A Review on Toxicity and Challenges in Transferability of Surface-functionalized Metallic Nanoparticles from Animal Models to Humans
1Department of Pharmaceutics, Faculty of Pharmacy, Jinnah Sindh Medical University, Karachi 75510, Pakistan
2Department of Pharmaceutics and Bioavailability and Bioequivalence Research Facility, Faculty of Pharmacy and Pharmaceutical Sciences, University of Karachi, Karachi 75270, Pakistan
3Food and Feed Safety Laboratory, Food and Marine Resources Research Centre, PCSIR Laboratories Complex, Shahrah-e-Salimuzzaman Siddiqui, Off University Road, Sindh 74200, Pakistan
4Department of Pharmaceutics, Faculty of Pharmacy, Benazir Bhutto Shaheed University, Lyari, Karachi 75660, Pakistan
*Correspondence to: Rabia Ismail Yousuf, E-mail: rabia_pharmaceutics@yahoo.com, riyousuf@uok.edu.pk
Received: December 30 2020; Revised: March 3 2021; Accepted: April 17 2021; Published Online: May 10 2021
Cite this paper:
Muhammad Arif Asghar, Rabia Ismail Yousuf, Muhammad Harris Shoaib, Muhammad Asif Asghar and Nazish Mumtaz. A Review on Toxicity and Challenges in Transferability of Surface-functionalized Metallic Nanoparticles from Animal Models to Humans. BIO Integration 2021; 2(2): 71–80.
DOI: 10.15212/bioi-2020-0047. Available at: https://bio-integration.org/
Download citation
© 2021 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
The unique size and surface morphology of nanoparticles (NPs) have substantially influenced all aspects of human life, making nanotechnology a novel and promising field for various applications in biomedical sciences. Metallic NPs have gained immense interest over the last few decades due to their promising optical, electrical, and biological properties. However, the aggregation and the toxic nature of these NPs have restricted their utilization in more optimized applications. The optimum selection of biopolymers and biological macromolecules for surface functionalization of metallic NPs will significantly improve their biological applicability and biocompatibility. The present mini-review attempts to stress the overview of recent strategies involved in surface functionalization of metallic NPs, their specific biomedical applications, and comparison of their in vitro, ex vivo, and in vivo toxicities with non-functionalized metallic NPs. In addition, this review also discusses the various challenges for metallic NPs to undergo human clinical trials.
Keywords
Biomedical applications, comparative toxicity, human clinical trials, metallic nanoparticles, nanotechnology, surface functionalization.
An Editorial Note to this article was published on February 24 2022.
Nanotechnology
The term nanotechnology was first introduced in 1959 by an American scientist, Dr. Richard Feynman, in his physics lecture and stated that “There’s plenty of room at the bottom.” He floated an idea in the mind of other scientists on research on the atomic level for new development in the field of science and technology. Because of the unique characteristics attributed to the surface morphology and size distribution of nanoparticles (NPs), scientists from different fields have focused on nanotechnology. From 2017 to 2019, the industrial market value of nanoscience and nanotechnology was found in the range of 2 to 3 billion dollars. However, the average compound annual growth rate is increasing to 29.5% from 2017 through 2021, which could be used as basis to predict that the industrial market value of nanoscience and nanotechnology might reach 7.3 billion dollars in 2023 [1]. The size of NPs is found in the range of 1–100 nm, and it can be classified into four different dimensions, i.e., zero dimension, one dimension, two dimensions, or three dimensions [2]. The acceptance of nanomaterials is highly appreciable, due to their size variability, excellent dispensable properties, high mobility, and specific mechanical, magnetic, and optical strength, which make them different from the microscopic-range materials [3, 4].
The utilization of nanotechnology has been found in numerous fields of science and technology such as biological and biomedical sciences, bioengineering, environmental remediation fields, material technology, and food and agriculture sciences [5, 6]. It is used in in vitro, ex vivo, and in vivo applications as antibacterial and antifungal agents, fluorescent labels, antimicrobial agents, transfection labels, cell imaging, drug delivery, biosensors, electrochemical devices, and energy storage diagnostic and therapeutic agents [7, 8].
The field of nanotechnology is also increasing our daily life expectancy by providing new opportunities to control different imaginable diseases. Different types of metallic NPs, particularly gold, silver, platinum, and zinc NPs are extensively used in different sectors of the food and pharmaceutical industries. Metallic NPs have excellent and unique physicochemical properties such as good chemical stability, high thermal stability, optical properties, electronic properties, remarkable photocatalytic activity, and low cost [9]. They can be surface-functionalized or coated with other biomolecules or biopolymers to create biocompatible and unique NPs with desired properties. Due to these unique physicochemical characterisitics of these metallic NPs, they could have several biomedical applications, including drug delivery, protein purification, bacterial detection, contamination decorporation, enzyme immobilization, hyperthermia, and many others [10]. At present, these metallic NPs are also used in toothpaste, coatings of sanitary ware, sunscreens, water cleaning, and food/feed and cosmetic products that are all directly in contact with the skin of humans and animals [11, 12].
This review is presented in three sections. The first section highlights the importance of nanotechnology and its applications in biomedical fields; the second section describes the low toxicity of surface-functionalized metallic NPs over non-modified metallic NPs. The last section of this review focuses on the challenges in transferability of surface-functionalized NPs from animal models to humans.
Surface functionalization of metallic NPs
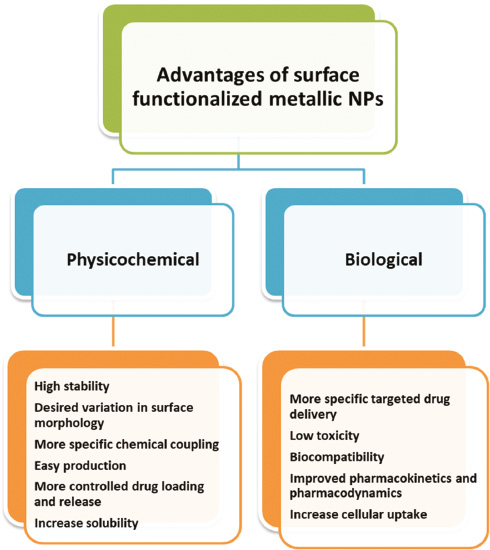
Bio-functionalized metallic NPs are highly versatile nano-structured materials that have enormous potentials to be utilized in various applications such as bio-imaging, optoelectronics, biosensing, photonics, and nanomedicine. Several physicochemical and biological advantages have been reported for surface-modified metallic NPs (Figure 1).
Figure 1 Physicochemical and biological advantages of surface-functionalized metallic nanoparticles (NPs) [13, 14].
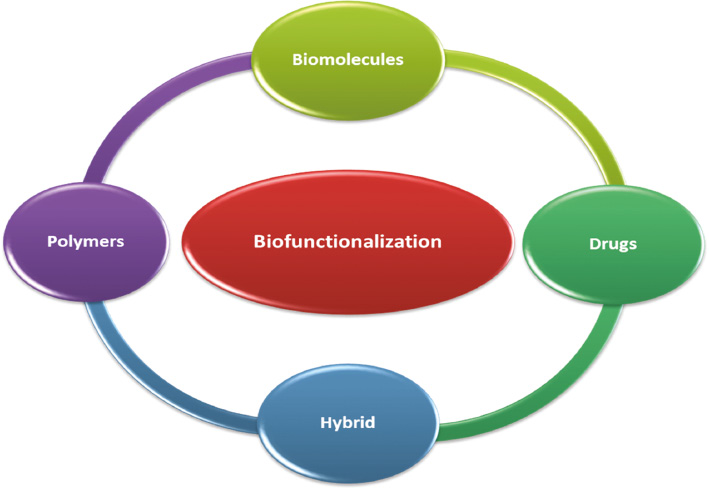
Besides surface functionalization of metallic NPs with different biological molecules, polymers or drugs can modify their bio-distribution and toxicity profile substantially in the case of targeted drug delivery to the mononuclear phagocyte system (MPS) such as in the lungs, bone marrow, liver, and spleen. Whenever NPs are administered intravenously, the host immune system can recognize them and rapidly eliminate them from the general circulation by phagocytes [15]. Apart from NP size, the hydrophobicity of the synthesized NPs determines the blood components level that binds this surface (e.g., opsonins). Hence, surface hydrophobicity influences the in vivo fate of NPs [16]. Indeed, conventional NPs are rapidly opsonized and eliminated by the MPS [17]. To increase the chances of the desired therapeutic outcomes in targeted drug delivery systems, the opsonization and elimination rate of in vivo circulatory NPs should be minimized. This can be efficiently achieved by surface functionalization of metallic NPs with hydrophilic biodegradable polymers (e.g., polyethylene oxide, poloxamine, polyethylene glycol [PEG], chitosan, carboxymethyl cellulose, polysorbate 80, and polyoxamer), biomolecules (e.g., proteins, peptides, antibodies), drugs, or different biopolymers used in combination as hybrid (Figure 2).
Figure 2 Types of biocompatible components used for surface functionalization of metallic nanoparticles [20, 22, 23].
In general, metal-cored NPs stabilized with hydrophobic ligand such as gold, quantum dots, and iron oxide are easily entrapped in a PEG derivative’s micellular shell of lipids–PEG. The bulky PEG molecule prevents plasma proteins adsorption and uptake by the macrophages by acting as a steric barrier, resulting in longer-time body circulation NPs with improved overall bio-distribution and biocompability [18]. Although surface-functionalized polymeric NPs can be synthesized readily in a consistent manner with respects to their size and chemical composition in comparison with any other NP system, they are subject to clearance through reticuloendothelial system [19]. Therefore, several new strategies for designing surface functionalization of polymeric NPs in order to enhance overall biodistribution and biocompatibility are reported. PEGylation is perhaps the most commonly used method for the improvement of bio-distribution and biocompatibility by surface modification of polymeric nanocapsule [20]. Few studies have been reported on the preparation of targeted NPs using PEGylation techniques that include functional PEG chains, specifically the preparation of poly(ecaprolactone)-PEG and poly(lactic-acid)-PEG copolymers with biotinylated end groups. Other systems use PLA-PEG-maleimide copolymers to synthesize NPs that can be bound to thiol-containing targeting agents. However, it is usually necessary to add cysteines to increase the possibility of reducing their activity. However, PEG addition for surface functionalization of polymeric nanocapsule has some limitations. For example, disparities can occur in surface densities when larger-size synthetic molecules are attached to particle surface, creating an uneven distribution of targeting moieties. Furthermore, low-molecular-weight PEG destroys or modifies the structure of NPs systems in body tissues due to its water-soluble properties, which hinders the targeting of target-specific delivery system [21]. PEG also provides substantial benefits, such as reduced antigenicity and immunogenicity of the drug after surface functionalization with NPs.
Their synthesis is very simple, which, coupled with their desirable physicochemical and biological characteristics, appreciable biocompatibility, and low in vivo toxicity, makes these functionalized NPs highly attractive for researchers for the aforementioned applications. The functionalization of biological macromolecules and biopolymers with different types of nanomaterials in a controlled way enhances greater activity and has a tremendous biological application in pharmaceutical sciences such as biosensing, imaging, and various chronic diseases treatment [24, 25]. A wide variety of organic molecules with different sizes, compositions, and complexity are present in nature, which gives function and structure for the different biological processes. Small molecules, such as vitamins, lipids, sugar, and peptides, and larger molecules, such as DNA, RNA, proteins, and enzymes, are examples of organic molecules that are used for various biological processes.
In addition, the functionalization of biopolymers on NPs surfaces reduced the opsonization of NPs by different complements and other serum factors [26]. However, the structural and intermediate configurations of different biopolymers can also alter the biological properties of polymeric NPs. For instance, brush-like PEG molecules reduced the complement activation and phagocytosis, whereas PEG molecules with mushroom-like surface favored phagocytosis and also potent complement activators [27]. At present, nanoscale effects and interaction mechanisms between metallic NPs and bio-macromolecules have not been well studied or understood. Ruckenstein et al. reported that although many metallic NPs possess excellent physicochemical properties, they do not have appropriate properties for surface functionalization applied for specific applications [28]. Hence, surface functionalization of NPs must be done in a controlled manner with conjugated molecules that change the structure, morphology, and surface while maintaining the overall mechanical integrity of metals.
Biomedical applications of surface-functionalized metallic NPs
Several applications of metallic NPs in the field of science and technology, especially in biomedical science, are an emerging area of applied research. The physicochemical and optical properties of metallic NPs were susceptible to surface functionalization, thereby suggesting potential candidates in the detection and biomedical applications. Few of the recently reported surface-functionalized metallic NPs are listed in Table 1 with their biomedical applications.
Table 1 Surface-functionalized Metallic Nanoparticles with Their Applications
| S. no | NPs | Conjugated with | Size and shape | Application | Reference |
|---|---|---|---|---|---|
| 1 | Silver | Chitosan+alginate | 50–70 nm; round | Anti-bacterial | [29] |
| 2 | Silver | Chitosan | 30–40 nm; spherical | Anti-bacterial, anti-coagulant | [30] |
| 3 | Silver | Catechol+chitosan | 40–70 nm; quasi–shaped | Anti-bacterial | [31] |
| 4 | Silver | Chitosan+antibiotics | 80–120 nm; quasi–shaped | Anti-bacterial | [32] |
| 5 | Gold | Poly-lactic acid+poly-ethylene glycol | 216 nm; lamellar structure | Anti-inflammatory | [33] |
| 6 | Silver | Glycolic acid | 30–40 nm; spherical | Treatment of skin cancer cells | [34] |
| 7 | Silver | Polyvinylpyrrolidone+antibiotics | 60–70 nm; quasi shaped | Anti-bacterial | [35] |
| 8 | Gold | Hyaluronic acid | 14–19 nm; spherical | Hyaluronidase inhibitor | [36] |
| 9 | Silver | Xylase | 20–35 nm; spherical | Detection of mercury level in blood | [37] |
| 10 | Silver | Dextran | 10–14 nm; spherical | Detection of insulin level in blood | [38] |
| 11 | Silver | Guar gum | 6–10 nm; spherical | Detection of ammonia level in blood | [39] |
| 12 | Iron | β-Cyclodextrin-dextran-g-stearic acid | 60–100 nm; micelles | Cancer cells imaging | [40] |
| 13 | Gold | Cellobiose | 12–35 nm; spherical | Measurement of activity | [41] |
| 14 | Gold | Antibodies | 30–58 nm; spherical | Treatment of pancreatic carcinoma | [42] |
| 15 | Silver | Heparin | 60–90 nm; spherical | Anti-angiogenesis | [43] |
Further, the highly significant antimicrobial effects of surface-functionalized metallic NPs, suggest the utilization of nanoconjugates as novel antibacterial agents [44]. The surface-functionalized metallic NPs have been reported to have improved intracellular uptake and biocompatibility for drug delivery [45]. At present, cancer is the most prominent factor of mortality and morbidity globally. More than 90 million people suffer from different types of cancer, and the number is continually increasing despite the numerous therapeutic efforts [46]. Because of the plasmonic and unique surface-mobilized nature of functionalized metallic NPs, they are useful for highly specific targeting and imaging of cell surface or specific site of actions for applications in cancer therapies [47]. The labeling of biomolecules with NPs has improved the visualization of cellular components in vivo using electron microscopy [48]. An investigation used polyvinyl alcohol–functionalized silver NPs for detecting labeled DNA for breast cancer gene [49]. In the last few decades, viruses, including HIV, Zika virus, influenza virus, and many others, have become a serious concern globally because of their potential to cause pandemics. In a study, dose-dependent surface-modified NPs were markedly attached to the surface of HIV, thereby inhibiting the binding of the virus to host cells [50]. The metallic NPs coated with biocompatible nanoconjugates readily bind to the living cells surface while maintaining the morphology of normal cells, i.e., neurite extensions [51]. After tissue injuries, the healing process is a complex physiological process with overlapping phases of tissue inflammation and remodeling, the rate of which is substantially affected by the size, depth, and especially the type of the injury as well as bacterial infections [52]. Several polymeric functionalized metallic NPs with highly significant wound healing properties have been reported [53, 54]. Another study has also observed that biopolymer-functionalized metallic NPs enhanced the stability of nanocomposite in aqueous dispersion, which is substantially more important for thin film deposition or for use as imaging probes [55].
Toxicity of functionalized metallic NPs versus non-functionalized metallic NPs
Although the use of metallic NPs has gained much attention in industrial and biomedical sciences, several reports have also been mentioned their toxicity and adverse effects on the cellular components and sometimes on the overall biological systems [56, 57]. Currently, NPs have been studied for cell toxicity, genotoxicity, and immunotoxicity. The cytotoxicity of metallic NPs is generally associated with the comfortable oxidation of metallic ions, which are relatively toxic for cellular components. Since they have a greater surface area and higher chemical reactivity, it results in increased production of reactive oxygen species [58]. There are several other suggested mechanisms for metallic NPs cytotoxicity, such as contamination with the toxic elements, physicochemical properties, high surface charge, fibrous structure, and generation of radical species [59, 60]. Many researchers have agreed that NPs interfere with the detection systems or with assay materials. It addition, it has been reported that metallic NPs produced size-dependent toxicity, as silver NPs with a diameter of 10 nm showed greater tendency to penetrate into the cellular systems of living beings in comparisons with silver NPs with greater diameters (i.e., 20–100 nm) [61]. Similarly, NPs show variability in their toxicity levels at different aspect ratios depending on their shape and dimensions. For example, 10-μm asbestos fibers with a three-dimensional structure cause lung cancer, whereas shorter asbestos fibers with a two-dimensional structure cause mesothelioma; meanwhile, 2-μm fibers with one dimension cause asbestosis [10]. Typically, NP toxicities also increase with greater surface area particle. It can also be observed that NPs with the same dose react with human cells in different manners [62].
Hence, because of the reported toxic effects of metallic NPs, researchers continuously modify the dose, size, shape, surface morphology, and other properties of metallic NPs that can cause in vitro or in vivo toxicities through surface functionalization of metallic NPs using different types of bio-polymers, biomolecules, and other non-toxic conjugated systems. These physicochemical factors of metallic NPs are exceptionally important for their toxicological profile, bio-distribution, accumulation inside body tissues, metabolism, and elimination [63]. Many researchers have reported the toxicity profile of metallic NPs after surface functionalization using different conjugated materials for various applications (Table 2). The results of these studies indicated that the toxicity of various metallic NPs has been reduced after functionalization with different biological molecules, biopolymers, and drugs.
Table 2 Reported Toxicity of Surface-functionalized Metallic NPs in Comparison with Non-functionalized NPs
| S.no | Metallic NPs | Toxicity models | Dose | Results | Reference |
|---|---|---|---|---|---|
| 1 | DOX-coated gold NPs | In vivo | 10 mg/kg | Lowest systemic toxicity compared with free DOX | [64] |
| 2 | Carbon-coated magnetic NPs | In vivo | 10 ppm | Coated NPs had less neurobehavioral toxicities compared with the magnetic NPs alone | [65] |
| 3 | n-Octyltriethoxysilane-coated iron oxide NPs | In vitro | 32 μg | Coated metallic NPs had low cytotoxic effects | [66] |
| 4 | Asparaginase enzyme–functionalized metallic NPs | In vitro | 50 μg | Three-fold increased thermal stability from free enzyme and retained 90% activity | [67] |
| 5 | Tamoxifen-loaded metallic NPs | In vitro | 100 mL | Low cellular toxicity compared with free NPs and drug | [68] |
| 6 | Antibody-conjugated metallic NPs | In vitro | 1 μM | Cytotoxic activity was decreased in in vitro studies after conjugation | [69] |
| 7 | Salicylic acid–loaded iron NPs | Ex vivo | 0.15 mL | Coated metallic NPs had no embolic risk, on a safety IV administration | [70] |
| 8 | Chitosan-coated iron NPs | In vitro | 4 μg | INPs triggers more toxic effects in living cells compared with coated NPs | [71] |
| 9 | Antibody-coated metallic NPs | In vitro | 10 mg | Low cellular toxicity compared with non-coated NPs | [72] |
| 10 | Starch-dextran–coated metallic NPs | In vitro | 0.5 mg | Coated metallic NPs had maximum interaction and entered a cell without cytotoxic effect | [73] |
| 11 | Chitosan-coated silver NPs | In vivo | 90 μg | Low hepatotoxicity at higher doses | [32] |
| 12 | Dimercaptosuccinic acid–coated iron oxide | In vitro | 0.4 mg | Drug-targeted without effect on cell morphology and cell viability | [74] |
NPs, nanoparticles; DOX, doxorubicin; IV, intravenous.
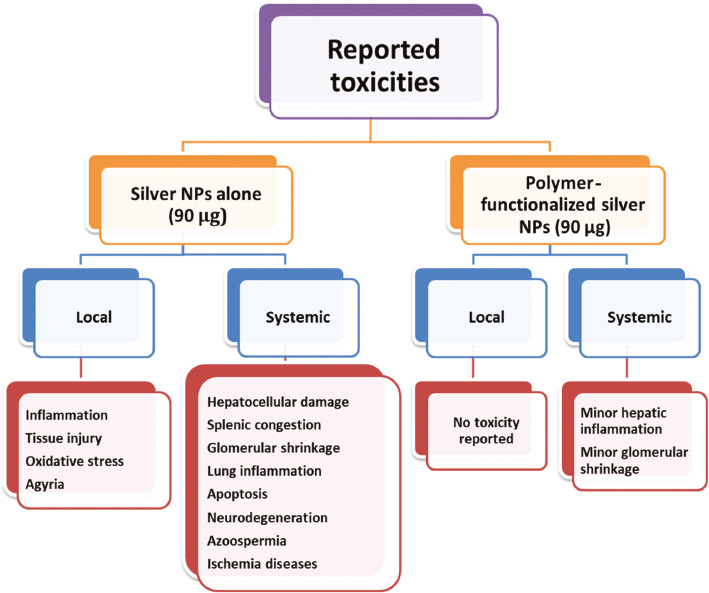
According to the results of previous studies, orally administered polymeric-coated silver NPs (chitosan and polyethylene glycol) produced low systemic toxic effects; meanwhile, compared with silver NP alone at the same dose, i.e., 90 μg/kg, polymeric-coated silver NPs showed no local toxic effects (Figure 3).
Figure 3 Reported local and systemic toxicity of chitosan and polyethylene glycol–coated silver nanoparticles (NPs) in comparison with silver NPs alone [32, 75–77].
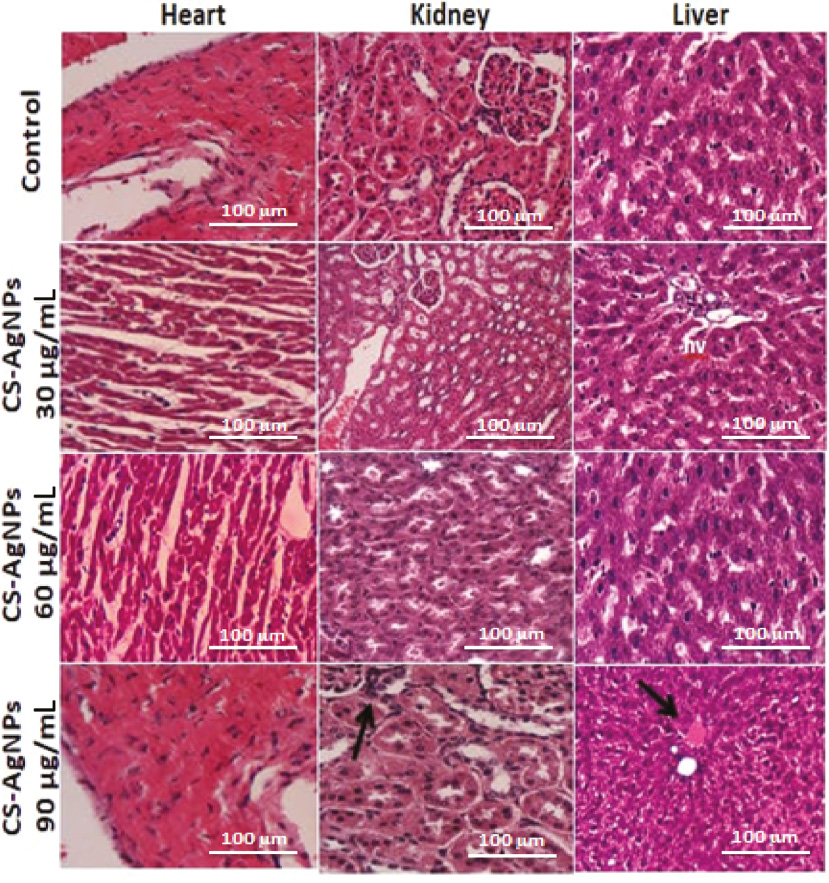
Furthermore, significantly lower rates of hemolysis were observed when gold NPs functionalized with gum karaya and xanthan gum were used, compared with using gold NPs alone at concentrations of 200 μg/mL [78, 79]. Metallic NPs functionalized with porphyrin and pectin produced no significant effect in kidney cells, even at higher concentrations, i.e., 100 μM [80, 81]. Additionally, a significantly higher percentage of macrophage cell viability was found after exposure to polymeric functionalized metallic NPs than the metallic NPs themselves [82]. A previous report clarified that metallic NPs with smaller diameter (10 nm) tend to penetrate the biological membrane and produce more toxic effects at the deepest layers of the stratum corneum; meanwhile, no local toxicity was reported for polymeric functionalized metallic NPs [83]. The findings of the oral toxicity study on rats model also demonstrated that gellan gum–functionalized NPs had limited effects on the biochemical and hematological indexes at a dose of 1500 ppm [84]. Identical findings were also observed in an in vivo toxicity study in a zebrafish model [85]. Worthington et al. observed that the human alveolar epithelial cell viability was significantly much higher after inhalation of chitosan-coated copper NPs in comparison with exposure of non-functionalized copper NPs [86]. Similarly, citrate-coated gold NPs showed no toxicity on embryonal fibroblasts in the MTT assay up to the maximum concentration; however, such high concentrations of non-functionalized gold NPs induced significant changes in cell morphology [87]. Furthermore, quantum dots showed no cytotoxicity on fibroblasts using impedance microscopy [88]. Chitosan-coated silver NPs produced minimum hepatic cellular changes, and with therapeutic concentration, no changes were found in the heart, kidney, or liver tissues of rats (Figure 4) [32]. Hence, based on available toxicity data and their experimental models, it may be concluded that several metallic NPs are more valuable and less toxic to biological systems after surface functionalization with different conjugated systems principally with bio-polymers.
Figure 4 Histopathological examinations of the heart, kidney, and liver of male rats after administration of chitosan-coated silver NPs at different doses [32].
Challenges in transferability of surface-functionalized NPs from animals models to humans
Despite several potential advantages of surface-functionalized NPs, the utilization of these unique NPs systems for tissue targeting in human body system has not been widely embraced in comparison with other classes of medicines, including antibody fragments, liposomal systems, and many others. Currently, all surface-modified NPs are at the preclinical or initial discovery stage. The reason behind the above phenomenon is slightly complex and needs to be explored. Animal models are generally used for the evaluation of potential pharmacological activities in nanomaterial prior to human clinical trials. Even various animals testing are needed prior to human exposure [89]. However, the transfer of animal testing to human trials has several restrictions and challenges. Müller et al. interpreted the challenges as being “lost in translation” [90]. At present, drug candidates testing are commonly reported on mouse models studies prior to clinical trials. However, it is already established that certain biological responses in rodent models are not transferable to or comparable with larger animals and human clinical trials [89]. Concerning the biomedical applications of nanocarriers as drug delivery systems, the behavior assessment in biological fluids is the most critical prior to the evaluation of the pharmacological effect [91]. Most NP systems are formulated to be administered intravenously; thus, their first exposure is the blood plasma with biological material; then, NPs systems will cover with biological macromolecules or physically adsorbed proteins [92]. This new complex produced undesirable responses such as nanocarrier aggregation and rapid clearance from the blood stream, accumulation in specific tissues due to unspecific cell uptakes, and inflammatory responses of the body [93]. The physicochemical properties of nanomaterial, including their shape, size, charge, hydrophilicity, and, more importantly, surface functionalization, affect the formation of this complex [91]. Therefore, there is an urgent need to understand and modify these physicochemical properties during the formation of nanomaterials for clinical applications. Importantly, surface functionalization of NPs systems was found to significantly influence cellular uptake in biological systems [94]. In reported literatures, a significant connection between NP aggregation in biological fluid and in vivo bio-distribution [95, 96]. Therefore, based on the above circumstances, we suggest that surface-functionalized NPs should be first evaluated in animal model using plasma cell line type to ensure the safe transferability of this unique drug delivery systems from animal trials to in vivo human experiments. Additionally, the same animal trials, at least in vitro tests and blood plasma studies, should also be carried out with human cell lines and these obtained outcomes should be compared in the respective animal studies. This could provide possible predictions on whether the stability of NPs in the blood is the same in human systems. Ideally, this is the most suitable way for the smooth transfer of surface-functionalized NPs from animal models to human clinical trials.
Conclusions and future perspectives
As research on metallic NPs has exponentially increased, appropriate awareness of multifunctional hosts for surface functionalization of metallic NPs has increased in scientific fields. Using different bio-polymers, bio-molecules, and many other biological macromolecules exhibited enormous potential for the surface functionalization of metallic NPs owing to their advantages such as low toxicity, biocompatibility, and high target site selectivity. This mini-review creates a comprehensive insight into the approaches and efforts applied for surface functionalization of metallic NPs. According to the reported literature, surface-modified metallic NPs have been proven to have low toxicity and high intracellular uptake for drug delivery. There is significant space available for the exploration of effective surface functionalization and coupling with metallic NPs to produce a stable, bioactive, and highly biocompatible interface. Considering the applications of metallic NPs in various biomedical fields, there is a substantial need for new Food and Administration approval that is free of bias for in-vivo pharmacological and toxicological models for surface-functionalized metallic NPs studies. It is hoped that this mini-review would help provide current knowledge regarding the recent progress and toxicity of surface-functionalized metallic NPs and thus lead to more nano-conjugated systems with low toxicity that can be applied for drug delivery and treatment of various diseases.
References
- Mousa SA, Bawa R, Audette GF. The road from nanomedicine to precision medicine. Boca Raton, Florida, United States: CRC Press; 2020.
- Asghar MA, Asghar MA, Rehman AA, Khan K, Zehravi M, et al. Synthesis and characterization of graphene oxide nanoparticles and their antimicrobial and adsorption activity against aspergillus and aflatoxins. Lat Am J Pharm 2019;38:1036-44.
- Asghar MA, Zahir E, Shahid SM, Khan MN, Asghar MA, et al. Iron, copper and silver nanoparticles: green synthesis using green and black tea leaves extracts and evaluation of antibacterial, antifungal and aflatoxin B1 adsorption activity. LWT 2018;90:98-107. [DOI: 10.1016/j.lwt.2017.12.009]
- Zhang L. Applications, challenges and development of nanomaterials and nanotechnology. J Chem Soc Pak 2020;42:658-66.
- Jamkhande PG, Ghule NW, Bamer AH, Kalaskar MG. Metal nanoparticles synthesis: an overview on methods of preparation, advantages and disadvantages, and applications. J Drug Deliv Sci Technol 2019;53:101174. [DOI: 10.1016/j.jddst.2019.101174]
- Asghar MA, Asghar MA. Green synthesized and characterized copper nanoparticles using various new plants extracts aggravate microbial cell membrane damage after interaction with lipopolysaccharide. Int J Biol Macromol 2020;160:1168-76. [PMID: 32464203 DOI: 10.1016/j.ijbiomac.2020.05.198]
- Han X, Xu K, Taratula O, Farsad K. Applications of nanoparticles in biomedical imaging. Nanoscale 2019;11:799-819. [PMID: 30603750 DOI: 10.1039/c8nr07769j]
- Asghar MA, Zahir E, Asghar MA, Iqbal J, Rehman AA. Facile, one-pot biosynthesis and characterization of iron, copper and silver nanoparticles using Syzygium cumini leaf extract: as an effective antimicrobial and aflatoxin B1 adsorption agents. PLoS One 2020;15:e0234964. [PMID: 32614844 DOI: 10.1371/journal.pone.0234964]
- Huang S-H, Juang R-S. Biochemical and biomedical applications of multifunctional magnetic nanoparticles: a review. J. Nanoparticle Res 2011;13:4411-30. [DOI: 10.1007/s11051-011-0551-4]
- Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol 2018;9:1050-74. [PMID: 29719757 DOI: 10.3762/bjnano.9.98]
- Urzedo AL, Gonçalves MC, Nascimento MHM, Lombello CB, Nakazato G, et al. Cytotoxicity and antibacterial activity of alginate hydrogel containing nitric oxide donor and silver nanoparticles for topical applications. ACS Biomater Sci Eng 2020;6:2117-34. [PMID: 33455338 DOI: 10.1021/acsbiomaterials.9b01685]
- Khawaja H, Zahir E, Asghar MA, Asghar MA. Graphene oxide decorated with cellulose and copper nanoparticle as an efficient adsorbent for the removal of malachite green. Int J Biol Macromol 2021;167:23-34. [PMID: 33259838 DOI: 10.1016/j.ijbiomac.2020.11.137]
- Delfi M, Ghomi M, Zarrabi A, Mohammadinejad R, Taraghdari ZB, et al. Functionalization of polymers and nanomaterials for biomedical applications: antimicrobial platforms and drug carriers. Prosthesis 2020;2:117-39. [DOI: 10.3390/prosthesis2020012]
- Naskar A, Kim K-S. Nanomaterials as delivery vehicles and components of new strategies to combat bacterial infections: advantages and limitations. Microorganisms 2019;7:356. [PMID: 31527443 DOI: 10.3390/microorganisms7090356]
- Sofias AM, Toner YC, Meerwaldt AE, van Leent MMT, Soultanidis G, et al. Tumor targeting by αvβ3-integrin specific lipid nanoparticles occurs via phagocyte hitchhiking. ACS Nano 2020;14:7832-46. [PMID: 32413260 DOI: 10.1021/acsnano.9b08693]
- Zhao Z, Ukidve A, Krishnan V, Mitragotri S. Effect of physicochemical and surface properties on in vivo fate of drug nanocarriers. Adv Drug Deliv Rev 2019;143:3-21. [PMID: 30639257 DOI: 10.1016/j.addr.2019.01.002]
- Liu Q, Guan J, Qin L, Zhang X, Mao S. Physicochemical properties affecting the fate of nanoparticles in pulmonary drug delivery. Drug Discov Today 2020;25:150-9. [PMID: 31600580 DOI: 10.1016/j.drudis.2019.09.023]
- Parveen S, Sahoo SK. Long circulating chitosan/PEG blended PLGA nanoparticle for tumor drug delivery. Eur J Pharmacol 2011;670:372-83. [PMID: 21951969 DOI: 10.1016/j.ejphar.2011.09.023]
- Reddy LH, Arias JL, Nicolas J, Couvreur P. Magnetic nanoparticles: design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem Rev 2012;112:5818-78. [PMID: 23043508 DOI: 10.1021/cr300068p]
- Weingart J, Vabbilisetty P, Sun X-L. Membrane mimetic surface functionalization of nanoparticles: methods and applications. Adv Colloid Interface Sci 2013;197:68-84. [PMID: 23688632 DOI: 10.1016/j.cis.2013.04.003]
- Jahan ST, Sadat SMA, Walliser M, Haddadi A. Targeted therapeutic nanoparticles: an immense promise to fight against cancer. J Drug Deliv 2017;2017:9090325. [PMID: 29464123 DOI: 10.1155/2017/9090325]
- Oh J-H, Park DH, Joo JH, Lee JS. Recent advances in chemical functionalization of nanoparticles with biomolecules for analytical applications. Anal Bioanal Chem 2015;407:8627-45. [PMID: 26329278 DOI: 10.1007/s00216-015-8981-y]
- Khawaja H, Zahir E, Asghar MA, Asghar MA. Graphene oxide, chitosan and silver nanocomposite as a highly effective antibacterial agent against pathogenic strains. Colloids Surf, A Physicochem Eng Asp 2018;555:246-55. [DOI: 10.1016/j.colsurfa.2018.06.052]
- Hong T, Liu W, Li M, Chen C. Recent advances in the fabrication and application of nanomaterial-based enzymatic microsystems in chemical and biological sciences. Anal Chim Acta 2019;1067:31-47. [PMID: 31047147 DOI: 10.1016/j.aca.2019.02.031]
- Chen Y, Du M, Yu J, Rao L, Chen X, et al. Nanobiohybrids: a synergistic integration of bacteria and nanomaterials in cancer therapy. BIO Integration 2020;1:25-36. [DOI: 10.15212/bioi-2020-0008]
- Lu L, Kang S, Sun C, Sun C, Guo Z, et al. Multifunctional nanoparticles in precise cancer treatment: considerations in design and functionalization of nanocarriers. Curr Top Med Chem 2020;20:2427-41. [PMID: 32842941 DOI: 10.2174/1568026620666200825170030]
- Hu J, Sheng Y, Shi J, Yu B, Yu Z, et al. Long circulating polymeric nanoparticles for gene/drug delivery. Curr Drug Metab 2018;19:723-38. [PMID: 29219050 DOI: 10.2174/1389200219666171207120643]
- Ruckenstein E, Li H, Cheng C. Solution and surface polymerization. New York: CRC Press; 2019. [DOI: 10.1201/9780429027420]
- Bilal M, Rasheed T, Iqbal HMN, Li C, Hu H, et al. Development of silver nanoparticles loaded chitosan-alginate constructs with biomedical potentialities. Int J Biol Macromol 2017;105:393-400. [PMID: 28705499 DOI: 10.1016/j.ijbiomac.2017.07.047]
- Asghar MA, Yousuf RI, Shoaib MH, Asghar MA. Antibacterial, anticoagulant and cytotoxic evaluation of biocompatible nanocomposite of chitosan loaded green synthesized bioinspired silver nanoparticles. Int J Biol Macromol 2020;160:934-43. [PMID: 32470586 DOI: 10.1016/j.ijbiomac.2020.05.197]
- Huang X, Bao X, Liu Y, Wang Z, Hu Q. Catechol-functional chitosan/silver nanoparticle composite as a highly effective antibacterial agent with species-specific mechanisms. Sci Rep 2017;7:1-10. [PMID: 28500325 DOI: 10.1038/s41598-017-02008-4]
- Asghar MA, Yousuf RI, Shoaib MH, Asghar MA, Ansar S, et al. Synergistic nanocomposites of different antibiotics coupled with green synthesized chitosan-based silver nanoparticles: characterization, antibacterial, in vivo toxicological and biodistribution studies. Int J Nanomed 2020;15:7841. [PMID: 33116504 DOI: 10.2147/IJN.S274987]
- Kamalakannan R, Mani G, Muthusamy P, Susaimanickam AA, Kyobum K. Caffeine-loaded gold nanoparticles conjugated with PLA-PEG-PLA copolymer for in vitro cytotoxicity and anti-inflammatory activity. J Ind Eng Chem 2017;51:113-21. [DOI: 10.1016/j.jiec.2017.02.021]
- Kumar M, Wangoo N, Gondil VS, Pandey SK, Lalhall A, et al. Glycolic acid functionalized silver nanoparticles: a novel approach towards generation of effective antibacterial agent against skin infections. J Drug Deliv Sci Technol 2020;60:102074. [DOI: 10.1016/j.jddst.2020.102074]
- Kaur A, Kumar R. Enhanced bactericidal efficacy of polymer stabilized silver nanoparticles in conjugation with different classes of antibiotics. RSC Adv 2019;9:1095-105. [DOI: 10.1039/C8RA07980C]
- Shen M-Y, Chao C-F, Wu Y-J, Wu Y-H, Chin-Ping Huang C-P, et al. A design for fast and effective screening of hyaluronidase inhibitor using gold nanoparticles. Sens Actuators B Chem 2013;181:605-10. [DOI: 10.1016/j.snb.2013.02.054]
- Luo Y, Shen S, Luo J, Wang X, Suna R. Green synthesis of silver nanoparticles in xylan solution via Tollens reaction and their detection for Hg 2+. Nanoscale 2015;7:690-700. [DOI: 10.1039/C4NR05999A]
- Davidović S, Lazic V, Vukoje I, Papan J, Anhrenkiel SP, et al. Dextran coated silver nanoparticles—chemical sensor for selective cysteine detection. Colloids Surf B 2017;160:184-191. [DOI: 10.1016/j.colsurfb.2017.09.031]
- Pandey S, Goswami GK, Nanda KK. Green synthesis of biopolymer–silver nanoparticle nanocomposite: an optical sensor for ammonia detection. Int J Biol Macromol 2012;51:583-9. [PMID: 22750580 DOI: 10.1016/j.ijbiomac.2012.06.033]
- Su H, Liu Y, Wang D, Wu C, Xia C, et al. Amphiphilic starlike dextran wrapped superparamagnetic iron oxide nanoparticle clsuters as effective magnetic resonance imaging probes. Biomaterials 2013;34:1193-203. [PMID: 23168385 DOI: 10.1016/j.biomaterials.2012.10.056]
- Lai C, Zeng G-M, Huang D-L, Zhao M-H, Wei Z, et al. Synthesis of gold–cellobiose nanocomposites for colorimetric measurement of cellobiase activity. Spectrochim Acta A 2014;132:369-74. [DOI: 10.1016/j.saa.2014.04.091]
- Eck W, Craig G, Sigdel A, Ritter G, Old LJ, et al. PEGylated gold nanoparticles conjugated to monoclonal F19 antibodies as targeted labeling agents for human pancreatic carcinoma tissue. ACS Nano 2008;2:2263-72. [PMID: 19206392 DOI: 10.1021/nn800429d]
- Kemp MM, Kumar A, Mousa S, Dyskin E, Yalcin M, et al. Gold and silver nanoparticles conjugated with heparin derivative possess anti-angiogenesis properties. Nanotechnology 2009;20:455104. [PMID: 19822927 DOI: 10.1088/0957-4484/20/45/455104]
- Saratale RG, Karuppusamy I, Saratale GD, Pugazhendhi A, Kumar G, et al. A comprehensive review on green nanomaterials using biological systems: recent perception and their future applications. Colloids Surf B Biointerfaces 2018;170:20-35. [PMID: 29860217 DOI: 10.1016/j.colsurfb.2018.05.045]
- Jahangirian H, Kalantari K, Izadiyan Z, Rafiee-Moghaddam R, Shameli K, et al. A review of small molecules and drug delivery applications using gold and iron nanoparticles. Int Nanomed 2019;14:1633. [PMID: 30880970 DOI: 10.2147/IJN.S184723]
- Saginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, et al. Epidemiology of bladder cancer. Med Sci 2020;8:15. [PMID: 32183076 DOI: 10.3390/medsci8010015]
- Xie Z, Fan T, An J, Choi W, Duo Y, et al. Emerging combination strategies with phototherapy in cancer nanomedicine. Chem Soc Rev 2020;49:8065-87. [PMID: 32567633 DOI: 10.1039/d0cs00215a]
- Nakamura M, Hayashi K, Nakamura J, Mochizuki C, Murakami T, et al. Near-infrared fluorescent thiol-organosilica nanoparticles that are functionalized with IR-820 and their applications for long-term imaging of in situ labeled cells and depth-dependent tumor in vivo imaging. Chem Mater 2020;32:7201-14. [DOI: 10.1021/acs.chemmater.0c01414]
- Elbaz NM, Ziko L, Mamdouh W. Core-shell silver/polymeric nanoparticles-based combinatorial therapy against breast cancer in-vitro. Sci Rep 2016;6:30729. [PMID: 27491622 DOI: 10.1038/srep30729]
- Budhadev D, Poole E, Nehlmeier I, Liu Y, Hooper J, et al. Glycan-gold nanoparticles as multifunctional probes for multivalent lectin–carbohydrate binding: implications for blocking virus infection and nanoparticle assembly. J Am Chem Soc 2020;142:18022-34. [PMID: 32935985 DOI: 10.1021/jacs.0c06793]
- Zhao J, Santino F, Giacomini D, Gentilucci L. Integrin-targeting peptides for the design of functional cell-responsive biomaterials. Biomedicines 2020;8:307. [PMID: 32854363 DOI: 10.3390/biomedicines8090307]
- Tottoli EM, Dorati R, Genta I, Chiesa E, Pisani S, et al. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020;12:735. [PMID: 32764269 DOI: 10.3390/pharmaceutics12080735]
- Thanh NT, Hieu MH, Phuong NTM, Thuan TDB, Thu HNT, et al. Optimization and characterization of electrospun polycaprolactone coated with gelatin-silver nanoparticles for wound healing application. Mater Sci Eng: C Mater Biol Appl 2018;91:318-29. [PMID: 30033261 DOI: 10.1016/j.msec.2018.05.039]
- Wang Y, Dou C, He G, Ban L, Huang L, et al. Biomedical potential of ultrafine ag nanoparticles coated on poly (gamma-glutamic acid) hydrogel with special reference to wound healing. Nanomaterials 2018;8:324. [PMID: 29757942 DOI: 10.3390/nano8050324]
- Punetha VD, Rana S, Yoo HJ, Chaurasia A, McLeskey Jr JT, et al. Functionalization of carbon nanomaterials for advanced polymer nanocomposites: a comparison study between CNT and graphene. Prog Polym Sci 2017;67:1-47. [DOI: 10.1016/j.progpolymsci.2016.12.010]
- Yao Y, Zang Y, Qu J, Tang M, Zhang T. The toxicity of metallic nanoparticles on liver: the subcellular damages, mechanisms, and outcomes. Int J Nanomedicine 2019;14:8787-804. [PMID: 31806972 DOI: 10.2147/IJN.S212907]
- Wang M, Lai X, Shao L, Li L. Evaluation of immunoresponses and cytotoxicity from skin exposure to metallic nanoparticles. Int J Nanomedicine 2018;13:4445-59. [PMID: 30122919 DOI: 10.2147/IJN.S170745]
- Abdal Dayem A, Hossain MK, Lee SB, Kim K, Saha SK, et al. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int J Mol Sci 2017;18:120. [PMID: 28075405 DOI: 10.3390/ijms18010120]
- Niazi JH, Gu MB. Toxicity of metallic nanoparticles in microorganisms-a review, In Atmospheric and biological environmental monitoring. Dordrecht: Springer; 2009. pp. 193-206.
- Li YF, Chen C. Fate and toxicity of metallic and metal-containing nanoparticles for biomedical applications. Small 2011;7:2965-80. [PMID: 21932238 DOI: 10.1002/smll.201101059]
- Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem 2019;12:908-31. [DOI: 10.1016/j.arabjc.2017.05.011]
- Saleh TA. Nanomaterials: classification, properties, and environmental toxicities. Environ Technol Innov 2020;20:101067. [DOI: 10.1016/j.eti.2020.101067]
- Chen W, Wu C. Synthesis, functionalization, and applications of metal–organic frameworks in biomedicine. Dalton Trans 2018;47:2114-33. [PMID: 29369314 DOI: 10.1039/c7dt04116k]
- Elbialy NS, Fathy MM, Khalil WM. Doxorubicin loaded magnetic gold nanoparticles for in vivo targeted drug delivery. Int J Pharm 2015;490:190-9. [PMID: 25997662 DOI: 10.1016/j.ijpharm.2015.05.032]
- Malhotra N, Audira G, Chen J-R, Siregar P, Hsu H-S, et al. Surface modification of magnetic nanoparticles by carbon-coating can increase its biosafety: evidences from biochemical and neurobehavioral tests in zebrafish. Molecules 2020;25:2256. [PMID: 32403340 DOI: 10.3390/molecules25092256]
- Ma W, Gehret PM, Hoff RE, Kelly LP, Suh WH. The investigation into the toxic potential of iron oxide nanoparticles utilizing rat pheochromocytoma and human neural stem cells. Nanomaterials 2019;9:453. [PMID: 30889833 DOI: 10.3390/nano9030453]
- Alam S, Ahmad R, Pranaw K, Mishra P, Khare SK. Asparaginase conjugated magnetic nanoparticles used for reducing acrylamide formation in food model system. Bioresour Technol 2018;269:121-6. [PMID: 30157443 DOI: 10.1016/j.biortech.2018.08.095]
- Nosrati H, Rashidi N, Danafar H, Manjili HK. Anticancer activity of tamoxifen loaded tyrosine decorated biocompatible Fe 3 O 4 magnetic nanoparticles against breast cancer cell lines. J Inorg Organomet Polym Mater 2018;28:1178-86. [DOI: 10.1007/s10904-017-0758-7]
- Trabulo S, Aires A, Aicher A, Heeschen C, Cortajarena AL. Multifunctionalized iron oxide nanoparticles for selective targeting of pancreatic cancer cells. Biochim Biophys Acta (BBA)-Gen Subj 2017;1861:1597-605. [PMID: 28161480 DOI: 10.1016/j.bbagen.2017.01.035]
- Buteică S, Mihăiescu DE, Rogoveanu I, Mărgăritescu DN, Mîndrilă I, et al. Chick chorioallantoic membrane model as a preclinical tool for nanoparticles biology study. Rom Biotechnol Lett 2016;21:11684-90.
- Shukla S, Jadaun A, Arora V, Sinha RK, Biyani N, et al. In vitro toxicity assessment of chitosan oligosaccharide coated iron oxide nanoparticles. Toxicol Rep 2015;2:27-39. [PMID: 28962334 DOI: 10.1016/j.toxrep.2014.11.002]
- Tse BW-C, Cowin GJ, Soekmadji C, Jovanvic L, Vasireddy RS, et al. PSMA-targeting iron oxide magnetic nanoparticles enhance MRI of preclinical prostate cancer. Nanomedicine 2015;10:375-86. [PMID: 25407827 DOI: 10.2217/nnm.14.122]
- Marcus M, Karni M, Baranes K, Levy I, Alon N, et al. Iron oxide nanoparticles for neuronal cell applications: uptake study and magnetic manipulations. J Nanobiotechnol 2016;14:37. [PMID: 27179923 DOI: 10.1186/s12951-016-0190-0]
- Calero M, Chiappi M, Lazaro-Carrillo A, Rodriguez MJ, Chichon FJ, et al. Characterization of interaction of magnetic nanoparticles with breast cancer cells. J Nanobiotechnol 2015;13:1-15. [PMID: 25880445 DOI: 10.1186/s12951-015-0073-9]
- Pinzaru I, Coricovac D, Dehelean C, Moacă E-A, Mioc M, et al. Stable PEG-coated silver nanoparticles—a comprehensive toxicological profile. Food Chem Toxicol 2018;111:546-56. [PMID: 29191727 DOI: 10.1016/j.fct.2017.11.051]
- Bilberg K, Hovgaard MB, Besenbacher F, Baatrup E. In vivo toxicity of silver nanoparticles and silver ions in zebrafish (Danio rerio). J Toxicol 2012;2012. [DOI: 10.1056/NEJMoa1008108]
- van der Zande M, Vandebriel RJ, Van Doren E, Kramer E, Rivera ZH, et al. Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day oral exposure. ACS Nano 2012;6:7427-42. [PMID: 21247310 DOI: 10.1021/nn302649p]
- Pooja D, Panyaram S, Kulhari H, Reddy B, Rachamalla SS, et al. Natural polysaccharide functionalized gold nanoparticles as biocompatible drug delivery carrier. Int J Biol Macromol 2015;80:48-56. [PMID: 26093321 DOI: 10.1016/j.ijbiomac.2015.06.022]
- Pooja D, Panyaram S, Kulhari H, Rachamalla SS, Sistla R. Xanthan gum stabilized gold nanoparticles: characterization, biocompatibility, stability and cytotoxicity. Carbohydr Polym 2014;110:1-9. [DOI: 10.1016/j.carbpol.2014.03.041]
- Venkatpurwar V, Mali V, Bodhankar S, Pokharkar V. In vitro cytotoxicity and in vivo sub-acute oral toxicity assessment of porphyran reduced gold nanoparticles. Toxicol Environ Chem 2012;94:1357-67. [DOI: 10.1080/02772248.2012.697731]
- Reena K, Balashanmugam P, Gajendiran M, Arul Antony S. Synthesis of leucas aspera extract loaded Gold-PLA-PEG-PLA amphiphilic copolymer nanoconjugates: in vitro cytotoxicity and anti-inflammatory activity studies. J Nanosci Nanotechnol 2016;16:4762-70. [PMID: 27483820 DOI: 10.1166/jnn.2016.12404]
- Li JJ, Muralikrishnan S, Ng C-T, Yung L-YL, Bay B-H. Nanoparticle-induced pulmonary toxicity. Exp Biol Med 2010;235:1025-33. [PMID: 20719818 DOI: 10.1258/ebm.2010.010021]
- Baroli B, Ennas MG, Loffredo F, Isola M, Pinna R, et al. Penetration of metallic nanoparticles in human full-thickness skin. J Invest Dermatol 2007;127:1701-12. [PMID: 17380118 DOI: 10.1038/sj.jid.5700733]
- Dhar S, Mali V, Bodhankar S, Shiras A, Prasad BLV, et al. Biocompatible gellan gum-reduced gold nanoparticles: cellular uptake and subacute oral toxicity studies. J Appl Toxicol 2011;31:411-20. [PMID: 21089158 DOI: 10.1002/jat.1595]
- Bar-Ilan O, Albrecht RM, Fako VE, Furgeson DY. Toxicity assessments of multisized gold and silver nanoparticles in zebrafish embryos. Small 2009;5:1897-910. [PMID: 19437466 DOI: 10.1002/smll.200801716]
- Worthington KL, Adamcakova-Dodd A, Wongrakpanich A, Mudunkotuwa IA, Mapuskar KA, et al. Chitosan coating of copper nanoparticles reduces in vitro toxicity and increases inflammation in the lung. Nanotechnology 2013;24:395101. [PMID: 24008224 DOI: 10.1088/0957-4484/24/39/395101]
- Qu Y, Lü X. Aqueous synthesis of gold nanoparticles and their cytotoxicity in human dermal fibroblasts—fetal. Biomed Mater 2009;4:025007. [PMID: 19258699 DOI: 10.1088/1748-6041/4/2/025007]
- Male KB, Lachance B, Hrapovic S, Sunahara G, Luong JHT. Assessment of cytotoxicity of quantum dots and gold nanoparticles using cell-based impedance spectroscopy. Anal Chem 2008;80:5487-93. [PMID: 18553941 DOI: 10.1021/ac8004555]
- Oberdörster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Particle and Fibre Toxicol 2005;2:1-35. [PMID: 16209704 DOI: 10.1186/1743-8977-2-8]
- Müller LK, Simon J, Rosenauer C, Mailänder V, Morsbach S, et al. The transferability from animal models to humans: challenges regarding aggregation and protein corona formation of nanoparticles. Biomacromolecules 2018;19:374-85. [PMID: 29286657 DOI: 10.1021/acs.biomac.7b01472]
- Abd Ellah NH, Abouelmagd SA. Surface functionalization of polymeric nanoparticles for tumor drug delivery: approaches and challenges. Expert Opin Drug Deli 2017;14:201-14. [PMID: 27426638 DOI: 10.1080/17425247.2016.1213238]
- Lacerda SHDP, Park JJ, Meuse C, Pristinski D, Becker ML, et al. Interaction of gold nanoparticles with common human blood proteins. ACS Nano 2010;4:365-79. [PMID: 20020753 DOI: 10.1021/nn9011187]
- Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm 2008;5:505-15. [PMID: 18672949 DOI: 10.1021/mp800051m]
- Mout R, Moyano DF, Rana S, Rotello VM. Surface functionalization of nanoparticles for nanomedicine. Chem Soc Rev 2012;41:2539-44. [PMID: 22310807 DOI: 10.1039/c2cs15294k]
- Guerrini L, Alvarez-Puebla RA, Pazos-Perez N. Surface modifications of nanoparticles for stability in biological fluids. Materials 2018;11:1154. [PMID: 29986436 DOI: 10.3390/ma11071154]
- Fonseca-Gomes J, Loureiro JA, Tanqueiro SR, Mouro FM, Ruivo P, et al. In vivo bio-distribution and toxicity evaluation of polymeric and lipid-based nanoparticles: a potential approach for chronic diseases treatment. Int J Nanomed 2020;15:8609. [PMID: 33177821 DOI: 10.2147/IJN.S267007]