infiltration mode and biological function of the new anti-tumor target TNXB. The ability to select the most appropriate immunotherapeutic strategy for patients with cancer on the basis of extensive insight regarding their immune infiltration status will be crucial.
Herein, we first found that TNXB expression was significantly higher in glioma tissues than normal brain tissues, and was directly correlated with glioma WHO grade. TNXB overexpression was strongly associated with the clinicopathological features of patients with glioma and thus may provide a promising independent biomarker for poor prognosis among these patients. More importantly, TNXB expression was positively associated with the levels of tumor-infiltrating B cells, CD4+ T cells, macrophages, neutrophils, dendritic cells and TAMs in diverse types of cancer. Notably, TNXB expression was significantly associated with common immune-checkpoint molecules (e.g., PD-1, PD-L1, CTLA4, TIM-3, LAG3, PDCDILG2, TIGIT and Siglec-15). GO and KEGG pathway enrichment analyses also indicated that TNXB participates in the immune response, humoral immune response and interferon-gamma-mediated signaling pathways. These results suggested that, owing to the strong association between TNXB overexpression and immune escape of gliomas, TNXB may be used as a biomarker for validating the status of immune infiltration in diverse types of cancer.
Tenascins are multimeric glycoproteins that can be divided into five subtypes: Tenascin-C, Tenascin-R, Tenascin-X, TNXB and Tenascin-N. To date, no study has focused on TNXB expression in gliomas. TNXB regulates interactions between cells and the extracellular matrix and may boost the growth of epithelial tumors [16]. Recently, numerous researchers have reported that TNXB expression is strongly associated with cancer development [26]. Yuan et al. first reported that TNXB might serve as a new diagnostic biomarker of malignant mesothelioma in the differential diagnosis of various types of cancer including those in the serosal cavities, particularly in differential diagnosis between tumors and ovarian/peritoneal serous carcinoma [27]. Yan et al. have found that lncRNA LINC01305 silencing suppresses epithelial-mesenchymal transition, and invasion and migration by targeting TNXB, thus repressing the PI3K/Akt signaling pathway in cervical cancer cells [18]. This result is consistent with our finding that TXNB might play a crucial role in the malignant biological behavior of gliomas.
In recent years, immunosuppressive drugs have markedly attracted researchers’ attention in the treatment of melanoma, because of their ability to significantly prolong the survival of patients with advanced melanoma [28]. However, the effective response rate in patients with melanoma is less than 30% [29]. Tumor-infiltrating immune cells are essential for the tumor immune-regulation network and cancer development. Treg cells are imperative in maintaining immune homeostasis by repressing abnormal/excessive immune responses to self- or non-self-antigens [30]. Additionally, Treg cells promote tumor progression by secreting transforming growth factor-β (TGF-β) [31, 32]. Dendritic cells additionally enhance tumor metastasis by recruiting Treg cells and decreasing the cytotoxicity of CD8+T cells [33]. Because of the inhibition of immune-cell function, patients are unable to generate an effective anti-tumor immune response, in agreement with the significant positive correlation observed between TNXB overexpression and tumor-infiltrating immune cells, except for CD8+ T cells in LGG.
Moreover, we unexpectedly detected a strong correlation between TNXB and TAM expression. TAMs have immunosuppressive and tumor-promoting roles. Additional roles of TAMs in tumor occurrence and development are as follows: i) promoting tumor cell growth by secreting EGF and CCL18; ii) meditating invasive and metastatic ability by releasing factors involved in cancer metastasis, such as MMPs, uPA and uPAR; iii) participating in the escape of tumor-infiltrating immune cells by producing immunosuppressive mediators (e.g., IL-10, PGE2 and TGF-β); and participating in the growth of lymphatic vessels by expressing VEGF [34–37]. Therefore, further attention must be paid to TAMs as an important target for tumor metastasis. The dynamic changes in the tumor immune microenvironment, immune-checkpoint molecules, tumor-infiltrating immune cells and the TMB are extremely important in the immune response. Therefore, additional accurate biomarkers must be developed for individualized cancer immunotherapy. Moreover, further research is warranted to determine whether TNXB overexpression in glioma tissues might mediate a tumor malignant phenotype through an immuno-inflammatory mechanism.
Conclusion
We first detected upregulated TNXB expression in patients with glioma, which increased with tumor grade. High TNXB expression markedly correlated with poor prognosis and thus may provide a promising independent prognostic biomarker for glioma. Moreover, a relationship between TNXB expression and the levels of tumor-infiltrating immune cells was observed in several tumor types and was strongly associated with common immune-checkpoint molecules. TNXB molecules that participate in the immune response and neutrophils are therefore activated.
Acknowledgements
We thank Professor Wang for the R software script.
Competing interests
There is no conflict of interest.
Financial support
The Postdoctoral Research Foundation of China (F121NF0018) supported this work.
Declarations of interest
None
Supplementary Figures and Tables
Click here to download Supplementary Figures and Tables.
References
- Claus EB, Walsh KM, Wiencke JK, Molinaro AM, Wiemels JL, et al. Survival and low-grade glioma: the emergence of genetic information. Neurosurg Focus 2015;38:E6. [PMID: 25552286 DOI: 10.3171/2014.10.focus12367]
- Li K, Ouyang L, He M, Luo M, Cai W, et al. IDH1 R132H mutation regulates glioma chemosensitivity through Nrf2 pathway. Oncotarget 2017;8:28865-28879. [PMID: 28427200 DOI: 10.18632/oncotarget.15868]
- Zhao Q, Yu J, Meng X. A good start of immunotherapy in esophageal cancer. Cancer Med 2019;8:4519-4526. [PMID: 31231980 DOI: 10.1002/cam4.2336]
- Hu H, Wang Z, Li M, Zeng F, Wang K, et al. Gene expression and methylation analyses suggest dctd as a prognostic factor in malignant glioma. Sci Rep 2017;7:11568. [PMID: 28912488 DOI: 10.1038/s41598-017-11962-y]
- Bennani-Baiti N, Thanarajasingam G, Ansell S. Checkpoint inhibitors for the treatment of hodgkin lymphoma. Expert Rev Clin Immunol 2016;12:673-679. [PMID: 26818843 DOI: 10.1586/1744666x.2016.1147350]
- Weller M, Roth P, Preusser M, Wick W, Reardon DA, et al. Vaccine-based immunotherapeutic approaches to gliomas and beyond. Nat Rev Neurol 2017;13:363-374. [PMID: 28497804 DOI: 10.1038/nrneurol.2017.64]
- Soularue E, Lepage P, Colombel JF, Coutzac C, Faleck D, et al. Enterocolitis due to immune checkpoint inhibitors: a systematic review. Gut 2018;67:2056-2067. [PMID: 30131322 DOI: 10.1136/gutjnl-2018-316948]
- Vanichapol T, Chiangjong W, Panachan J, Anurathapan U, Chutipongtanate S, et al. Secretory high-mobility group box 1 protein affects regulatory T cell differentiation in neuroblastoma microenvironment In vitro. J Oncol 2018;2018:7946021. [PMID: 30643519 DOI: 10.1155/2018/7946021]
- Zhang B, Shen R, Cheng S, Feng L. Immune microenvironments differ in immune characteristics and outcome of glioblastoma multiforme. Cancer Med 2019;8:2897-2907. [PMID: 31038851 DOI: 10.1002/cam4.2192]
- Chen Q, Xia R, Zheng W, Zhang L, Li P, et al. Metronomic paclitaxel improves the efficacy of PD-1 monoclonal antibodies in breast cancer by transforming the tumor immune microenvironment. Am J Transl Res 2020;12:519-530. [PMID: 32194900].
- Zhu C, Kong Z, Wang B, Cheng W, Wu A, et al. ITGB3/CD61: a hub modulator and target in the tumor microenvironment. Am J Transl Res 2019;11:7195-7208. [PMID: 31934272]
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013;19:1423-1437. [PMID: 24202395 DOI: 10.1038/nm.3394]
- Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases–elimination, equilibrium and escape. Curr Opin Immunol 2014;27:16-25. [PMID: 24531241 DOI: 10.1016/j.coi.2014.01.004]
- Wu H, Zhang X, Han D, Cao J, Tian J. Tumour-associated macrophages mediate the invasion and metastasis of bladder cancer cells through CXCL8. Peer J 2020;8:e8721. [PMID: 32201645 DOI: 10.7717/peerj.8721]
- Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017;168:707-723. [PMID: 28187290 DOI: 10.1016/j.cell.2017.01.017]
- Tucker RP, Chiquet-Ehrismann R. The regulation of tenascin expression by tissue microenvironments. Biochim Biophys Acta 2009;1793:888-892. [PMID: 19162090 DOI: 10.1016/j.bbamcr.2008.12.012]
- Nakayama K, Seike M, Noro R, Takeuchi S, Matsuda K, et al. Tenascin XB is a novel diagnostic marker for malignant mesothelioma. Anticancer Res 2019;39:627-633. [PMID: 30711938 DOI: 10.21873/anticanres.13156]
- Yan SP, Chu DX, Qiu HF, Xie Y, Wang CF, et al. LncRNA LINC01305 silencing inhibits cell epithelial-mesenchymal transition in cervical cancer by inhibiting TNXB-mediated PI3K/Akt signalling pathway. J Cell Mol Med 2019;23:2656-2666. [PMID: 30697971 DOI: 10.1111/jcmm.14161]
- Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 2019;47:D60-d613. [PMID: 30476243 DOI: 10.1093/nar/gky1131]
- Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, et al. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res 2010;38:W214-220. [PMID: 20576703 DOI: 10.1093/nar/gkq537]
- Li T, Fan J, Wang B, Traugh N, Chen Q, et al. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res 2017;77:e108-e110. [PMID: 29092952 DOI: 10.1158/0008-5472.can-17-0307]
- Cui Y, Chen H, Xi R, Cui H, Zhao Y, et al. Whole-genome sequencing of 508 patients identifies key molecular features associated with poor prognosis in esophageal squamous cell carcinoma. Cell Res 2020;30:902-913. [PMID: 32398863 DOI: 10.1038/s41422-020-0333-6]
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015;348:69-74. [DOI: 10.1126/science.aaa4971]
- Pfaar O, Robinson DS, Sager A, Emuzyte R. Immunotherapy with depigmented-polymerized mixed tree pollen extract: a clinical trial and responder analysis. Allergy 2010;65:1614-1621. [PMID: 20645937 DOI: 10.1111/j.1398-9995.2010.02413.x]
- Ranasinghe SL, Rivera V, Boyle GM, McManus DP. Kunitz type protease inhibitor from the canine tapeworm as a potential therapeutic for melanoma. Sci Rep 2019;9:16207. [PMID: 31700040 DOI: 10.1038/s41598-019-52609-4]
- Roll L, Faissner A. Tenascins in CNS lesions. Semin Cell Dev Biol 2019;89:118-124. [PMID: 30287388 DOI: 10.1016/j.semcdb.2018.09.012]
- Yuan Y, Nymoen DA, Stavnes HT, Rosnes AK, Bjørang O, et al. Tenascin-X is a novel diagnostic marker of malignant mesothelioma. Am J Surg Pathol 2009;33:1673-1682. [PMID: 19738457 DOI: 10.1097/PAS.0b013e3181b6bde3]
- Herrera-Rios D, Mughal SS, Teuber-Hanselmann S, Pierscianek D, Sucker A, et al. Macrophages/Microglia Represent the Major Source of Indolamine 2,3-Dioxygenase Expression in Melanoma Metastases of the Brain. Front Immunol 2020;11:120. [PMID: 32117271 DOI: 10.3389/fimmu.2020.00120]
- Tomita Y, Fukasawa S, Shinohara N, Kitamura H, Oya M, et al. Nivolumab versus everolimus in advanced renal cell carcinoma: Japanese subgroup 3-year follow-up analysis from the Phase III CheckMate 025 study. Jpn J Clin Oncol 2019;49:506-514. [PMID: 30941424 DOI: 10.1093/jjco/hyz026]
- Dürr C, Follo M, Idzko M, Reichardt W, Zeiser R. Graft-versus-host disease reduces regulatory T-cell migration into the tumour tissue. Immunology 2012;137:80-88. [PMID: 22681312 DOI: 10.1111/j.1365-2567.2012.03610.x]
- Zeng G, Jin L, Ying Q, Chen H, Thembinkosi MC, et al. Regulatory T cells in cancer immunotherapy: basic research outcomes and clinical directions. Cancer Manag Res 2020;12:10411-10421. [PMID: 33116895 DOI: 10.2147/cmar.s265828]
- Verma A, Mathur R, Farooque A, Kaul V, Gupta S, et al. T-regulatory cells in tumor progression and therapy. Cancer Manag Res 2019;11:10731-10747. [PMID: 31920383 DOI: 10.2147/cmar.s228887]
- Qi Z, Yan F, Chen D, Xing W, Li Q, et al. Identification of prognostic biomarkers and correlations with immune infiltrates among cGAS-STING in hepatocellular carcinoma. Biosci Rep 2020;40 . [PMID: 33006365 DOI: 10.1042/bsr20202603]
- Li J, Zeng Z, Jiang X, Zhang N, Gao Y, et al. Stromal microenvironment promoted infiltration in esophageal adenocarcinoma and squamous cell carcinoma: a multi-cohort gene-based analysis. Sci Rep 2020;10:18589. [DOI: 10.1038/s41598-020-75541-4]
- Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol 2019;12:76. [PMID: 31300030 DOI: 10.1186/s13045-019-0760-3]
- Olivier M, Gregory DJ, Forget G. Subversion mechanisms by which Leishmania parasites can escape the host immune response: a signaling point of view. Clin Microbiol Rev 2005;18:293-305. [PMID: 15831826 DOI: 10.1128/cmr.18.2.293-305.2005]
- Bogdan C, Gessner A, Solbach W, Röllinghoff M. Invasion, control and persistence of Leishmania parasites. Curr Opin Immunol 1996;8:517-525. [PMID: 8794010 DOI: 10.1016/s0952-7915(96)80040-9]