ADME-Tox Prediction and Molecular Docking Studies of Two Lead Flavonoids From the Roots of Tephrosia Egregia Sandw and the Gastroprotective Effects of Its Root Extract in Mice
Marcos Eber F. Rogério1, Hellíada V. Chaves2, Isabela R. Pinto3, Nayara A. de Sousa4, Kátia A. Ribeiro1, Dina Andressa M. Monteiro4, Antonio Alfredo R. e Silva2, Ângela Martha C. Arriaga6, Maria Valdeline S. Teixeira6, Antônia T. A. Pimenta6, Roberta Jeane B. Jorge5, Helyson Lucas B. Braz5, Vicente de Paulo T. Pinto1,4, Maria Elisabete Amaral de Moraes5, Virgínia C. C. Girão7 and Mirna Marques Bezerra4,5,*
1Master of Biotechnology Degree Program, Federal University of Ceará, Sobral, Ceará, Brazil
2School of Dentistry, Federal University of Ceará, Sobral, Ceará, Brazil
3School of Dentistry, University Center INTA–UNINTA, Tianguá, Ceará, Brazil
4School of Medicine, Federal University of Ceará, Sobral, Ceará, Brazil
5Drug Research and Development Center (NPDM), Federal University of Ceará, Fortaleza, Ceará, Brazil
6Department of Organic and Inorganic Chemistry, Science Centre, Federal University of Ceará, Fortaleza, Ceará, Brazil
7Department of Morphology, Faculty of Medicine, Federal University of Ceara, Fortaleza, Ceara, Brazil
Equal contribution: Bezerra Mirna Marques
*Correspondence to: Mirna Marques Bezerra, E-mail: mirna@ufc.br.
Received: December 3 2021; Revised: February 10 2022; Accepted: February 23 2022; Published Online: April 18 2022
Cite this paper:
Marcos Eber F. Rogério, Hellíada V. Chaves, Isabela R. Pinto, Nayara A. de Sousa, Kátia A. Ribeiro, Dina Andressa M. Monteiro, Antonio Alfredo R. e Silva, Ângela Martha C. Arriaga, Maria Valdeline S. Teixeira, Antônia T. A. Pimenta, Roberta Jeane B. Jorge, Helyson Lucas B. Braz, Vicente de Paulo T. Pinto, Maria Elisabete Amaral de Moraes, Virgínia C. C. Girão and Mirna Marques Bezerra. ADME-Tox Prediction and Molecular Docking Studies of Two Lead Flavonoids From the Roots of Tephrosia Egregia Sandw and the Gastroprotective Effects of Its Root Extract in Mice. BIO Integration 2022; 3(2): 43–52.
DOI: 10.15212/bioi-2021-0035. Available at: https://bio-integration.org/
Download citation
© 2022 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
Background: This study aimed to predict the pharmacokinetic and toxicological properties of lead flavonoids from the roots of T. egregia [praecansone A (1) and pongachalcone (2)], and to assess the gastroprotective effects and possible underlying mechanisms of the root extract in mice.
Methods: Quantitative and qualitative data for in silico absorption, distribution, metabolism, and excretion (ADME) analyses of the two flavonoids were acquired from the SwissADME database. Toxicity assessment was performed with the ProTox-II server. To evaluate the putative interactions of both flavonoids with opioid receptors and NO protein, we acquired structures of the targets (μ, κ, and δ-opioid receptors, and iNOS) in Homo sapiens from https://www.rcsb.org/. For docking studies, AutoDock 4.2 was used for ligand and target arrangement, and AutoDock Vina was used for calculations. For in vivo assays, mice were pretreated (per os) with T. egregia (2, 20, or 200 mg/kg). After 60 min, 99.9% ethanol (0.2 mL) was injected (per os). At 30 min after ethanol injection, the mice were euthanized, and the gastric damage, gastric levels of hemoglobin, glutathione content, and activity of superoxide dismutase and catalase were evaluated. To elucidate T. egregia mechanisms, we used misoprostol, a prostaglandin analog; indomethacin, an inhibitor of prostaglandin synthesis; L-arginine, an NO precursor; L-NAME, an antagonist of NO synthase; naloxone, an opioid antagonist; and morphine, an opioid agonist.
Results: In silico results showed that flavonoids (1) and (2) had favorable ADME properties and toxicity profiles, and exhibited satisfactory binding energies data (below −6.0 kcal/mol) when docked into their targets (μ, κ, and δ-opioid receptors, and iNOS). T. egregia decreased the ethanol-induced gastric damage and hemoglobin levels, and increased the glutathione content, and activity of superoxide dismutase and catalase. Naloxone and L-NAME, but not indomethacin, prevented T. egregia’s effects, thus suggesting that opioid receptors and NO are involved in T. egregia’s efficacy.
Conclusions: Flavonoids (1) and (2) exhibited favorable pharmacokinetic properties, showing high lethal dose, 50% (LD50; 3,800 and 2,500 mg/kg, respectively) values. Neither flavonoid was found to be hepatotoxic, carcinogenic, or cytotoxic to human cells. In vivo assays indicated that T. egregia ameliorated oxidative stress levels, and its mechanism is at least partially based on opioid receptors and NO. T. egregia may therefore be considered as a new gastroprotective strategy.
Keywords
Flavonoids, in silico, natural products, oxidative stress.
Introduction
Gastric ulcer is a common disease globally, which poses an economic burden [1, 2]. Protective factors [secretion, bicarbonate production, blood flow, production of nitric oxide (NO) and prostaglandins, and enzymatic and non-enzymatic antioxidant systems] and risk factors (hydrochloric acid, pepsin, Helicobacter pylori infection, nonsteroidal anti-inflammatory drugs, psychological stress, free radicals, and excessive intake of alcohol) modulate the pathophysiology of gastric ulcers [3–5].
Drugs currently used to treat gastric ulcers include antibiotics (in cases of H. pylori infection), histamine receptor antagonists (ranitidine), proton-pump inhibitors (omeprazole), and cytoprotective agents (bismuth and sucralfate). However, several adverse effects and limitations of these treatments have been reported. Therefore, the management of gastric ulcers is an issue in clinical medicine, and new approaches to treat this disease are needed to overcome the adverse effects associated with conventional treatment [6, 7]. In this regard, plant-based natural products used in traditional medicine have been applied in the treatment of several illnesses.
Tephrosia egregia Sandw (T. egregia), a plant in the Fabaceae family, is found mainly in tropical and sub-tropical regions [8]. Our group and others have demonstrated the occurrence of flavonoids in the roots of T. egregia, including pongaflavone and praecansone B [8], 12a-hydroxyrotenone [9], praecansone A [10], 2′,6′-dimethoxy-4′,5′-(2′,2″-dimethyl)-pyranochalcone, [11], pongachalcone [12], and maackiain [13]. Among these compounds, the lead flavonoids from the ethanolic extract of T. egregia roots are praecansone A and pongachalcone [8]. Numerous biological activities of Tephrosia species have been described in the literature, including, antitumor, estrogenic, antiprotozoal, antimicrobial, larvicidal, anti-inflammatory, and antioxidant functions [14–20]. Nevertheless, the literature lacks studies addressing the gastroprotective effects of T. egregia.
Therefore, in this study, we performed in silico assays to predict the pharmacokinetic and toxicological properties of the lead flavonoids from the roots of T. egregia [praecansone A (1) and pongachalcone (2)]. In addition, we evaluated the possible healing effects of T. egregia on gastric ulcers induced by ethanol in mice, and we assessed the possible mechanism of action, investigating the involvement of prostaglandins, NO, and opioid receptors.
Methods
In silico studies: ADME-toxicity prediction and molecular docking
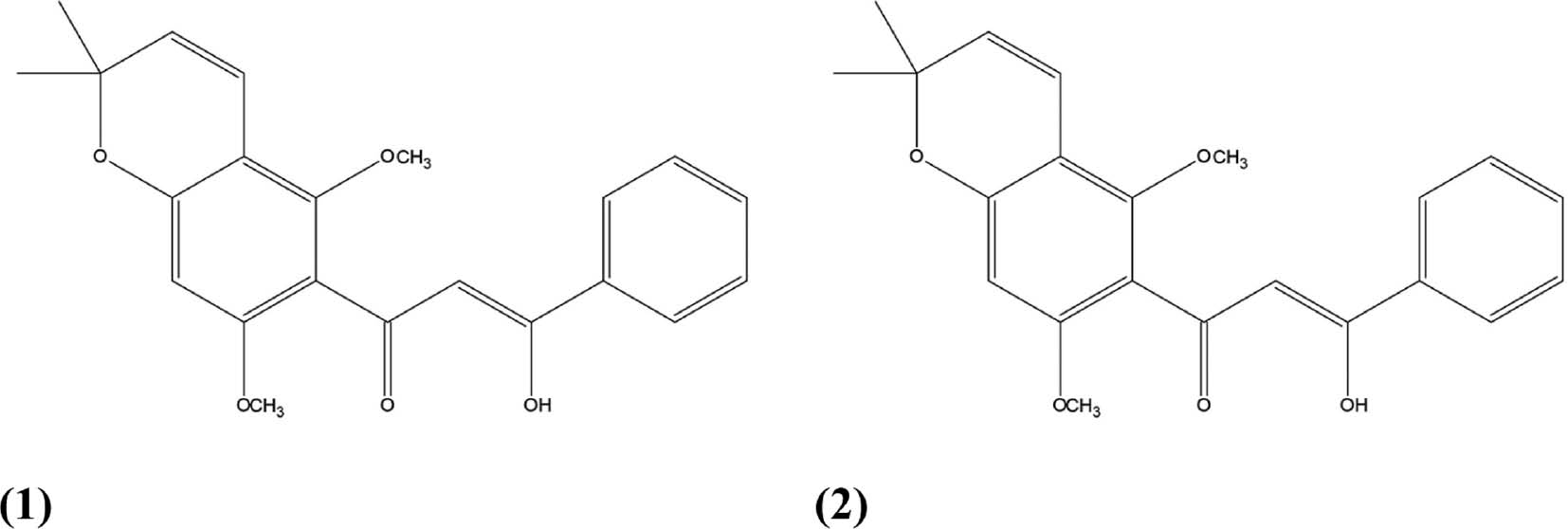
During drug-development research, substantial efforts have attempted to limit animal use in determining the acute oral toxicity of new compounds, in agreement with the “3Rs” principle [21]. Therefore, to predict the pharmacokinetic and toxicological properties of T. egregia as a drug candidate, we performed in silico absorption, distribution, metabolism, excretion, and toxicity (ADME-T) studies of the two best-known and most abundant flavonoids in the roots of T. egregia: praecansone A (1) [10], and pongachalcone (2) [12]. Figure 1 shows the chemical structures of these two compounds. In addition, to evaluate the pharmacodynamic phase, we assessed the putative interactions of both flavonoids with opioid receptors (μ, κ, and δ-opioid receptors) and inducible NO synthase (iNOS) protein structures, through molecular docking assays evaluating the compound-protein interactions.
Figure 1 Chemical structure of praecansone A (1) and pongachalcone (2).
The structures of flavonoids (1) and (2) were modeled three-dimensionally in Avogadro 1.1.2 software [22] and geometrically optimized through density functional theory with the correlation functional B3LYP and base 6-31G(d) in GAMESS software [23].
The prediction of the pharmacokinetic profiles of flavonoids (1) and (2) was evaluated according to Lipinski’s “rule of five” and ADME parameters. According to Lipinski’s rule, in general, to be active orally, a drug must meet at least four of the following five principles: molecular mass less than 500 g/mol, high lipophilicity (octanol–water partition coefficient less than 5), fewer than five H-bond donors, fewer than ten H-bond acceptors, and molar refractivity between 40 and 130 cm3·mol−1 [24]. Another related criterion, polar surface area <140 Å2, was added later [25]. Quantitative and qualitative values were obtained from the SwissADME online server (http://www.swissadme.ch/).
In silico toxicity assessment of flavonoids (1) and (2) was performed with the ProTox-II server [26].
To evaluate the putative interactions of flavonoids (1) and (2) with opioid receptors and NO protein, we downloaded structures (targets) of iNOS (PDB: 3NQS) and the opioid receptors mu (μ; PDB: 4DK1), kappa (k; PDB: 4DJH), and delta (δ; PDB: 6PT3) of Homo sapiens from the Protein Data Bank (https://www.rcsb.org/).
All structures were defined by the X-ray diffraction technique with a resolution between 1.20 and 3.10 Å. The positions of the coupling sockets were based on the native ligand of the macromolecules and the Web Server Computed Atlas of Surface Topography of proteins (http://sts.bioe.uic.edu/castp/calculation.html). For molecular docking simulations, AutoDock tools v4.2 was used to prepare the ligands and targets [27], and AutoDock Vina 1.1.2 was used to perform the calculations [28]. Results were visualized with AutoDock tools, Discovery Studio v4.5 [29], and UCSF Chimera X [30].
Plant material and extract preparation from T. egregia roots
The extract of T. egregia roots used in the experiments was obtained from the Department of Organic and Inorganic Chemistry at the Federal University of Ceará. The specimen was collected in the flowering stage on July 22, 2014, at Icaraí beach (Caucaia, Ceará, Brazil; longitude −38.6563 and latitude −3.73454). Botanical identification was performed by the Department of Biology at the Federal University of Ceará. A voucher specimen has been deposited at the Herbário Prisco Bezerra of the Federal University of Ceará, under registration number 55945.
The roots of T. egregia were extracted by continual maceration with ethanol (3 × 3.0 L) at room temperature. The resulting solution was filtered and concentrated under reduced pressure with a rotary evaporator, thus resulting in 1.13 g of root extract. Fresh dilution of the macerated extract in 0.9% NaCl solution was prepared on the day of the experiment and injected orally at different doses, as described below.
Animals
Swiss mice (female; 25–30 g) from the vivarium of the Federal University of Ceará were housed under temperature (22 ± 2°C) and light control (12/12 h light/dark cycle), and were given water and food ad libitum. According to ample-size calculation w based on α = 0.05 and a study power of 0.8, the experimental protocol was performed with seven mice per group. All experimental protocols were performed to minimize animal suffering and the number of animals used, in accordance with the Guide for the Care and Use of Laboratory Animals from the Brazilian Society of Science in Laboratory Animals. The experimental protocol was approved by the Institutional Animal Care and Use Committee from the Federal University of Ceará, Campus of Sobral (process No. 01/2016).
Pre-clinical trials
Ethanol-induced gastropathy
Mice received (per os) vehicle (0.9% saline), T. egregia (2, 20, or 200 mg/kg), or ranitidine (80 mg/kg), included as a positive control treatment. After 60 min, 99.9% ethanol (0.2 mL/animal) was injected (per os). The saline-treated group consisted of naive animals. At 30 min after ethanol challenge, mice were euthanized, and stomach samples were removed, opened along the greater curvature, washed with saline, fixed on glass plates, and photographed (Motorola Razr HD, KDA20.117) at a resolution of 1280 × 720 pixels. Gastric lesions were measured and compared with the total area of each stomach in Image J 1.42q®. The method was performed as previously described [31]. The evaluation of gastric hemoglobin (Hb) levels, glutathione (GSH) content, and superoxide dismutase (SOD) and catalase (CAT) activity was performed in unfixed stomach samples, as described below.
Assessment of the antioxidant activity of T. egregia
Gastric Hb determination
Gastric Hb levels were measured with a standard kit containing Drabkin’s reagent, according to the manufacturer’s instructions. Briefly, slices of fresh gastric tissue (50–100 mg) were homogenized in Drabkin’s reagent solution (100 mg/mL) and centrifuged twice at 10,000 rpm (10 min). The supernatant was then collected, and the Hb concentration was determined on the basis of absorbance at 540 nm, as compared with a standard Hb dilution. Results are expressed in μg Hb/100 mg tissue.
Assessment of GSH content, and gastric SOD and CAT activity
GSH content in the gastric mucosa was measured as described previously [32]. Gastric samples were homogenized in freezing 0.02 M EDTA solution (10%). Then 400 μL of tissue homogenate was mixed in glass tubes with distilled water (320 μL) and 80 μL of 50% (w/v) trichloroacetic acid, and centrifuged (3000 rpm, 15 min, 4°C). Tris buffer (800 μL, 0.4 M, pH 8.9) and 5.5-dithio-bis (2-nitrobenzoic acid; 20 μL, 0.01 M,) were added to the supernatants and mixed. After shaking, the absorbance at 412 nm was measured (5 min). Results are expressed in micrograms GSH per gram wet tissue.
The SOD activity was measured as previously described [33]. The reactant was 50 mM phosphate buffer, 0.1 mM EDTA, and 19.5 mM L-methionine, pH 7.8. A homogenate solution of 10% of gastric fresh tissue in phosphate-buffered saline was prepared. This homogenate was centrifuged twice, first for 10 min at 3,600 rpm and 4° C, and then for 20 min at 12,000 rpm and 4°C. The supernatant was removed between each centrifugation. In the dark, 30 μL of supernatant was mixed with 1 mL of reactant (described above). Then 150 μL of 750 μM NBT and 300 μL of 1 μM riboflavin were added. After 15 min of exposure to fluorescent light (15 W), the tubes with the mixture were read in a spectrophotometer at 560 nm. The results are expressed as μSOD per μg tissue protein.
The CAT activity was evaluated according to methods described previously [34]. Briefly, stomach homogenate was diluted to 10% in reaction medium (diluted H2O2, Tris-HCl buffer, 5 mM EDTA, pH 8, in milliQ H2O). The enzymatic activity was measured at 230 nm with a spectrophotometer (during 6 min). Results are expressed in μM per min per μg protein.
Probing T. egregia’s mechanism of action
Analysis of NO involvement in T. egregia-mediated gastric protection
Mice were pretreated (per os) with vehicle (0.3 mL/30 g), T. egregia (200 mg/kg), or the NOS substrate L-arginine [600 mg/kg; intraperitoneal (i.p.)]. Thirty minutes later, 99.9% ethanol (0.2 mL/animal) was administered by gavage. In another set of experiments, L-NAME (20 mg/kg), a nonspecific inhibitor of NOS, was injected (i.p.) 15 min before vehicle, T. egregia, or L-arginine injection. Thirty minutes after ethanol challenge, mice were euthanized, and gastric tissue samples were collected and analyzed as described previously.
Study of prostaglandin involvement in T. egregia-mediated gastric protection
Mice were pretreated (per os) with T. egregia (200 mg/kg), the prostaglandin analogue misoprostol (50 mg/kg), or saline (0.3 mL/30 g) 1 h before ethanol injection. Indomethacin (10 mg/kg), a prostaglandin synthesis inhibitor, was injected (per os) 2 hours before injection of T. egregia, misoprostol, or saline. Thirty minutes after ethanol administration, mice were euthanized, and gastric tissue samples were collected and analyzed as described previously.
Examination of opioid receptor involvement in T. egregia-mediated gastric protection
First, to examine the involvement of opioid receptors in the gastroprotective effect of T. egregia, we injected mice (i.p.) with naloxone (4 mg/kg), a non-selective opioid receptor antagonist. After 15 min, the mice were treated with morphine (5 mg/kg, subcutaneous), T. egregia (200 mg/kg, per os), or saline (0.3 mL/30 g; per os), followed by ethanol (0.2 mL). Thirty minutes after the ethanol challenge, the mice were euthanized, and gastric tissue samples were collected and analyzed as described previously.
Drugs and chemicals
Ranitidine was obtained from Aché Pharmaceutical (Guarulhos, SP, Brazil). Morphine and naloxone were purchased from Cristália Pharmaceutical Chemicals (Itapira, SP, Brazil). Indomethacin, Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME), and L-arginine methyl ester dihydrochloride (L-Arg) were purchased from Sigma Aldrich (St. Louis, MO, USA). Ethanol and formaldehyde were obtained from Vetec Química Fina Ltda (Duque de Caxias, RJ, Brazil). Misoprostol was acquired from Biolab Searle (Independência, SP, Brazil). All drugs were solubilized in sterile saline solution (0.9% NaCl). The enzymatic kits used for evaluation of tissue Hb were purchased from Labtest Diagnosis (Lagoa Santa, MG, Brazil).
Statistical analysis
Results are expressed as mean ± standard error of the mean. ANOVA followed by Bonferroni test was used to compare means. P < 0.05 was considered significant. The analyses were performed in GraphPad Prism 6, San Diego, CA, USA.
Results
In silico predicted ADME properties of two flavonoids [praecansone A (1) and pongachalcone (2)] isolated from T. egregia roots
ADME assessment is a major method for characterization of pharmacokinetic profiles. Physicochemical characteristics and the ADME parameters of flavonoids (1) and (2) are shown in Tables 1 and 2.
Table 1 Prediction of Physicochemical Parameters of Two Flavonoids (praecansone A and pongachalcone) Isolated from Tephrosia egregia Sandw Roots
| Physicochemical parameter | Praecansone A–(1) | Pongachalcone–(2) |
|---|---|---|
| Molecular formula | C22H22O5 | C12H8N2 |
| Molecular weight | 366.41 g/mol | 180.21 g/mol |
| Lipophilicity | 1.44 log P | 1.78 log P |
| No of acceptor H-bond | 5 | 5 |
| No of donor H-bond | 1 | 1 |
| Molar refractivity | 104.9 cm3·mol−1 | 57.04 cm3·mol−1 |
| Polar surface area | 64.99 Å2 | 25.78 Å2 |
Table 2 Prediction of Pharmacokinetic Properties (Absorption, Distribution, Metabolism, and Excretion – ADME) of Two Flavonoids (praecansone A and pongachalcone) Isolated from Tephrosia egregia Sandw Roots
| Pharmacokinetic properties | Praecansone A–(1) | Pongachalcone–(2) |
|---|---|---|
| Gastrointestinal absorption | + | + |
| BBB permeability | + | + |
| P-gp | + | + |
| CYP1A2 inhibitor | + | + |
| CYP2C9 inhibitor | + | + |
| CYP2C19 inhibitor | + | + |
| CYP2D6 inhibitor | − | + |
| CYP3A4 inhibitor | + | + |
BBB permeability: Blood brain barrier permeability; P-gp: P-glycoprotein (P-gp) substrate.
An established method to assess drug-likeness is evaluation according to Lipinski’s rule. Flavonoids (1) and (2) showed favorable results when evaluated with Lipinski’s rule (Table 1). The molecular weights of both compounds are below 500 g/mol, and the lipophilicity (log P) is less than 5 for both compounds. Flavonoids (1) and (2) have fewer than ten H-bond acceptors and fewer than five H-bond donors. The molar refractivity is between 40 and 130 cm3·mol−1 (flavonoid (1) = 104.9 and flavonoid (2) = 57.04), and the polar surface area is <140 Å2 (flavonoid (1) = 64.99 Å2 and flavonoid (2) = 25.78 Å2). Because flavonoids (1) and (2) pass Lipinski’s rule of five, both are candidate compounds categorized as possible active drugs with oral administration.
The pharmacokinetic properties of flavonoids (1) and (2), shown in Table 2, indicated high gastrointestinal absorption of both compounds. In addition, our data suggested that both flavonoids (1) and (2) can cross the blood brain barrier. Moreover, both compounds appear to interact with the substrate P-glycoprotein (P-gp), an efflux transporter that mediates the active transport of drugs from the intracellular to the extracellular compartment. Additionally, in silico prediction of CYP–ligand interactions was performed to evaluate the inhibitory potency of flavonoids (1) and (2) toward five human CYP isoforms (CYP1A2, 2C9, 2C19, 2D6, and 3A4). Both compounds inhibited the enzymatic activity of CYP isoforms, thus suggesting an approach for rapid prediction of CYP inhibitory activity through in silico assays. These isoforms were selected because they are responsible for almost all metabolic reactions (90%), including the metabolism of clinically used drugs and carcinogens [35]. Therefore, flavonoids (1) and (2) have favorable ADME properties.
Prediction of the toxicity of the two flavonoids (praecansone A and pongachalcone) isolated from T. egregia roots
An essential stage during drug development is the evaluation of the toxicity of new candidate drugs. The toxicity of flavonoids (1) and (2) isolated from T. egregia roots was predicted with in silico tools. The LD50, hepatotoxicity, carcinogenicity, immunotoxicity, mutagenicity, and cytotoxicity were predicted (Table 3). On the basis of the consensus of the results, the compounds were determined to be positive or negative for particular toxicity endpoints. With the ProTox-II webserver, flavonoids (1) and (2) were predicted to be in toxicity class 5 (2,000 < LD50 ≤ 5,000, indicating low toxicity) for acute oral toxicity [26]. Flavonoid (1) had a higher LD50 (3,800 mg/kg) than flavonoid (2) (2,500 mg/kg), with a prediction accuracy of 70.1% and 73.5%, respectively (Table 3). Toxicity prediction by ProTox-II indicated that flavonoids (1) and (2) are not hepatotoxic. The two flavonoids are not carcinogenic or cytotoxic to human cells (Table 3).
Table 3 Prediction of Toxicity of Two Flavonoids (Praecansone A and Pongachalcone) Isolated from T. Egregia Roots
| Toxicity prediction model | Praecansone A–(1) | Pongachalcone–(2) |
|---|---|---|
| LD50 | 3,800 mg/kg | 2,500 mg/kg |
| Toxicity class | 5 | 5 |
| Hepatotoxicity | – | – |
| Carcinogenicity | – | – |
| Immunotoxicity | + (20%)* | + (12%)* |
| Mutagenicity | – | – |
| Cytotoxicity | – | – |
| Prediction accuracy | 70.1% | 73.5% |
LD50: lethal dose, 50%. *Probability percentage.
Immunotoxicity is an adverse effect of xenobiotics on the immune system [26]. We used a model of immunotoxicity based on immune cell cytotoxicity data from the U.S. National Cancer Institute’s public database. In this model, growth inhibition values <10 μM toward the B-cell line RPMI-8226 are considered to indicate toxicity of compounds [36].
In the overall immunotoxicity parameter, a structure-based probability was detected by the system of 20% chance for flavonoid (1) and 12% chance for flavonoid (2). The ProTox-II immunotoxicity prediction model has an accuracy of 74.00% in cross-validation and 70.00% in external validation [26]. Overall, both compounds displayed favorable toxicity profiles.
Molecular docking
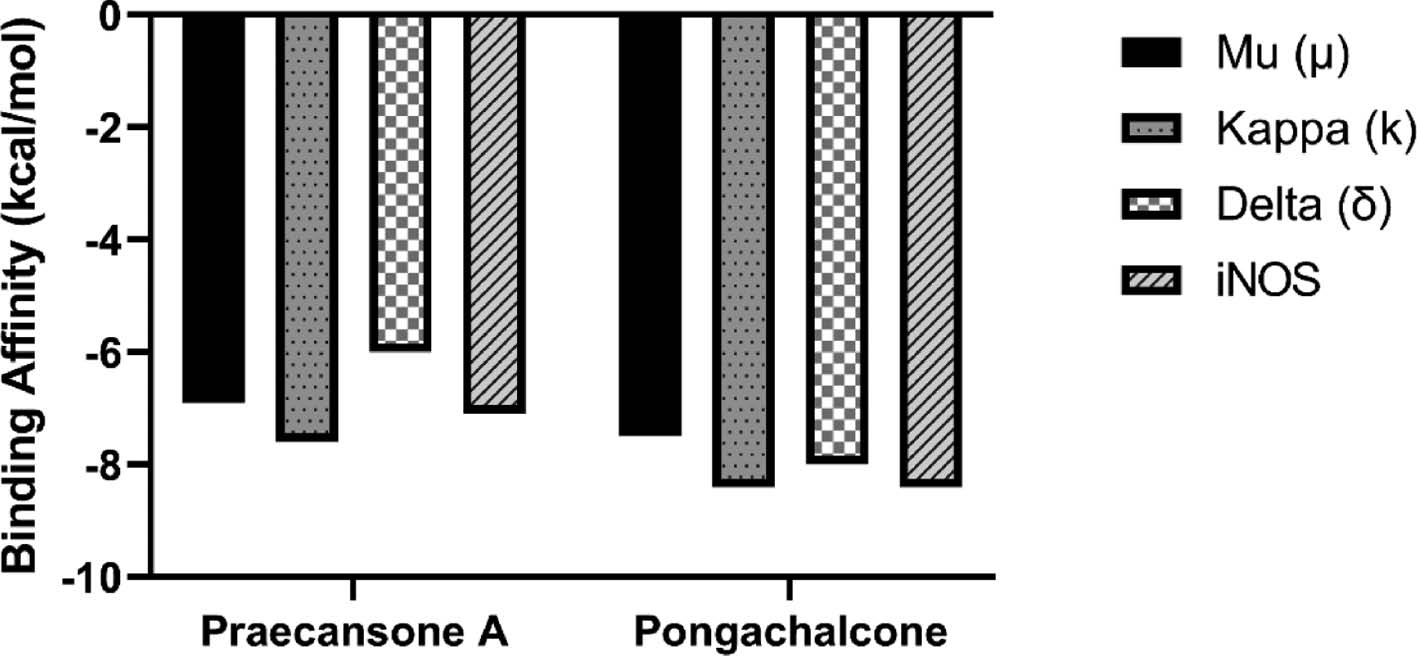
Flavonoids (1) and (2) showed interesting interactions and satisfactory binding energy data when docked into their targets (below −6.0 kcal/mol; Figure 2). The docking results for flavonoid (1) were −6.95 kcal/mol for the μ-opioid receptor, −7.6 kcal/mol for the κ-opioid receptor, −6.0 kcal/mol for the δ-opioid receptor, and −7.1 kcal/mol for iNOS protein (Figure 2). Furthermore, the results for interaction with flavonoid (2) were −7.5 kcal/mol for the μ-opioid receptor, −8.4 kcal/mol for the κ-opioid receptor, −8.0 kcal/mol for the δ-opioid receptor, and −8.4 kcal/mol for iNOS (Figure 2).
Figure 2 Graphical representation of binding energies (ΔG, in kcal/mol) of molecular docking between the ligands (flavonoids) praecansone A and pongachalcone and the targets μ-opioid receptor, κ-opioid receptor, δ-opioid receptor, and iNOS protein.
Effects of T. egregia in an ethanol-induced gastropathy model
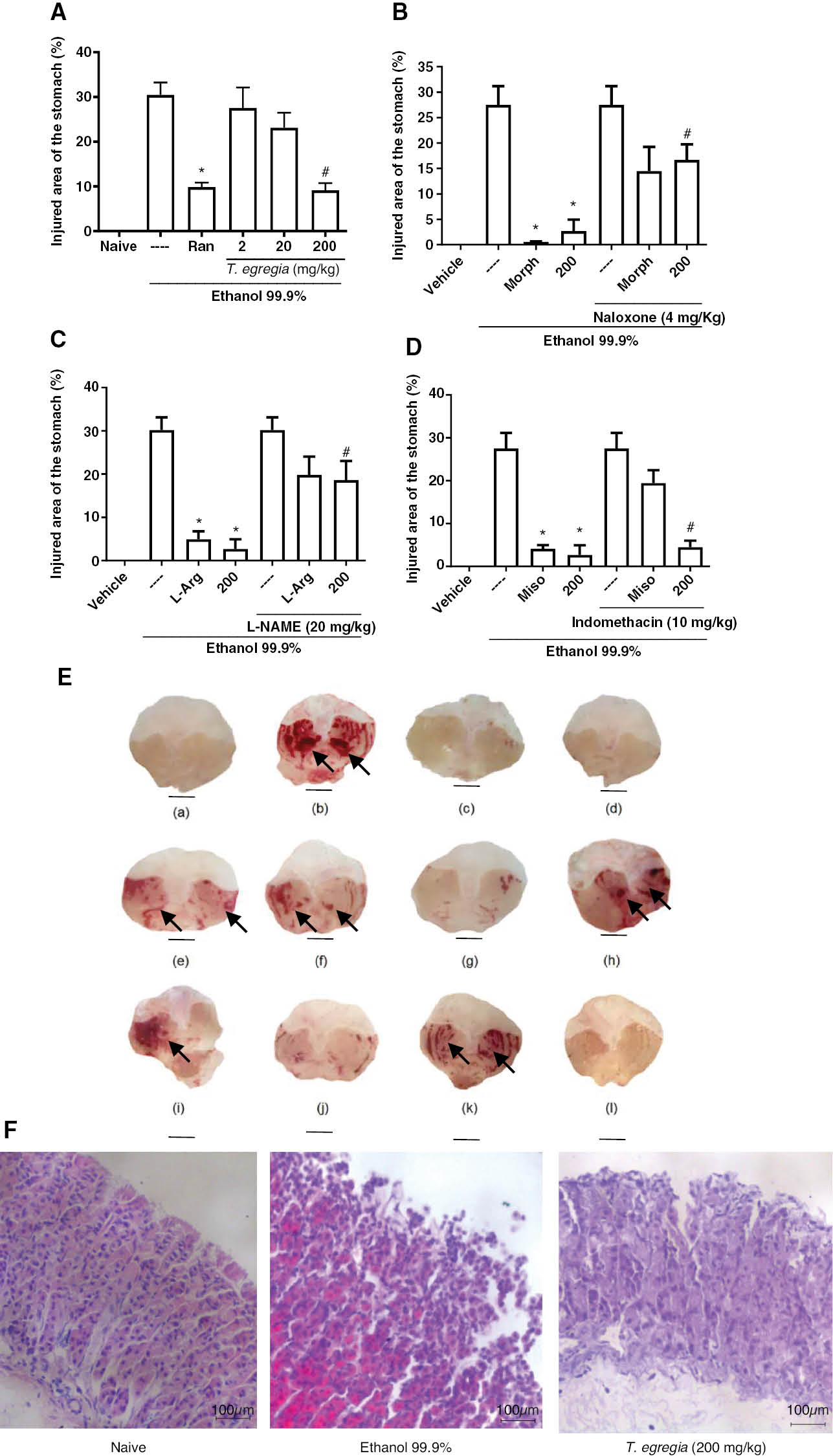
The treatment with T. egregia (200 mg/kg) decreased (P < 0.05) gastric damage (9.13 ± 1.6) below that observed in groups receiving only vehicle (30.46 ± 2.74; Figure 3A). Interestingly, the findings for the groups receiving T. egregia (200 mg/kg) and ranitidine (9.83 ± 1.01) were similar (P > 0.05). Macroscopic images of the gastroprotective effects of T. egregia (200 mg/kg) against ethanol-induced gastric damage are shown in Figure 3E-c. Figure 3F illustrates the effect of T. egregia (200 mg/kg) on the histopathological (H&E; magnification ×200) features of gastric mucosa from mice after ethanol challenge. The T. egregia (200 mg/kg) group showed a preserved epithelial lining, whereas the ethanol 99.9% group displayed loss of integrity of the epithelial lining.
Figure 3 Effects of T. egregia on ethanol-induced gastric damage (A) and the involvement of opioid receptors (B), NO (C), and prostaglandins (D) on its gastroprotective effect. (A) Mice were treated (per os) with ranitidine (Ran; 80 mg/kg) or T. egregia (2, 20, or 200 mg/kg). After 30 min or 1 h, absolute ethanol (0.2 ml per mice) was administered (per os). The untreated group (—) received (per os) saline before ethanol. The vehicle group (naive) contained unchallenged mice (treated with only saline). Gastric damage was quantified after 30 min. The results are expressed as mean± S.E.M. for each group of at least 7 mice. *P < 0.05 in relation to the challenged group (—). ANOVA followed by Bonferroni’s multiple comparison test. (B) *P < 0.05 with respect to the ethanol-challenged group (—); #P < 0.05 with respect to morphine alone. (C) *P < 0.05 with respect to the ethanol-challenged group (—); #P < 0.05 with respect to L-Arg alone; (D) *P < 0.01 with respect to the ethanol-challenged group (—); #P > 0.05 with respect to T. egregia alone. Morph = morphine; 200 = mice treated with T. egregia at 200 mg/kg. (E) Macroscopic images of the gastric mucosa illustrating T. egregia’s gastroprotective effects on ethanol-induced gastric damage and its possible mechanisms. (a) Naive animals (vehicle group). (b) Ethanol group. (c) T. egregia (200 mg/kg). (d) Morphine. (e) Naloxone + morphine. (f) Naloxone + T. egregia (200 mg/kg). (g) L-Arg. (h) L-NAME (20 mg/kg) + L-Arg. (i) L-NAME (20 mg/kg) + T. egregia (200 mg/kg). (j) Misoprostol. (k) Indomethacin + misoprostol. (l) Indomethacin + T. egregia (200 mg/kg). The black arrows indicate extensive areas of gastric mucosa damage (hemorrhagic and hyperemic mucosa). (F) Effect of T. egregia (200 mg/kg) on the histopathological (H&E) features of gastric mucosa from mice after ethanol (99.9%) challenge. Naive: control group showing preserved epithelial lining and simple cylindrical epithelium with well-defined morphological features. Ethanol 99.9%: group showing loss of integrity of the epithelial lining and discontinuous, simple cylindrical epithelium. T. egregia (200 mg/kg): group showing mostly preserved epithelial lining and simple cylindrical epithelium with few discontinuous areas (magnification ×200).
Tissue Hb assessment
Table 4 shows that T. egregia (200 mg/kg) decreased (P < 0.05) the gastric Hb to levels below those in the groups receiving only the vehicle.
Table 4 Effects of T. Egregia on Gastric Hb Concentration After Ethanol (99.9%) Challenge
| Experimental groups | Tissue Hb (μg/100 mg tissue) |
|---|---|
| Unchallenged (vehicle) | 9.4 ± 0.7 |
| Ethanol challenged | 16.9 ± 0.10 |
| Ethanol + T. egregia (2 mg/kg) | 10.7 ± 0.9 |
| Ethanol + T. egregia (20 mg/kg) | 10.8 ± 1.5 |
| Ethanol + T. egregia (200 mg/kg) | 8.4 ± 0.6* |
Table shows mean ± S.E.M. ANOVA followed by Bonferroni’s multiple comparison test. *P < 0.05 compared with the ethanol-challenged group.
Evaluation of GSH content, and gastric SOD and CAT activity
The antioxidant effects of T. egregia on gastric damage induced by ethanol challenge are depicted in Table 5. T. egregia pre-treatment (200 mg/kg), compared with vehicle alone, resulted in significantly higher (P < 0.05) GSH content, and SOD and CAT activity.
Table 5 Antioxidant Effects of T. Egregia on Ethanol-Induced Gastric Damage
| Experimental groups | Unchallenged (vehicle) | Ethanol challenged | T. egregia |
|---|---|---|---|
| GSH (μg per g tissue ± S.E.M.) |
1,237 ± 55.75 | 802.8 ± 75.69 | 1,596 ± 153.5* |
| SOD (μSOD per μg protein ± S.E.M.) |
2.398 ± 0.4942 | 1.019 ± 0.09951 | 2.085 ± 0.2554* |
| CAT (mM per min per mg protein ± S.E.M.) |
193 ± 37.04 | 40.75 ± 7.296 | 203.9 ± 39.44* |
The values are represented as means ± S.E.M. for seven mice per group. *Significantly different (P < 0.05) from the ethanol-challenged group (ANOVA, Bonferroni).
Opioid receptors and NO involvement in T. egregia-mediated gastric protection
T. egregia (200 mg/kg) and morphine pre-treatment protected (P < 0.05) the gastric mucosa of the mice against ethanol damage (2.67 ± 2.2 and 0.53 ± 0.1 versus 27.5 ± 3.65, respectively). However, naloxone (4 mg/kg) pre-administration (i.p.), compared with individual T. egregia or morphine treatments, reversed the gastroprotective effects of both T. egregia (16.67 ± 3.07) and morphine (14.47 ± 4.7; Figure 3B). Similarly, L-arginine administration (4.94 ± 1.82; P < 0.05) protected the gastric mucosa in mice exposed to ethanol challenge. Finally, L-NAME pretreatment (20 mg/kg), compared with T. egregia alone, reversed (P < 0.05) T. egregia’s gastroprotective effects (18.6 ± 4.43; Figure 3C). Figure 3E depicts macroscopic images of the gastric mucosa indicating the involvement of opioid receptors (3E-d, 3E-e, and 3E-f) and NO (3E-g, 3E-h, and 3E-i) in the gastroprotective effects of T. egregia (200 mg/kg).
T. egregia’s gastroprotective effects do not involve prostaglandin synthesis
Figure 3D shows that indomethacin (10 mg/kg) followed by ethanol injection produced hemorrhagic lesions that were somewhat diminished by misoprostol treatment (per os; 50 μg/kg). The gastroprotective effect of T. egregia occurred regardless of indomethacin injection, thus suggesting that its efficacy does not involve prostaglandin synthesis. Macroscopic images showing the absence of the involvement of prostaglandins in the gastroprotective effects of T. egregia (200 mg/kg) are shown in Figure 3E (3E-j, 3E-k, and 3E-l).
Discussion
This study revealed the gastroprotective effects of the extract of T. egregia roots in mice subjected to ethanol challenge. Because a main phase in drug development involves evaluation of the pharmacokinetics, toxicology, and pharmacodynamics of a drug candidate, we performed in silico ADME and toxicity prediction of two lead flavonoids from the roots of T. egregia. In addition, we found evidence that that the gastroprotective and antioxidant activity of T. egregia is mediated at least partly by opioid receptors and NO.
The Tephrosia genus (family Fabaceae) is mainly found in regions with high temperatures [37]. Phytochemical investigations revealed several phytoconstituents in the Tephrosia genus with biological activities including anti-diabetic, anti-ulcer, anti-diarrheal, wound healing, anti-inflammatory, insecticidal, anti-viral, anti-protozoal, anti-fungal, and anti-plasmodial effects [19]. However, very few data are available regarding the pharmacokinetics, toxicology, and mechanism of action of T. egregia and its phytoconstituents.
Drug discovery is a complex process requiring analyses of several parameters (safety, pharmacokinetics, and efficacy) of new drug candidates. During drug research, substantial efforts have been made to decrease animal use in determining the acute oral toxicity of new compounds, in agreement with the “3Rs” principle [21]. In this regard, computational software has contributed to predicting the features and parameters (pharmacokinetics, toxicology, and pharmacodynamics of drug candidates) needed for the development of new drug candidates. In the present study, in silico results showed that the lead flavonoids from the roots of T. egregia [praecansone A (1) and pongachalcone (2)] had favorable ADME properties and toxicity profiles, and exhibited satisfactory binding energies (below −6.0 kcal/mol) data when docked into their targets (μ-, κ-, and δ-opioid receptors, and iNOS).
Through in vivo assays, we evaluated gastric Hb levels, a sign of mucosal bleeding. In fact, gastric damage has been associated with elevated levels of Hb [38]. Herein, an increase in Hb levels was observed after ethanol challenge, but was diminished after treatment with T. egregia (200 mg/kg).
Oxidative stress plays a key role in the pathophysiology of gastric disorders [39]. Similarly to other findings [40], our results indicated the involvement of oxidative stress in gastric pathology, thereby indicating the potential role of antioxidants as gastroprotective agents for gastric ulceration [41]. Ethanol reacts with cell membranes and consequently increases lipid peroxidation and the production of hydroxyl radicals and superoxide anions, which may induce direct oxidative damage [42]. In the present study, ethanol challenge decreased the activity of the antioxidant enzymes SOD and CAT, but these effects were prevented by T. egregia (200 mg/kg) treatment. Notably, we observed no differences (P > 0.05) in the SOD and CAT values between animals treated with T. egregia and naive animals. These results suggested that T. egregia has therapeutic potential in ameliorating ethanol-induced gastric injury by alleviating oxidative stress. Intracellular anti-oxidative systems protect the gastric mucosa against oxidative stress [43].
Prostaglandins have important roles in the gastrointestinal tract, modulating mucosal defense by inhibiting acid secretion, increasing the amount of mucus, and promoting vasodilation [44]. Because prostaglandin synthesis may be blocked by nonsteroidal anti-inflammatory drugs, the administration of these agents is associated with gastric damage [45]. Our current data showed that T. egregia gastroprotection occurred independently of pretreatment with indomethacin, a non-steroidal anti-inflammatory agent, thus demonstrating that the gastroprotective effects do not occur through prostaglandins.
Substantial evidence indicates that NO acts as a dual signaling molecule in both physiological and pathophysiological processes. NO’s roles in gastric mucosa integrity, protection, and ulcer healing have been described [46]. In fact, NO is a vasodilatory molecule that may control gastric blood flow and gastric mucosal barrier integrity—important elements of gastric ulcer healing [47]. In the present study, L-NAME, a non-selective competitive inhibitor of NOS, decreased the gastroprotective effect of T. egregia, thus suggesting the role of NO in T. egregia gastroprotective effect.
To evaluate the putative involvement of opioid receptors in the gastroprotective effect of T. egregia, we used naloxone, a non-selective opioid antagonist. Naloxone significantly prevented the effect of morphine, the standard opioid agonist, and consequently reversed the gastroprotective effects. Similarly, in the presence of naloxone, no gastroprotective effects of T. egregia were observed, thus suggesting that T. egregia’s effect in ameliorating ethanol-induced gastric damage in mice appears to depend on the activation of opioid receptors. The activation of supraspinal δ- and μ-opioid receptors has been associated with greater production of NO in a model of ethanol-induced gastric damage [48], thus suggesting that activation of opioid receptors and mucosal NO may be involved in the gastroprotective effect of T. egregia.
Conclusion
Our data suggest that T. egregia has gastroprotective potential in mice, which may be partly due to its antioxidant efficiency, and the involvement of opioid receptors and NO. Considering the numerous mechanisms through which the gastric mucosa may resist injury, we believe that T. egregia may serve as an innovative technology to develop new strategies for improving ulcer healing.
Acknowledgements
This work was supported by Brazilian grants from Conselho Nacional de Pesquisa (CNPq) and Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (Funcap). Vicente de Paulo Teixeira Pinto is a Funcap/Ceara/Brazil investigator.
Author contributions
Conceptualization: Mirna M. Bezerra, Hellíada V. Chaves, and Antonio Alfredo R. e Silva. Methods: Marcos Eber F. Rogério, Isabela R. Pinto, Nayara A. de Sousa, Kátia A. Ribeiro, and Dina Andressa M. Monteiro. Formal analysis and investigation: Roberta Jeane B. Jorge, and Helyson Lucas B. Braz. Writing—original draft preparation: Mirna M. Bezerra, Isabela R. Pinto, and Nayara A. de Sousa. Writing—review and editing: Helliada V. Chaves and Maria Elisabete A. de Moraes. Funding acquisition: Mirna M. Bezerra, Hellíada V. Chaves, and Vicente de Paulo T. Pinto. Resources: Ângela Martha C. Arriaga, Maria Valdeline S. Teixeira, and Antônia T. A. Pimenta. Supervision: Mirna M. Bezerra, Hellíada V. Chaves, and Vicente de Paulo T. Pinto.
References
- Fahmi AA, Abdur-rahman M, Aboul-naser AF, Hamed MA, Abd-alla H I, et al. Pulicaria crispa mitigates gastric ulcer induced by ethanol in rats: role of treatment and auto healing. Biomarkers 2019;24:286-94 . [PMID: 30512969 DOI: 10.1080/1354750X.2018.1556340]
- Rahman Z, Dwivedi DK, Jena GB. Ethanol-induced gastric ulcer in rats and intervention of tert-butylhydroquinone: involvement of Nrf2/HO-1 signalling pathway. Hum Exp Toxicol 2019;39:547-62. [PMID: 31876185 DOI: 10.1177/0960327119895559]
- Fazalda A, Quraisiah A, Azlina MFN. Antiulcer effect of honey in nonsteroidal anti-inflammatory drugs induced gastric ulcer model in rats: a systematic review. Evid Based Complement Alternat Med 2018;2018:1-12 . [PMID: 30105063 DOI: 10.1155/2018/7515692]
- Badr AM, El-Orabi NF, Ali RA. The implication of the crosstalk of Nrf2 with NOXs, and HMGB1 in ethanol-induced gastric ulcer: potential protective effect is afforded by Raspberry Ketone. PLoS One 2019;14:e0220548. [PMID: 31404064 DOI: 10.1371/journal.pone.0220548]
- Mousa AM, El-Sammad NM, Hassan SK, Madboli AENA, Hashim AN, et al. Antiulcerogenic effect of Cuphea ignea extract against ethanol-induced gastric ulcer in rats. BMC Complement Altern Med 2019;19:345. [PMID: 31791313: DOI: 10.1186/s12906-019-2760-9]
- Omayone TP, Salami AT, Olopade JO, Olaleye SB. Attenuation of ischemia-reperfusion-induced gastric ulcer by low-dose vanadium in male Wistar rats. Life Sci 2020;259:1-9. [PMID: 32800836 DOI: 10.1016/j.lfs.2020.118272]
- Kangwan N, Pintha K, Lekawanvijit S, Suttajit M. Rosmarinic. Acid enriched fraction from Perilla frutescens leaves strongly protects indomethacin-induced gastric ulcer in rats. BioMed Res Int 2019:9514703. [PMID: 30949513 DOI: 10.1155/2019/9514703]
- Teixeira MVS, Lima JQ, Pimenta ATA, Silva FRL, Oliveira MCF, et al. New flavone and other compounds from Tephrosia egregia: assessing the cytotoxic effect on human tumor cell lines. Rev Bras Farmacogn 2018;28:333-8. [DOI: 10.1016/j.bjp.2018.03.008]
- You Zhi L, Guanhua L, Xiaoyi W, Zhonchua L, Hanhoug X. Isolation and identification of insecticidal compounds from Tephrosia purpurea (Fabaceae) bark and their insecticidal activity. Acta Entomol Sin 2011;54:1368-76.
- Taurus PK, Machocho AK, Lang’at-Thoruw CC, Chhabra SC. Flavonoids from Tephrosia aequilata. Phytochemistry 2002;60:375-9. [PMID: 12031428 DOI: 10.1016/s0031-9422(02)00078-x]
- Ganapaty S, Srilakshmi GVK, Pannakal ST, Laatsh HA. A pyranochalconeand prenylflavanones from Tephrosia pulcherrima (Baker) drumm. Nat Prod Commun 2008;3:49-52. [DOI: 10.1177/1934578X0800300111]
- Andrei CC, Ferreira DT, Fraccione M, Moraes LAB, Carvalho MG, et al. C-prenylflavonoids from roots of Tephrosia tunicata. Phytochemistry 2000;55:799-804. [PMID: 11190399 DOI: 10.1016/s0031-9422(00)00371-x]
- Sato S, Takeo J, Aoyama C, Kawahara H. Na+-Glucose cotransporter (SGTL)inhibitory flavonoids from roots of Sophora flavescens. Bioorg Med Chem 2007;15:3445-9. [PMID: 17374486 DOI: 10.1016/j.bmc.2007.03.011]
- Arriaga AMC, Magalhães FEA, Feitosa EMA, Malcher GT, Andrade-Neto M, et al. Composition of the essential oil of Tephrosia egrégia Sandw. J Essent Oil Res 2005;17:451-2. [DOI: 10.1080/10412905.2005.9698960]
- Ribeiro WHF, Vasconcelos JN, Arriaga AMC, Oliveira MCF, Andrade-Neto M, et al. Tephrosia toxicaria Pers essential oil: chemical composition and larvicidal activity. Nat Prod Commun 2006;1:391-3. [DOI: 10.1177/1934578X06001005090]
- Vasconcelos JN, Lima JQ, Lemos TLG, Oliveira MCF, Almeida MMB, et al. Estudo químico e biológico de Tephrosia toxicaria Pers. Quimica Nova 2009;32:382-96. [DOI: 10.1590/S0100-40422009000200021]
- Juma WP, Akala HM, Eyase FL, Muiva LM, Heydenreich M, et al. Terpurinflavone: an antiplasmodial flavone from the stem of Tephrosia purpurea. Phytochemi Lett 2011;4:176-8. [DOI: 10.1016/j.phytol.2011.02.010]
- Do Val DR, Bezerra MM, Silva AAR, Pereira KMA, Rios LC, et al. Tephrosia toxicaria Pers. reduces temporomandibular joint inflammatory hypernociception: the involvement of the HO-1 pathway. Eur J Pain 2014;18:1280-9. [PMID: 24715714 DOI: 10.1002/j.1532-2149.2014.488.x]
- Chen Y, Yan T, Gao C, Cao W, Huang R. Natural products from the genus Tephrosia. Molecules 2014;19:1432-58. [PMID: 24473207 DOI: 10.3390/molecules19021432]
- Arriaga AMC, Lima JQ, Vasconcelos JN, Oliveira MCF, Lemos TLG, et al. Antioxidant and larvicidal activities of Tephrosia egregia Sandw against Aedes aegypti. Nat Prod Commun 2009;4:529-30. [PMID: 19475999]
- Russell WMS, Burch RL. The principles of humane experimental technique. Wheathampstead: Universities Federation for Animal Welfare; 1959.
- Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, et al. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminform 2012;4:17. [PMID: 22889332 DOI: 10.1186/1758-2946-4-17]
- Barca GMJ, Bertoni C, Carrington L, Datta D, De Silva N, et al. Recent developments in the general atomic and molecular electronic structure system. J Chem Phys 2020;21:154102. [PMID: 32321259 DOI: 10.1063/5.0005188]
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 1997;23:3-25. [PMID: 11259830 DOI: 10.1016/s0169-409x(00)00129-0]
- Veber DF, Johnson SR, Cheng H-Y, Smith BR, Ward KW, et al. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem 2002;45:2615-23. [PMID: 12036371 DOI: 10.1021/jm020017n]
- Banerjee P, Eckert AO, Schrey AK, Preissner R. ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucleic Acids Res 2018;46:W257-63. [PMID: 29718510 DOI: 10.1093/nar/gky318]
- Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 2009;30:2785-91. [PMID: 19399780 DOI: 10.1002/jcc.21256]
- Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 2010;31(2):455-61. [PMID: 19499576 DOI: 10.1002/jcc.21334]
- Biovia, Dassault Systèmes. Discovery Studio. BIOVIA Workbook, Release, 2020. V 4.5.
- Pettersen EF, Goddard TD, Huang CC, Meng EC, Couch GS, et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci 2021;30:70-82. [PMID: 32881101 DOI: 10.1002/pro.3943]
- Morimoto Y, Shimohara K, Oshima S, Sukamoto T. Effects of the new anti-ulcer agent KB-5492 on experimental gastric mucosal lesions and gastric mucosal defensive factors, as compared to those of teprenone and cimetidine. Jpn J Pharmacol 1991;57:495-505. [PMID: 1803065 DOI: 10.1254/jjp.57.495]
- Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 1968;25:192-205. [PMID: 4973948 DOI: 10.1016/0003-2697(68)90092-4]
- Sun Y, Oberley LW, LI Y. A simple method for clinical assay of superoxide dismutase. Clin Chem 1988;34:497-500. [PMID: 3349599 DOI: 10.1093/clinchem/34.3.497]
- Maehly AC, Chance B. The assay of catalases and proxidases. Methods Biochem Anal 1954;1:357-424. [PMID: 13193536 DOI: 10.1002/9780470110171.ch14]
- Nebert DW, Wikvall K, Miller WL. Human cytochromes P450 in health and disease. Philos Trans R Soc Lond B Biol Sci 2013;368:20120431. [PMID: 23297354 DOI: 10.1098/rstb.2012.0431]
- Schrey AK, Nickel-Seeber J, Drwal MN, Zwicker P, Schultze N, et al. Computational prediction of immune cell cytotoxicity. Food Chem Toxicol 2017;107:150-66. [PMID: 28558974 DOI: 10.1016/j.fct.2017.05.041]
- Al-Ghamdi FA. Morphological diversity of some tephrosia species (Fabaceae) in Saudi Arabia. Am J Plant Sci 2013;4:543-8. [DOI: 10.4236/ajps.2013.43070]
- Gong G, Zhao R, Zhu Y, Yu J, Wei B, et al. Gastroprotective effect of cirsilineol against hydrochloric acid/ethanol-induced gastric ulcer in rats. Korean J Physiol Pharmacol 2021;25:403-11. [PMID: 34448458 DOI: 10.4196/kjpp.2021.25.5.403]
- Pérez S, Taléns-Visconti R, Rius-Pérez S, Finamor I, Sastre J. Redox signaling in the gastrointestinal tract. Free Radic Biol Med 2017;104:75-103. [PMID: 28062361 DOI: 10.1016/j.freeradbiomed.2016.12.048]
- Ray A, Gulati K, Henke P. Stress gastric ulcers and cytoprotective strategies: perspectives and trends. Curr Pharm Des 2020;26:2982-90. [PMID: 32436823 DOI: 10.2174/1381612826666200521143203]
- Pinto IR, Chaves HV, Vasconcelos AS, de Sousa FCF, Santi-Gadelha T, et al. Antiulcer and antioxidant activity of a lectin from Mucuna pruriens seeds on ethanol-induced gastropathy: involvement of alpha-2 adrenoceptors and prostaglandins. Curr Pharm Des 2019;25:1430-9. [PMID: 31124421 DOI: 10.2174/1381612825666190524081433]
- Yu T, Yang Y, Kwak YS, Song GG, Kim MY, et al. Ginsenoside Rc from Panax ginseng exerts anti-inflammatory activity by targeting TANK binding kinase 1/interferon regulatory factor-3 and p38/ATF-2. J Ginseng Res 2017;41:127-33. [PMID: 28413316 DOI: 10.1016/j.jgr.2016.02.001]
- Ribeiro KA, Chaves HV, Pereira FSM, Pinto IR, Monteiro DAM, et al. Alpha-2 adrenergic and opioids receptors participation in mice gastroprotection of Abelmoschus esculentus lectin. Curr Pharm Des 2016;22:4736-42. [PMID: 26831461 DOI: 10.2174/1381612822666160201152438]
- Silva AAR, Bezerra MM, Chaves HV, Pinto VPT, Franco ES, et al. Protective effect of Chresta martii extract on ethanol-induced gastropathy depends on alpha-2 adrenoceptors pathways but not on nitric oxide, prostaglandins or opioids. J Ethnopharmacol 2012;142:206-12. [PMID: 22564358 DOI: 10.1016/j.jep.2012.04.042]
- Wallace JL. Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn’t the stomach digest itself? Physiol Rev 2008;88:1547-65. [PMID: 18923189 DOI: 10.1152/physrev.00004.2008]
- Magierowski M, Magierowska K, Kwiecien S, Brzozowski T. Gaseous mediators nitric oxide and hydrogen sulfide in the mechanism of gastrointestinal integrity, protection and ulcer healing. Molecules 2015;20:9099-123. [PMID: 25996214; PMCID: PMC6272495 DOI: 10.3390/molecules20059099]
- Liang TY, Deng RM, Li X, Xu X, Chen G. The role of nitric oxide in peptic ulcer: a narrative review. Med Gas Res 2021;11:42-5. [PMID: 33642337 DOI: 10.4103/2045-9912.310059]
- Gyires K, Müllner K, Rónai AZ. Activation of central opioid receptors may induce gastric mucosal defense in the rat. Journal of Physiology (Paris) 2001;95:189-96. [PMID: 11595436 DOI: 10.1016/s0928-4257(01)00024-9]