Toxicologic Profile and Anti-Nociceptive Effect of Two Semi-Synthetic Triterpene Derivatives from Combretum Leprosum in Mice
1Northeast Biotechnology Network (RENORBIO), Federal University of Ceará, Fortaleza, Ceará, Brazil
2School of Dentistry, Federal University of Ceará, Sobral, Ceará, Brazil
3Department of Organic and Inorganic Chemistry, Science Centre, Federal University of Ceará, Fortaleza, Ceará, Brazil
4School of Medicine, Federal University of Ceará, Sobral, Ceará, Brazil
5School of Medicine, University Center INTA–UNINTA, Sobral, Ceará, Brazil
6Postgraduate Program in Morphological Science, Department of Morphology, School of Medicine, Federal University of Ceará, Fortaleza, Ceará, Brazil
7Drug Research and Development Center (NPDM), Federal University of Ceará, Fortaleza, Ceará, Brazil
*Correspondence to: Mirna Marques Bezerra, School of Medicine, Federal University of Ceará (Campus Sobral), Av. Comandante Maurocélio Rocha Pontes, 100 – Jocely Dantas de Andrade Torres, Sobral, Ceará 62042-250, Brazil, E-mail: mirna@ufc.br
Received: April 1 2024; Revised: August 1 2024; Accepted: August 5 2024; Published Online: September 9 2024
Cite this paper:
Passos MJ, Chaves HV, Barbosa FG et al. Toxicologic Profile and Anti-Nociceptive Effect of Two Semi-Synthetic Triterpene Derivatives from Combretum Leprosum in Mice. BIO Integration 2024; 5: 1–10.
DOI: 10.15212/bioi-2024-0009. Available at: https://bio-integration.org/
Download citation
© 2024 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
Background and aim: Combretum leprosum Mart. serves as a medicinal plant in traditional Brazilian medicine. The beneficial effects of C. leprosum Mart. are attributed to the triterpene, 3β,6β,16β-trihydroxylup-20(29)-ene (CL-1). Herein we evaluate the toxicity of two semi-synthetic derivatives from CL-1 (CL-P2 and CL-P2A) in vitro and in vivo, and determine the efficacy in zymosan-induced writhing response and the putative mechanism of action.
Experimental procedure: Toxicity prediction was assessed using the PROTOX-II and ADMETlab 2.0 prediction tools, and SMILES codes for structure identification. In vitro cytotoxicity of the derivatives was tested using the sulforhodamine B assay in L929 and HaCaT cells at 24, 48, and 72 h. Mice received (oral gavage) CL-P2 or CL-P2A (10 mg/kg/d) for 14 days in in vivo toxicity assays. Blood samples and organs (stomach, liver, and kidneys) were collected for AST/ALT level determination and H&E staining, respectively. The anti-nociceptive effect of CL-P2 and CL-P2A (0.1, 1, or 10 mg/kg) was evaluated in the zymosan-induced writhing response. The peritoneal exudate was collected to determine myeloperoxidase (MPO) and superoxide dismutase (SOD) activity, and nitrite concentration.
Results: CL-P-2 and CL-P2A derivatives exhibited low cytotoxicity and did not change body mass, AST/ALT levels, or organ weight. The histopathologic analysis did not reveal significant changes in organs. Both derivatives inhibited the writhing response in a dose-dependent manner. In addition, both derivatives failed to reduce MPO activity. However, CL-P2A increased SOD activity and CL-P2 decreased nitrite/nitrate levels.
Conclusion: CL-P2 and CL-P2A were shown to exhibit anti-nociceptive effects without toxicity. Our data suggest that CL-P2 and CL-P2A efficacy is mediated, at least in part, via antioxidant activity by modulating nitrite/nitrate levels and SOD activity, respectively.
Keywords
Combretum leprosum, cytotoxicity, inflammation, oxidative stress, semi-synthetic derivatives.
Introduction
Combretum leprosum Mart. is a shrubby plant (2–3 m) found in regions of Africa, Asia, and Brazil. In northeast Brazil, C. leprosum Mart. is popularly known as “mofumbo, mufumbo, or pente de macaco.” Various plant parts have been used as anti-inflammatory agents in traditional medicine [1].
Species of the Combretaceae family have a range of pharmacologic activities, including contraceptive, anti-inflammatory, anti-proliferative, anti-ulcer, and anti-cholinesterase effects [2–5], making Combretaceae family members a potential source of novel bioactive compounds. Analysis of the Combretum genus showed flavonoids and triterpenes to be the chief components serving as natural sources for new drug development [6]. Facundo and colleagues (1993) isolated and characterized the pentacyclic triterpene, 3β,6β,16β-trihydroxylup-20(29)-ene [CL-1]; Figure 1) from C. leprosum leave extracts [7]. Indeed, the beneficial effects of this plant may be credited to the CL-1 triterpene [2, 8, 9]. Pharmacologic studies with CL-1 include the following: antimicrobial and leishmanicidal activities [9, 10]; anti-inflammatory effects [3]; anti-nociceptive effects [11]; anti-proliferative tumor cell activity [12]; antimicrobial effects [9]; and anti-ulcerogenic effects [4].
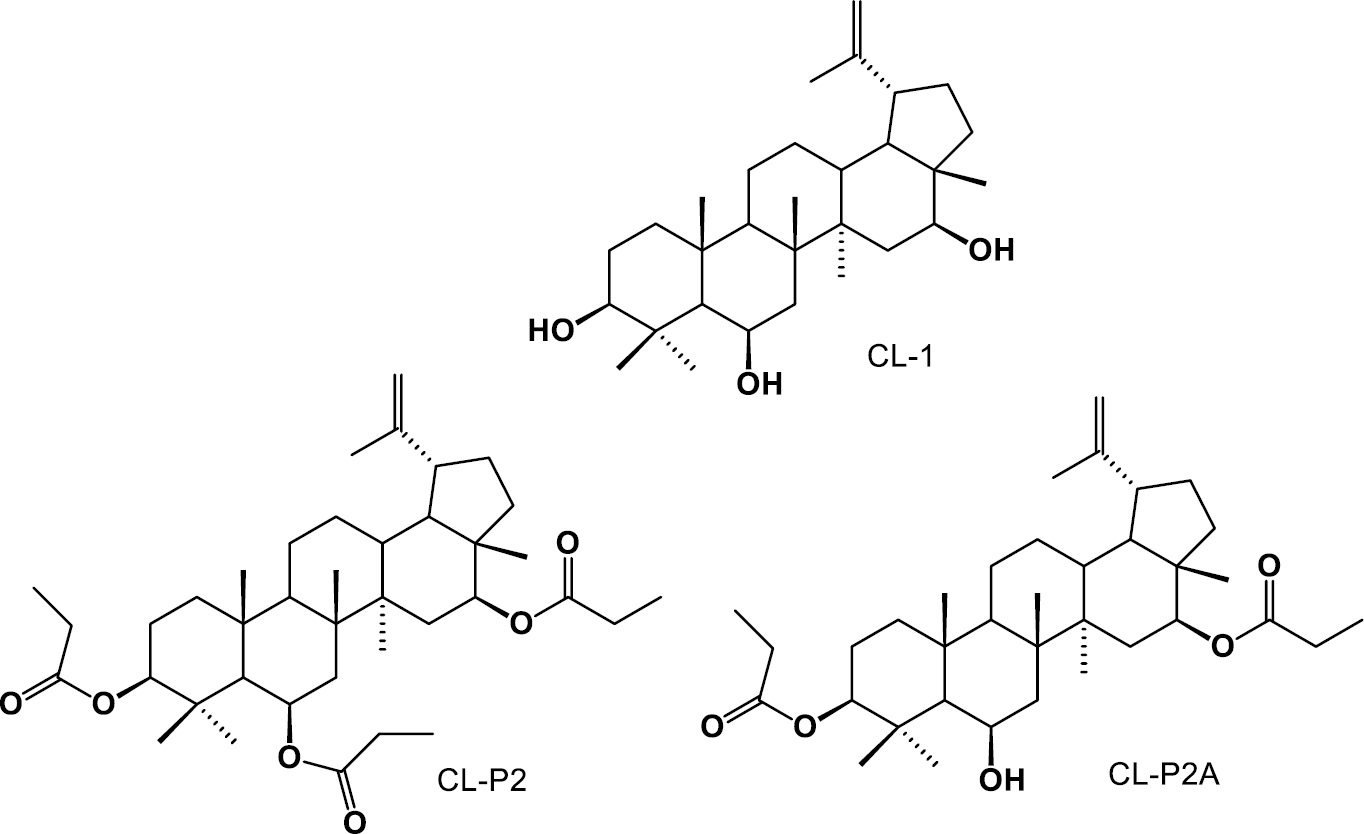
Figure 1 Chemicals structures of CL-1, CL-P2, and CL-P2A.
The structural modification of natural products with confirmed biological activities to obtain semi-synthetic derivatives during drug development may represent a promising approach. Our research group isolated and obtained six semi-synthetic derivatives of triterpene CL-1, as follows: dehydrated, CL-P1; diacylated, CL-P2A; triacylated, CL-P2; oxidized, CL-P3; hydrogenated, CL-P5; hydrazone, CL-P6; and oxime, CL-P9 [12].
Examining the toxicity and effectiveness of two derivatives (CL-P2 and CL-P2A) (Figure 1) of the triterpene CL-1 in vitro, in vivo, and in silico was the aim of the current study, given that C. leprosum is primarily used as a traditional medicine and that plant chemicals may aid in the search for new drugs. Although C. leprosum has been traditionally used by communities, its mechanism of action has not yet been elucidated. Longhi-Balbinot et al. (2012) [2], using the acetic acid-induced writhing model, demonstrated that the anti-inflammatory efficacy of CL-1 is associated with reduced cell migration and TNF-α levels. Furthermore, bearing in mind the previously reported anti-nociceptive [11] and anti-inflammatory [3] actions of CL-1, the efficacy and mechanism of action of CL-P2 and CL-P2A was determined in a classic model of the zymosan-induced writhing response.
Methodology
Obtaining and characterizing CL-P2 and CL-P2A
A 100-mg aliquot (0.218 mmol) of CL-1 was dissolved in 2.18 mL of CH2Cl2 in a 25-mL round bottom flask to obtain CL-P2 and CL-P2A derivatives. Then, 91 μL of CH3CH2COCl (propanoyl chloride), 26.59 mg of DMAP, and 31 μL of Et3N were added. The mixture was kept at room temperature while stirring and monitored by thin layer chromatography for 10 h. The reaction mixture was concentrated under reduced pressure, which provided 231 mg of product. After purification by flash chromatography, 79 mg (57.80%) of CL-P2 (triacylated product) and 37.4 mg (30.05%) of CL-P2A (diacylated product) were obtained using 13 g of silica gel (40–63 μM) and 400 mL of mixture hexane/acetate/methanol (9:0.5:0.5) as eluent.
3β,6β,16β-tripropionyloxylup-20(29)-ene (CL-P2)
The compound CL-P2 appeared as a yellowish resin with the following spectrometric data: 13C NMR (CDCl3, 75 MHz): δ 12.82 (C-28), 16.30 (C-27), 16.67 (C-26), 17.60 (C-24), 17.70 (C-25), 19.37 (C30), 21.11 (C-11), 23.92 (C-12), 24.82 (C-2), 27.60 (C-23), 29.81 (C-21), 33.66 (C-10), 36.76 (C-13), 36.96 (C-15), 37.69 (C-22), 37.98 (C-4), 38.65 (C-8), 40.06 (C-1), 40.42 (C-7), 44.41 (C-14), 47.48 (C-17), 47.63 (C-18), 47.94 (C19), 50.34 (C-9), 55.03 (C-5), 70.42 (C-6), 78.52 (C-16), 80.34 (C-3), 110.13 (C-29), 149.82 (C-20), 9.09, 28.11, 173.59 (propanoyl); δ 9.40, 28.14, 174.03 (propanoyl), δ 9.42, 28.65, 174.21 (propanoyl); 1H NMR (CDCl3, 300 MHz): δ 0.81 (s, CH3), 0.87 (s, CH3), 0.99 (s, CH3), 1.00 (s, CH3), 1.13 (9H, m, propanoyl), 1.20 (s, CH3), 1.21 (s, CH3), 1.66 (3H, s, H-30), 2.30 (6H, m, propanoyl), 4.41 (1H, dd, J = 11.3 and 4.5 Hz, H-16), 4.59 (1H, sl, H-29), 4.69 (1H, sl, H-29), 4.83 (1H, dd, J = 9.5 and 4.5 Hz, H-3), 5.51 (1H, sl, H-6); υmax 3070, 2943, 1735, 1642 cm−1; (+)-HR-ESI-MS m/z 649.44312 [M + Na]+ (calcd for C39H62O6Na, 649.89579).
6β-hydroxy-3β,16β-dipropionyloxylup-20(29)-ene (CL-P2A)
The compound CL-P2A appeared as a yellowish resin with the following spectrometric data: 13C NMR (CDCl3, 75 MHz): δ 12.74 (C-28), 16.19 (C-27), 16.87 (C-26), 17.72 (C-24), 18.20 (C-25), 19.27 (C-30), 20.98 (C-11), 23.92 (C-12), 24.75 (C-2), 27.54 (C-23), 29.72 (C-21), 33.55 (C-10), 36.52 (C-13), 36.71 (C-15), 37.60 (C-22), 38.75 (C-4), 40.11 (C-1), 40.40 (C-4), 42.14 (C-8), 44.32 (C-14), 47.36 (C-17), 47.53 (C-18), 47.90 (C-19), 50.52 (C-9), 55.66 (C5), 68.72 (C-6), 78.84 (C-16), 80.57 (C-3), 110.01 (C-29), 149.77 (C-20), 9.35, 28.10, 174.31, (propanoyl), 9.34, 28.07, 174.17, (propanoyl); 1H NMR (CDCl3, 300 MHz) δ 0.85 (3H, s, H-28), 0.93 (3H, s, H-27), 1.02 (3H, s, H-23), 1.14 (6H, m, propanoyl), 1.22 (6H, s, H-24 e H-25), 1.37 (3H, s, H-26), 1.68 (3H, s, H-30), 2.30 (4H, m, propanoyl), 4.43 (1H, dd, J = 11.2 and 4.5 Hz, H-16), 4.60 (1H, sl, H-29), 4.71 (1H, sl, H-29), 4.85 (1H, dd, J = 11.2 e 4.5 Hz, H-3), 4.51 (1H, sl, H-6); υmax 3072, 2945, 1733, 1643, 1189 cm−1; (-)-HR-ESI-MS m/z 605.39759 [M + Cl]− (calcd for C36H58O5Cl, 605.39783).
In silico toxicity
Toxicity prediction was assessed using the PROTOX-II [13] and ADMETlab 2.0 [14] prediction tools with SMILES codes for structure identification. Acute oral toxicity was determined using the toxicity class and mean lethal dose (LD50). Cardiotoxicity, nephrotoxicity, hematoxicity, and hepatoxicity were also predicted.
Cell culture
In vitro assays included cytotoxicity tests on immortalized human keratinocyte cells (HaCaT; CLS Cell Lines Service, Germany) and murine fibroblast L929 cells (clone 929; ATCC, Manassas, VA). DMEM (Gibco, USA) supplemented with 100 U/mL of penicillin, 100 μg/mL of streptomycin (Gibco), and 10% FBS (Gibco) were used to support cell growth (37°C in a 5% CO2 atmosphere).
Cytotoxicity assay
L929 and HaCaT (2 × 104 cells/mL) cells were plated in 96-well plates for 24 h and the groups were treated with CL-P2 (0.312–40 μg/mL) or CL-P2A (0.312–40 μg/mL) for 24–72 h. The vehicle group was the control group for the treatment of cells. The sulforhodamine B (SRB) staining method was used to quantity viable cells through an estimate of the total protein mass [15]. The cells were fixed with 10% trichloroacetic acid and incubated with SRB solution (0.4%) for 30 min. The excess dye was removed by repeated washing with 1% acetic acid. The protein-bound dye was dissolved in 10 mM Tris-base solution and the optical density was determined at 570 nm using a microplate autoreader (Multiskan FC, Thermo Scientific, Finland) [16]. The results are expressed as a percentage of cell viability.
Animals
Male Swiss mice (25–30 g) were housed in appropriate polypropylene cages and maintained on 12 h-12 h light-dark cycles with a constant room temperature of 25°C with water and food provided ad libitum. Every attempt was made to reduce the number of animals used and unnecessary suffering. The Institutional Animal Care and Use Committee from of the Federal University of Ceará (Campus Sobral) granted approval for the experimental protocol (permit number: 06/2017) in compliance with the rules issued by the Brazilian Society of Laboratory Animal Science (SBCAL).
Toxicologic assays
Animals were gavaged with CL-P2 or CL-P2A (10 mg/kg/ day) and the non-treated group (NT) were gavaged with the vehicle (saline) daily for 14 d. This dose was selected based on a previous report [17, 18].
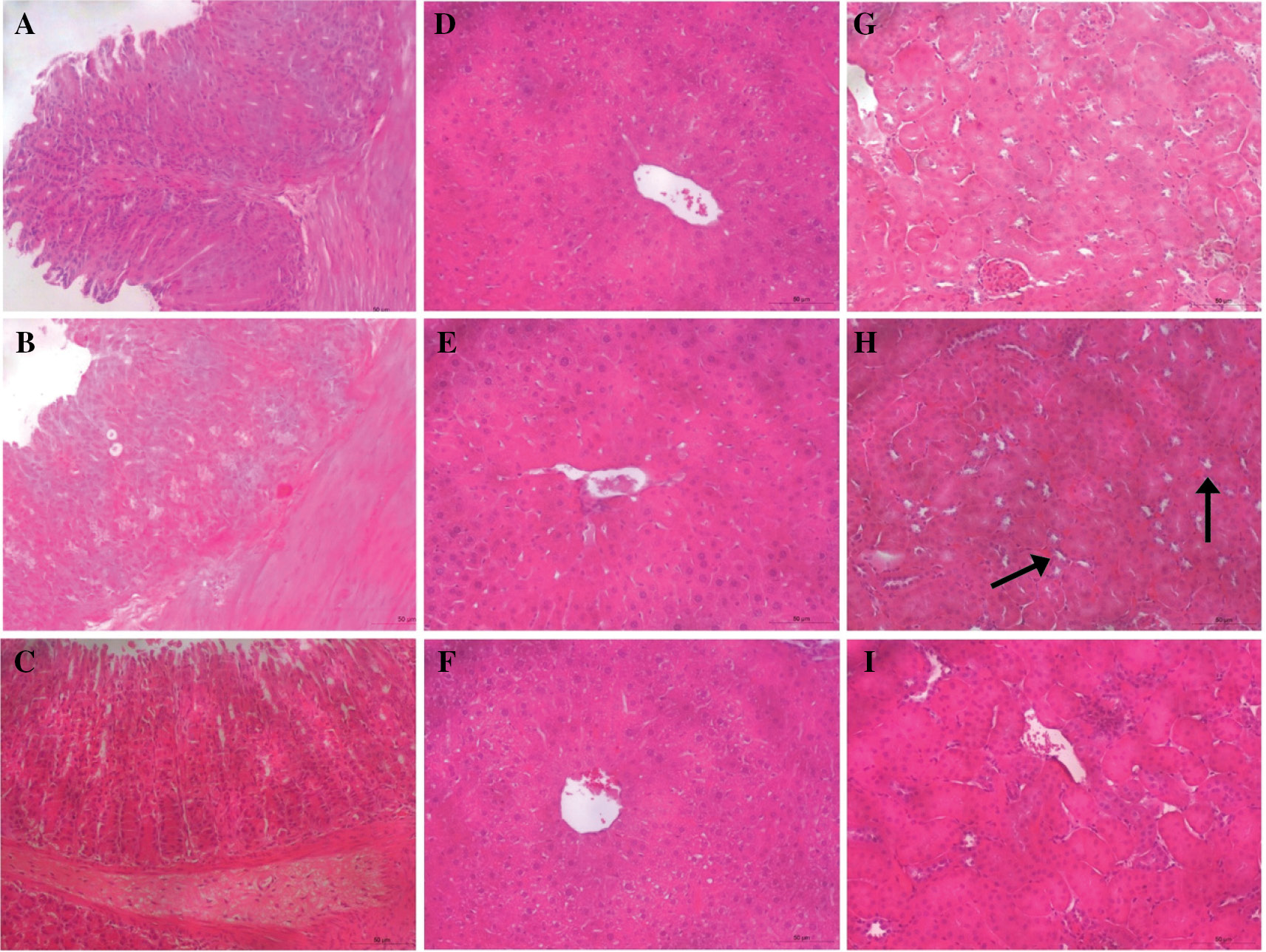
Daily weights and gavage with the derivatives were recorded for 14 d. Then, the mice were anesthetized with a combination of xylazine hydrochloride (10 mg/kg intraperitoneally [i.p.]) and ketamine (90 mg/kg i.p.), and blood samples were collected for aspartate aminotransferase (AST) and alanine aminotransferase (ALT) level determination, according to the manufacturer’s specifications (Labtest®, Lagoa Santa, MG, Brazil). The mice were euthanized and underwent a laparotomy for organ (stomach, liver, and kidneys) removal. The organs were weighed and a portion of each organ was processed for hematoxylin-eosin (H&E) staining. H&E-stained 5-μM sections were evaluated semi-quantitatively for loss of epithelial cells, hemorrhagic damage, inflammatory cells, vascular congestion, and edema with a 0–3 score for each parameter.
Measurement of anti-nociceptive activity: writhing test following an i.p. injection of zymosan
Nociceptive behavior was evaluated using the writhing model [19]. Briefly, zymosan (1 mg) was injected i.p. in mice. Mice were housed in a glass cylinder (30 cm in diameter and 45 cm in height) and the total number of writhing movements that occurred between 0 and 30 min after zymosan administration were counted.
Assessment of the anti-nociceptive effect of CL-P2 and CL-P2A
Mice were gavaged with vehicle (saline [NT group]), CL-P2 (0.1, 1, or 10 mg/kg) or CL-P2A (0.1, 1, or 10 mg/kg). Zymosan was injected ip 30 min later and the number of writhing move ents was counted. The mice were euthanized and the peritoneal cavity was washed with PBS. Aliquots of the fresh peritoneal exudate were collected to determine myeloperoxidase (MPO) and superoxide dismutase (SOD) activities, and the nitrite concentration.
Investigating the mechanism of action underlying CL-P2 and CL-P2A
Neutrophil involvement: MPO activity
MPO activity was assessed using the previously reported methodology [20]. Briefly, aliquots of the peritoneal exudates (10 μL) were mixed with 200 μL of a solution containing o-dianisidine dihydrochloride and 1% hydrogen peroxide (H2O2). MPO absorbance was measured at 450 nm. Data are expressed as MPO units/mL of peritoneal exudate.
Antioxidant activity: SOD activity and nitrite concentration
SOD activity was measured using the protocol previously described [19]. Peritoneal exudate (50 μL) was added to 1000 μL of reaction medium (250 mL phosphate buffer [50 mM], 250 μL of L-methionine (19.5 mM), and 250 μL of EDTA (100 μM). Then, 300 μL of riboflavin solution (1 μM) and 150 μL of nitro blue tetrazolium [NBT] (750 μM) were added to the solution. The material was exposed to light for 15 min. The absorbance was read at 560 nm using a spectrophotometer. The total protein content was determined with a commercial lab test kit (Total Proteins kit, Labtest®, Lagoa Santa, MG, Brazil). Data are shown as μg SOD/μg protein.
Nitrite concentrations was evaluated by the Griess reaction [21]. Briefly, 50 μL of the sample and 50 μL of Griess reagent [2% sulfanilamide in 5% phosphoric acid and 0.2% N-(1-naphthyl ethylenediamine dihydrochloride] were mixed. Absorbance was measured at 550 nm by spectrophotometry in an ELISA reader (Loccus, Cotia, SP, Brazil). Data are shown as μM of nitrite.
Statistical analysis
Data were normalized using the Shapiro-Wilk normality test. The results are shown as the mean ± SEM or median and range. ANOVA followed by the Tukey or Games-Howell test was used to compare means. The Kruskal–Wallis and Dunn tests were used to compare medians. A P < 0.05 was considered significant. Statistical analysis was performed using GraphPad Prism 8 (San Diego, CA, USA) and IBM SPSS Statistics for Windows (SPSS, version 20.0, IBM SPSS Inc., Chicago, IL, USA).
Results
Chemical structures
Figure 1 shows CL-1 and the two chemical derivatives of CL-1 (CL-P2 and CL-P2A). The semi-synthetic derivatives (triacylated CL-P2) and diacylated CL-P2A) were obtained from the pentacyclic triterpene (CL-1) isolated from C. leprosum flowers. No chemical and biological data have been reported for CL-P2 and CL-P2A.
In silico toxicity
Table 1 summarizes the in silico toxicity data. Due to the predicted LD50 of 5000 mg/kg, CL-P2 and CL-P2A were categorized as toxicity class 5 (minimal acute toxicity) [13]. Both compounds were categorized as inactive with respect to cardiotoxicity, indicating a very low risk of cardiac toxicity with probabilities of 0.72 for CL-P2A and 0.75 for CL-P2. Both compounds were considered inactive with respect to nephrotoxicity, indicating a small risk of kidney injury with a high probability of 0.94 for CL-P2 and 0.76 for CL-P2A. CL-P2 was classified as active with a probability of 0.44, while CL-P2A was considered inactive with a probability of 0.47; both compounds were classified as a moderate risk of hematologic toxicity based on the probability values. CL-P2 (probability of 0.56) and CL-P2A (probability of 0.79) were considered inactive with respect to hepatotoxicity.
Table 1 Predicted Toxicity Variables for CL-P2 and CL-P2A
| Variables | CL-P2 | Probability | CL-P2A | Probability |
|---|---|---|---|---|
| LD50 expected | 5000 mg/kg | – | 5000 mg/kg | – |
| Expected toxicity class | 5 | – | 5 | – |
| Cardiotoxicity | Inactive | 0.75 | Inactive | 0.72 |
| Nephrotoxicity | Inactive | 0.94 | Inactive | 0.76 |
| Hematoxicity | Active | 0.44 | Inactive | 0.47 |
| Hepatotoxicity | Inactive | 0.56 | Inactive | 0.79 |
In vitro assay: cytotoxicity evaluation of CL-P2 and CL-P2A
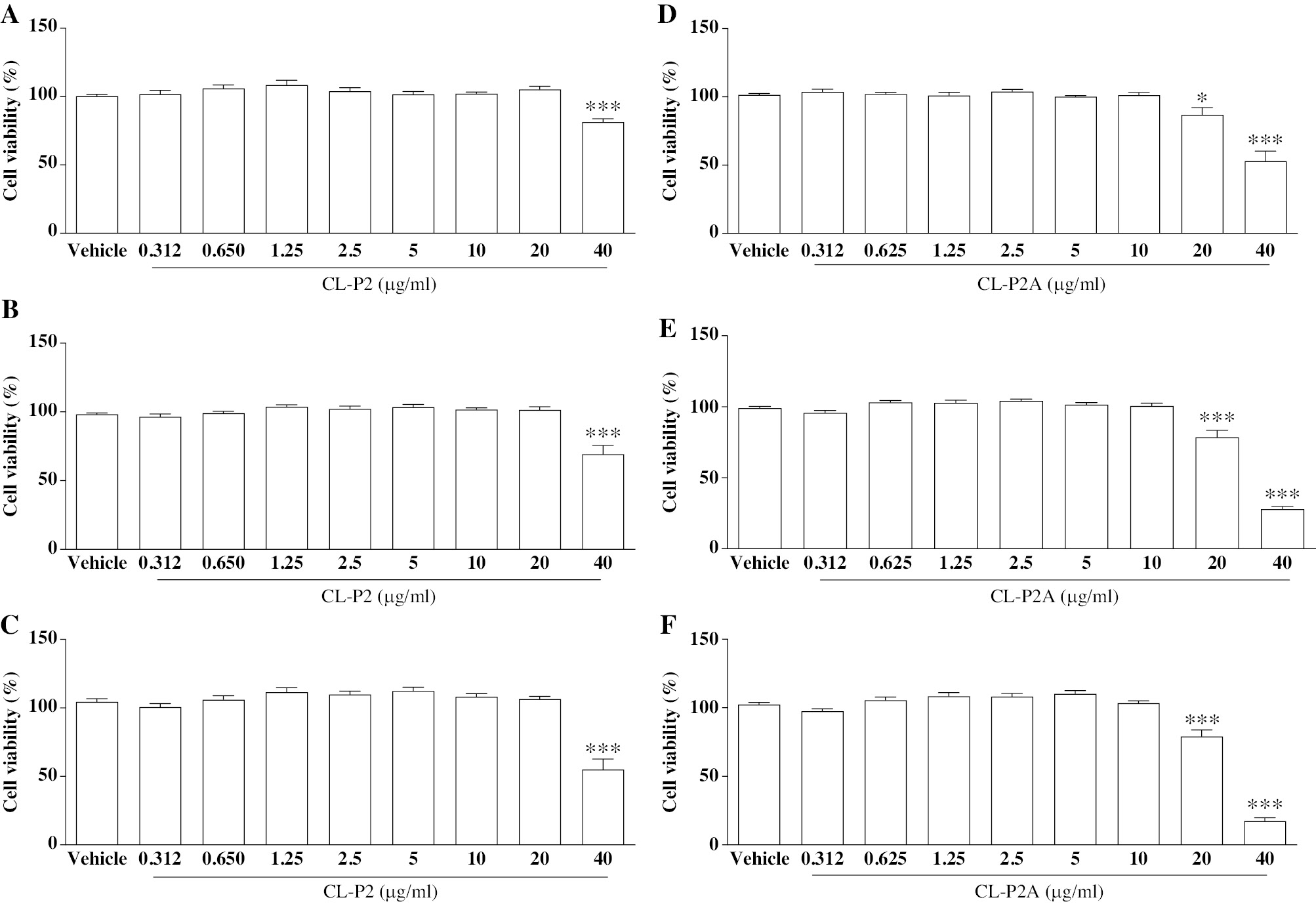
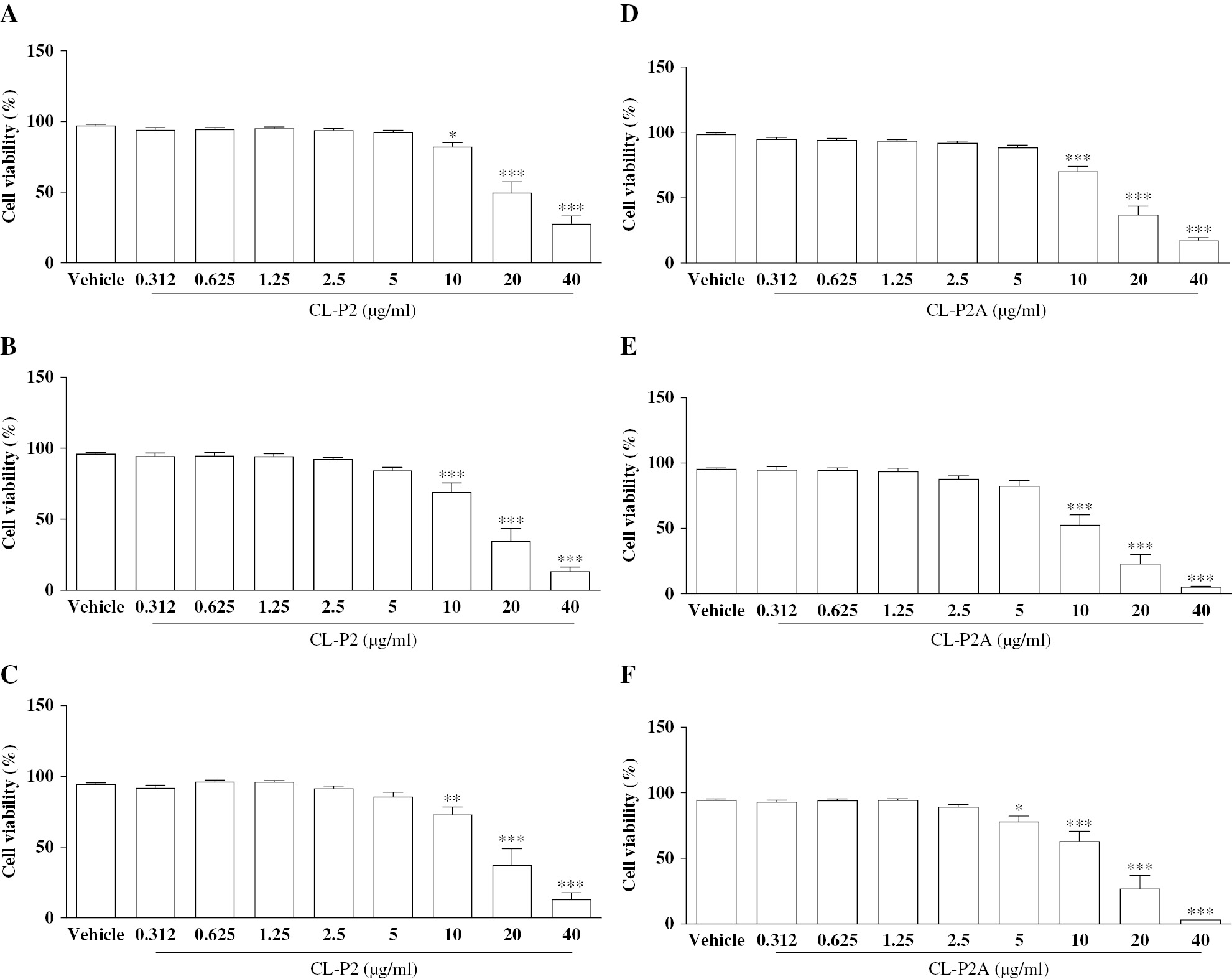
The cytotoxic activity of the CL-P2 and CL-P2A derivatives was evaluated using the SRB test. CL-P2 exhibited low cytotoxicity in HaCaT cells. The highest concentration of CL-P2 (40 μg/mL) reduced viable cells to 81.4%, 69.2%, and 54.9% compared to the vehicle group and 54.9% after 24, 48, and 72 h (P < 0.001; Figure 2A-C), respectively. However, CL-P2 caused an 82.4%, 49.7%, and 27.8% reduction in L929 cell growth at the highest concentrations (10, 20, and 40 μg/mL), respectively, at the shortest incubation time (24 h; Figure 3A) compared to the vehicle group (P < 0.05, P < 0.001, and P < 0.001, respectively). CL-P2 was shown to have a cytotoxic effect on L929 cells at 10, 20, and 40 μg/mL after 48 and 72 h (Figure 3B and C). The minimum inhibitory concentration (IC50) of the CL-P2 compound for HaCaT and L929 cells was 35.73 μg/mL and 10.5 μg/mL, respectively, after a 72-h incubation.
Figure 2 Effects of CL-P2 (A-C) and CL-P2A (D-F) on HaCaT cell (human epidermal keratinocyte line) viability by the SRB method after 24 (2A and 2D), 48 (2B and 2E), and 72 h (2C and 2F). Three independent experiments (n = 6/group) were performed and the results are expressed as the mean percentage ± SEM. *P < 0.05 and ***P < 0.001 represent a statistic difference compared to the vehicle group (ANOVA and Tukey’s test).
Figure 3 Effects of CL-P2 (A-C) and CL-P2A (D-F) on murine fibroblast (L929 cells) viability by the SRB method after 24 (2A and 2D), 48 (2B and 2E), and 72 h (2C and 2F). Three independent experiments (n = 6/group) were performed and the results are expressed as the mean percentage ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 represent a statistical difference compared to the vehicle group (ANOVA and Tukey’s test).
At a concentration of 20 μg/mL, CL-P2A reduced cell viability to 86.83% (P < 0.05) after 24 h, and 78.5% and 79.1% after 48 and 72 h, respectively, compared to vehicle group (P < 0.001; Figure 2D-F). In addition, CL-P2A reduced cell viability in HaCaT cells at the highest concentration (40 μg/ml) to 53%, 27.9%, and 17.3% after 24, 48, and 72 h, respectively, compared to the vehicle group (P < 0.001). Like CL-P2, L929 cell growth was reduced in the presence of the highest concentrations of CL-P2A (10, 20, and 40 μg/ml) compared to the vehicle group (P < 0.001) after 24, 48, and 72 h. Furthermore, CL-P2A (5 μg/mL) had a cytostatic effect with a reduction in cell viability of 78.1% after 72 h (Figure 3D-F; P < 0.05). The CL-P2A compound IC50 for HaCaT and L929 cells was 20.30 μg/mL and 7.61 μg/mL after a 72-h incubation, respectively.
Both derivatives exhibited low cytotoxicity, reduced the cell growth rate, and showed a cytostatic effect only at the highest concentrations.
In vivo toxicity assays
The toxicity parameters were evaluated to verify the safety of CL-P2 and CL-P2A in mixw. Body mass alterations, biochemical parameters (ALT and AST), organ weight, and histopathologic analyses were measured as side effect indices.
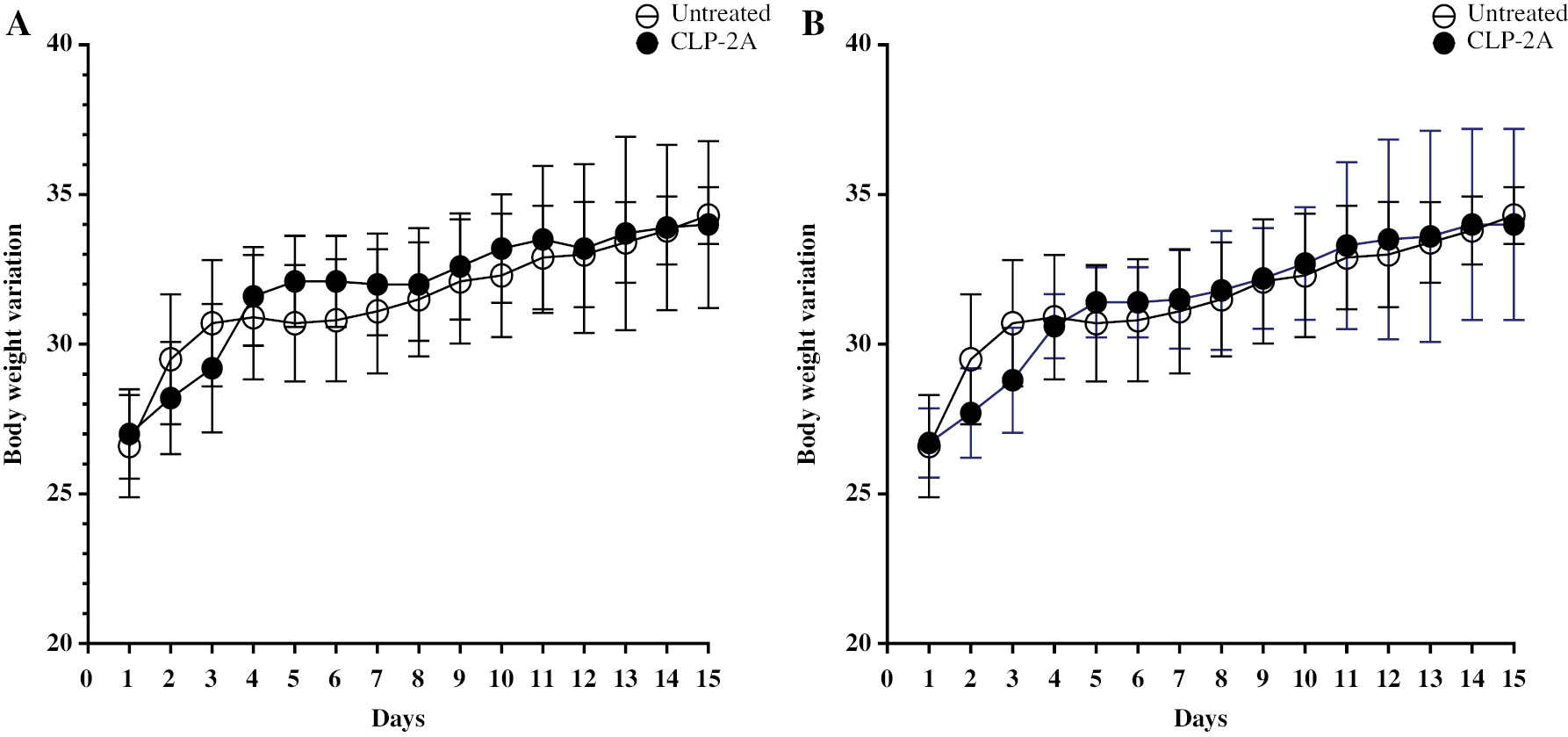
Loss of body weight is considered to be a marker of toxicity triggered by drugs [22]. The CL-P2 (Figure 4A) and CL-P2A group (Figure 4B) body mass variation curves were not statistically different (P > 0.05) from the NT. In addition, CL-P2 and CL-P2A treatment did not cause changes in liver enzyme levels (AST and ALT) compared to the vehicle group (Table 2). The derivatives did not alter organs weight (data not shown). To support these data, histopathologic analyses (H&E) of kidneys, liver, and stomach were performed. The histopathologic analyses did not reveal any important changes in the stomach, liver, and kidneys (Table 3 and Figure 5). These results strongly suggest that CL-P2 and CL-P2A did not produce systemic toxicity in vivo.
Figure 4 Body mass variation (g) in mice treated with CL-P2 (A) or CL-P2A (B) for 14 d. Mice received CL-P2 (10 mg/kg), CL-P2A (10 mg/kg), or vehicle per os for 14 d and were weighed daily. Data are represented as the mean ± S.E.M (t-test).
Table 2 Serum AST and ALT Levels After CL-P2 and CL-P2A Treatment
| Group | AST | ALT |
|---|---|---|
| Vehicle | 34.36 ± 5.54 | 43.61 ± 4.70 |
| CL-P2 (10 mg/kg) | 39.68 ± 7.15 | 55.84 ± 15.09 |
| CL-P2A (10 mg/kg) | 55.04 ± 5.54 | 42.96 ± 6.02 |
Data are represented as the mean ± S.E.M. (t-test).
Table 3 Effect of CL-P2 and CL-P2A Treatment on Histopathologic (H&E) Analysis of Stomach, Liver, and Kidneys
| Organs/Histopathologic Parameters | Groups | ||
|---|---|---|---|
| Vehicle | CLP-2 | CLP-2A | |
| Stomach | |||
| Loss of epithelial cells | 0.5 (0-2) | 1.5 (1-2) | 0 (1-1) |
| Hemorrhagic damage | 0 (0-1) | 0 (0-0) | 0 (0-0) |
| Edema | 0 (0-1) | 1 (0-1) | 1 (0-1) |
| Inflammatory cells | 0 (0-0) | 0 (0-0) | 0 (0-1) |
| Liver | |||
| Loss of epithelial cells | 0 (0-1) | 0.5 (0-1) | 0 (0-0) |
| Vascular congestion | 1 (0-2) | 2 (1-2) | 0 (1-1) |
| Hemorrhagic damage | 0 (0-0) | 1 (0-1) | 0 (0-1) |
| Edema | 0 (0-1) | 1 (1-1) | 0 (1-2) |
| Inflammatory cells | 0 (0-0) | 0 (0-0) | 0 (0-1) |
| Kidney | |||
| Loss of epithelial cells | 0 (0-0) | 0 (0-1) | 0 (0-0) |
| Necrosis | 0 (0-0) | 0 (0-1) | 0 (0-0) |
| Hemorrhagic damage | 0 (0-1) | 1 (1-2)a | 0 (1-2) |
| Edema | 0 (0-1) | 1 (0-2) | 0 (1-2) |
| Inflammatory cells | 0 (0-0) | 0 (0-0) | 0 (0-1) |
The data represent the median and range (n = 6 for each treatment).
aP < 0.05 versus vehicle group (Kruskal-Wallis and post hoc Dunn’s test).
Figure 5 Photomicrographs of organs from mice treated with CL-P2 or CL-P2A (10 mg/kg). (A) stomach, (D) liver, and (G) kidneys from mice that received vehicle (NT group). (B) stomach, (E) liver, and (H) kidneys from mice that received CL-P2. (C) stomach, (F) liver, and (I) kidneys from mice that received CL-P2A. Each treatment group was treated (oral gavage) daily for 14 d. (H) black arrows indicate area of hemorrhagic damage. Magnification 100×.
CL-P2 and CLP-2A efficacy against the zymosan-induced writhing response
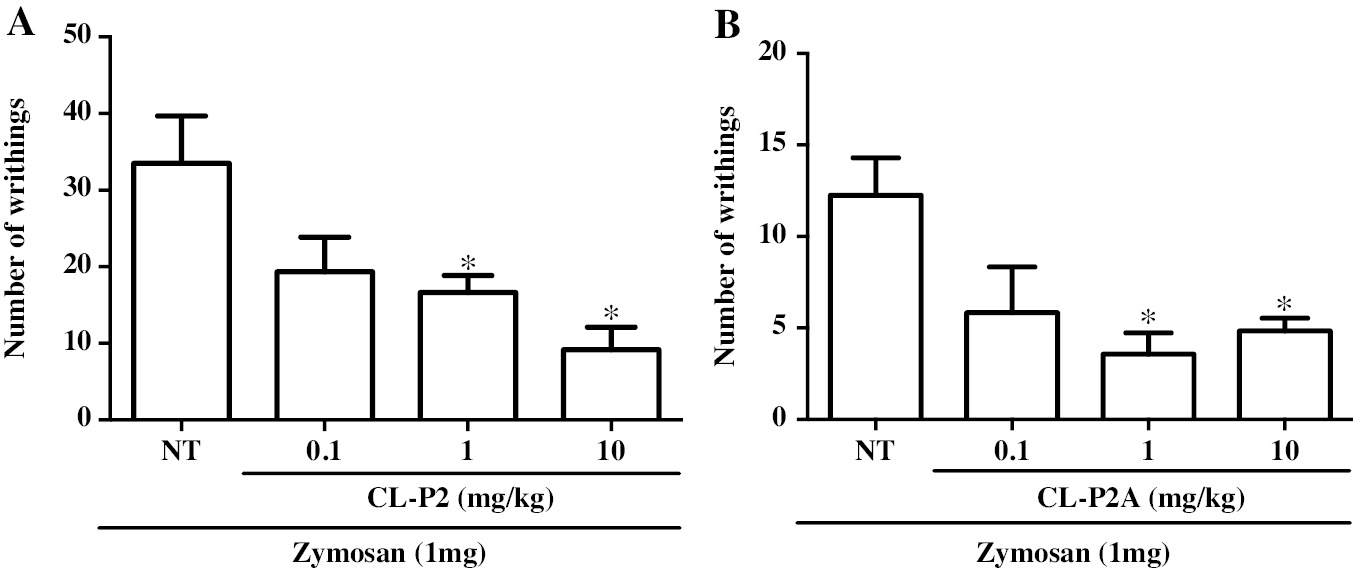
Zymosan (1 mg) induced a significant writhing response that was significantly (P < 0.05) inhibited by CL-P2 (1 and 10 mg/kg) by 50.24% and 72.63%, respectively, compared to the NT group (Figure 6A). Similarly, treatment with CL-P2A (1 or 10 mg/kg) reduced the zymosan effect (3.57 ± 1.17 and 4.83 ± 0.70, respectively; Figure 6B).
Figure 6 Effect of CL-P2 (A) and CL-P2A administration (B) on the zymosan-induced writhing response induced in mice. The number of writhing movements was determined between 0 and 30 min after injection (ip) of zymosan (1 mg/animal/250 μL). CLP-2 (0.1, 1 or 10 mg/kg) or CLP-2A (0.1, 1 or 10 mg/kg) was administered (oral gavage) 60 min before zymosan injection. Data are expressed as the mean ± SEM of 6 animals for each group *P < 0.05 indicates a significant difference from the untreated (NT) group (ANOVA and Tukey’s test).
Putative mechanisms involved in CL-P2 and CL-P2A efficacy
The possible involvement of neutrophils and oxidative stress (SOD and nitrite/nitrate) in the mechanism underlying CL-P2 and CL-P2A action was studied by measuring MPO and SOD activities, and nitrite/nitrate levels in peritoneal exudates in mice challenged with zymosan. Both derivatives failed to reduce MPO activity (Table 4). However, CL-P2A increased (P < 0.05) SOD activity and CL-P2 decreased (P < 0.05) nitrite/nitrate levels (Table 4).
Table 4 Effect of CL-P2 and CL-P2A on Myeloperoxidase (MPO) and Superoxide Dismutase (SOD) Activities, and Nitrite/nitrate Levels in the Peritoneal Exudates of Mice After Zymosan Injection
| Group | MPO Activity (MPO units/mL of Peritoneal Exudate) | SOD Activity (μg SOD/μg Protein) | Nitrite/nitrate Levels (μM) |
|---|---|---|---|
| Vehicle | 3.30 ± 0.29 | 1.68 ± 0.009 | 0.69 ± 0.023 |
| CL-P2 (10 mg/kg) | 3.39 ± 0.83 | 1.29 ± 0.093 | 0.60 ± 0.009a |
| CL-P2A (10 mg/kg) | 3.69 ± 0.29 | 2.24 ± 0.113a | 0.68 ± 0.016 |
Peritoneal exudates were collected 4 h after injection (i.p.) of zymosan (1 mg/animal/250 μL). CL-P2 (10 mg/kg) or CLP-2A (10 mg/kg) was administered 60 min before zymosan injection. Data represents the mean ± SEM (n = 7 mice per group; ANOVA andTukey’s test). aP < 0.05 compared to vehicle group.
Discussion
The development of modern medications may be influenced by the possible benefits of traditional herbal remedies. The main active components of botanical plants are progressively being isolated for use in pharmaceutical formulae for clinical purposes [23]. Herein, the toxicity and biological activities of semi-synthetic molecules (CL-P2 and CL-P2A) derived from the natural triterpene, 3β,6β,16β-trihydroxylup-20(29)-ene (CL-1), are reported for the first time base on in silico, in vitro, and in vivo experiments. The predicted toxicity suggested that CL-P2 and CL-P2A have favorable profiles. An in vitro assay showed low cytotoxicity for both semi-synthetic molecules in L929 and HaCaT cells. Furthermore, an in vivo assay showed that gavaged mice with both semi-synthetic molecules for 14 d did not cause toxicity or mortality.
The CL-P2 derivative had low cytotoxicity in HaCaT cells and only the highest concentration (40 μg/mL) reduced the viability of cells. However, CL-P2 had cytotoxic effects against L929 cells at the highest concentrations. Furthermore, the CL-P2A derivative reduced HaCaT viability at the highest concentrations (20 and 40 μg/mL). L929 cell growth was reduced in the presence of the highest concentrations of CL-P2A, like CL-P2. The triterpene compound class is considered to have low-to-moderate cytotoxic and potentially protective activity [24]. Sousa et al. [25] evaluated the C. leprosum extract, did not note mutagenic activity on mouse peritoneal macrophages at the tested concentrations.
After performing the MTT cell viability assay on peripheral blood mononuclear cells from healthy volunteers, Lacouth-Silva et al. [8] reported that CL-1 isolated from C. leprosum has moderate cytotoxicity. In addition, it was shown that CL-1 reduced the replication and survival of Leishmania amazonensis in host cells, while no cytotoxicity was observed on murine peritoneal macrophages [10]. Moreover, Horinouchi et al. [26] showed that adding CL-1 to the HaCaT cell culture reduced proliferation and induced cell apoptosis. Thus, considering these previous results and our current in vitro data, we suggest that both semi-synthetic derivatives exhibit minimal cytotoxicity.
An evaluation of CL-P2 and CL-P2A efficacy using an in vivo assay showed that both derivatives reduced zymosan-induced writhing movements, with the CL-P2 efficacy associated with reduced nitrite/nitrate levels. In contrast, CL-P2A efficacy was associated with increased SOD activity.
In silico assays are valuable for predictive toxicity assessment and can provide additional insight into the safety profile of compounds [27]. In the present study, in silico toxicity predicitions were confirmed using an in vivo toxicity assay. In fact, when evaluating the safety of both semi-synthetic derivative gavage in mice after 14 d of treatment, no signs of toxicity were noted. The following parameters were compared to mice that only received vehicle: body mass changes; biochemical parameters (ALT and AST); and H&E analysis of the kidneys, liver, and stomach,. Similarly, Sousa et al. [25] demonstrated no toxicity in mice treated with extracts and fractions from C. leprosum at different doses (250, 500, and 750 mg/kg) for 24, 48, and 72 h. These results suggest preclinical safety and efficacy for both semi-synthetic derivatives.
Some evidence suggests the potential of CL-1 as an anti-nociceptive, antioxidant, and anti-inflammatory agent [19, 26, 28]. Considering the mechanism of action, it was shown that CL-1 reduced total leukocyte migration (mainly neutrophils) induced by carrageenan in a peritonitis model [2]. Further, it was recently suggested that nitric oxide might modulate, at least in part, the anti-nociceptive effects of CL-1 [18]. Thus, the CL-P2 derivative reduced the number of writhing responses which corresponded to a reduction in nitrite levels. In contrast, the CL-P2A anti-nociceptive effect was associated with increased SOD activity without changes in MPO activity.
Although Longhi-Balbinot et al. [2] showed that CL-1 reduces inflammatory infiltration (mainly neutrophils), in the present study we showed that both derivatives (CL-P2 and CL-P2A) failed to reduce MPO activity. This fact may be related to the structural changes in CL-1 to produce CL-P2 and CL-P2A. Silva et al. [18] reported that the anti-nociceptive effect of CL-1 is modulated by nitric oxide, similar to the CL-P2 findings. Some authors have shown that Combretum possesses antioxidant properties [29–31]. Viau et al. [32] showed that C. leprosum has an antioxidant effect. In agreement with these data, we showed a modulatory effect of CL-P2 and CL-P2A on SOD activity and nitrite levels, suggesting an antioxidant activity for both compounds.
In conclusion, both derivatives (CL-P2 and CL-P2A) obtained from CL-1 reduced the zymosan-induced writhing response in mice without causing toxicity. The putative mechanism of antinociceptive action suggested that CL-P2 and CL-P2A efficacy was mediated, at least in part, via antioxidant activity by modulating nitrite/nitrate levels and SOD activity, respectively. Thus, considering the medicinal use of Combretum leprosum by the communities as an anti-inflammatory and healing agent, the current study aimed to generate strength of evidence for the development of new medicines based on prior knowledge based on successful community practices.
Acknowledgments
Hellíada Vasconcelos Chaves and Vicente de Paulo Teixeira Pinto are Funcap/Ceara/Brazil investigators. Mirna Marques Bezerra, Karuza Maria Alves Pereira, Nylane Maria N. de Alencar, Maria Elisabete A. de Moraes, and Paula Goes are CNPq/Brazil investigators.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Oliveira MM, Silva MM, Moreira TLM, Couto VF, Coelho YN, et al. O uso crônico de anti-inflamatórios não esteroidais e seus efeitos adversos. Rev Cad Med 2019;2(2):90-100.
- Longhi-Balbinot DT, Lanznaster D, Baggio CH, Silva MD, Cabrera CH, et al. Anti-inflammatory effect of triterpene 3β,6β,16β-tri-hidroxilup-20(29)-eno obtained from Combretum leprosum Mart & Eich in mice. J Ethnopharmacol 2012;142:59-64. [PMID: 22575213 DOI: 10.1016/j.jep.2012.04.013]
- Horinouchi CD, Mendes DA, Soley Bda S, Pietrovski EF, Facundo VA, et al. Combretum leprosum Mart. (Combretaceae): potential as an antiproliferative and anti-inflammatory agent. J Ethnopharmacol 2013;145(1):311-9. [PMID: 23159472 DOI: 10.1016/j.jep.2012.10.064]
- Nunes PH, Cavalcanti PM, Galvão SM, Martins MC. Antiulcerogenic activity of Combretum leprosum. Pharmazie 2009;64(1):58-62. [PMID: 19216233]
- Facundo VA, Rios KA, Medeiros CM, Militão JSLT, Miranda ALP, et al. Arjunolic acid in the ethanolic extract of Combretum leprosum root and its use as a potential multi-functional phytomedicine and drug for neurodegenerative disorders: anti-inflammatory and anticholinesterasic activities. J Braz Chem Soc 2005;16:1309-12. [DOI: 10.1590/S0103-50532005000800002]
- Lira SRS, Almeida RN, Almeida FRC, Oliveira FS, Duarte JC. Preliminary studies on the analgesic properties of the ethanol extract of Combretum leprosum. Pharm Biol 2002;40:213-5. [DOI: 10.1076/phbi.40.3.213.5837]
- Facundo VA, Andrade CHS, Silveira ER, Braz-Filho R, Huford CD. Triterpenes and flavonoids from C. leprosum.. Phytochemistry 1993;32:411-5. [DOI: 10.1016/S0031-9422(00)95005-2]
- Lacouth-Silva F, Xavier CV, da S Setúbal S, Pontes AS, Nery NM, et al. The effect of 3β, 6β, 16β-trihydroxylup-20(29)-ene lupane compound isolated from Combretum leprosum Mart. on peripheral blood mononuclear cells. BMC Complement Altern Med 2015;15(1):420. [PMID: 26608735 DOI: 10.1186/s12906-015-0948-1]
- Cruz BG, Rodrigues Teixeira AM, Silva PTD, Vasconcelos Evaristo FF, de Vaconcelos MA, et al. Antimicrobial activity of the lupane triterpene 3β,6β,16β-trihydroxylup-20(29)-ene isolated from Combretum leprosum Mart. J Med Microbiol 2019;68(10):1438-44. [PMID: 31385784 DOI: 10.1099/jmm.0.001056]
- Teles CB, Moreira-Dill LS, Silva Ade A, Facundo VA, de Azevedo WF Jr, et al. A lupane-triterpene isolated from Combretum leprosum Mart. fruit extracts that interferes with the intracellular development of Leishmania (L.) amazonensis in vitro. BMC Complement Altern Med 2015;15:165. [PMID: 26048712 DOI: 10.1186/s12906-015-0681-9]
- Silva FCO, Ferreira MKA, Silva AW, Matos MGC, Magalhães FEA, et al. Bioatividades de Triterpenos isolados de plantas: Uma breve revisão. Rev Virtual Quím 2020;12:1.
- Silva-Filho CJA, Freitas PGC, Oliveira FCE, Barbosa FG, Oliveira MCF, et al. Nanoencapsulation of triterpene 3β,6β,16β-trihydroxylup-20(29)-ene from Combretum leprosum as strategy to improve its cytotoxicity against cancer cell lines. Bioorg Med Chem Lett 2020;30(20):127469. [PMID: 32768650 DOI: 10.1016/j.bmcl.2020.127469]
- Banerjee P, Eckert AO, Schrey AK, Preissner R. ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucleic Acids Res 2018;46(W1):W257-W63. [PMID: 29718510 DOI: 10.1093/nar/gky318]
- Xiong G, Wu Z, Yi J, Fu L, Yang Z, et al. ADMETlab 2.0: an integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res 2021;49(W1):W5-W14. [PMID: 33893803 DOI: 10.1093/nar/gkab255]
- Papazisis KT, Geromichalos GD, Dimitriadis KA, Kortsaris AH. Optimization of the sulforhodamine B colorimetric assay. J Immunol Methods 1997;20:151-8. [PMID: 9433470 DOI: 10.1016/s0022-1759(97)00137-3]
- Houghton P, Fang R, Techatanawat I, Steventon G, Hylands PJ, et al. The sulphorhodamine (SRB) assay and other approaches to testing plant extracts and derived compounds for activities related to reputed anticancer activity. Methods 2007;42(4):377-87. [PMID: 17560325 DOI: 10.1016/j.ymeth.2007.01.003]
- Pietrovski EF, Rosa KA, Facundo VA, Rios K, Marques MC, et al. Antinociceptive properties of the ethanolic extract and of the triterpene 3β,6β,16β-trihidroxilup-20(29)-ene obtained from the flowers of Combretum leprosum in mice. Pharmacol Biochem Behav 2006;83:90-9. [PMID: 16458954 DOI: 10.1016/j.pbb.2005.12.010]
- Silva FCO, de Menezes JESA, Ferreira MKA, da Silva AW, Holanda CLA, et al. Antinociceptive activity of 3β-6β-16β-trihydroxylup-20(29)-ene triterpene isolated from Combretum leprosum leaves in adult zebrafish (Danio rerio). Biochem Biophys Res Commun 2020;3:362-7. [PMID: 32962857 DOI: 10.1016/j.bbrc.2020.07.107]
- Collier HO, Dinneen LC, Johnson CA, Schneider C, Schneider C. The abdominal constriction response and it’s suppression by analgesic drugs in the mouse. J Pharmacol 1968;32:295-310. [PMID: 4230818 DOI: 10.1111/j.1476-5381.1968.tb00973.x]
- Bradley PP, Christensen RD, Rothstein G. Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood 1982;60:618-22. [PMID: 6286012]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, et al. Analyses of nitrate, nitrite and [15N]nitrate in biological fluids. Anal Biochem 1982;126:131-8. [PMID: 7181105 DOI: 10.1016/0003-2697(82)90118-x]
- Ezeja MI, Anaga AO, Asuzu IU. Acute and sub-chronic toxicity profile of methanol leaf extract of Gouania longipetala in rats. J Ethnopharmacol 2014;15:1155-64. [PMID: 24384377 DOI: 10.1016/j.jep.2013.12.034]
- Xiuling Li, Liang S, Tan CH, Cao S, Xu X, et al. Nanocarriers in the enhancement of therapeutic efficacy of natural drugs. BIOI 2021;2(2):40-9. [DOI: 10.15212/bioi-2020-0040]
- Muffler H. Biotransformation of triterpenes. Proc Bioch 2011;46:1-15. [DOI: 10.1016/j.procbio.2010.07.015]
- Sousa HG, Uchôa VT, Cavalcanti SMG, de Almeida PM, Chaves MH, et al. Phytochemical screening, phenolic and flavonoid contents, antioxidant and cytogenotoxicity activities of Combretum leprosum Mart. (Combretaceae). J Toxicol Environ Health A 2021;19:399-417. [DOI: 10.1080/15287394.2021.1875345]
- Horinouchi CD, Mendes DA, Nolte S, Brito PS, Soley BD, et al. Antiproliferative and anti-inflammatory effects of 3β,6β,16β-Trihydroxylup-20(29)-ene on cutaneous inflammation. J Ethnopharmacol 2017;195:298-308. [PMID: 27880883 DOI: 10.1016/j.jep.2016.11.035]
- Hemmerich J, Ecker GF. In silico toxicology: from structure-activity relationships towards deep learning and adverse outcome pathways. Wiley Interdiscip Rev Comput Mol Sci 2020;10(4):e1475. [PMID: 35866138 DOI: 10.1002/wcms.1475]
- Coutinho MR, Oliveira LS, Evaristo FFV, Marinho MM, Marinho EM, et al. Pharmacological potential of the triterpene 3β,6β,16β-trihidroxilup-20 (29)-ene isolated from Combretum leprosum: a literature review. Fundam Clin Pharmacol 2022;36(3):486-93. [PMID: 34989452 DOI: 10.1111/fcp.12753]
- Ishola IO, Adeyemi OO, Agbaje EO, Tota S, Shukla R. Combretum mucronatum and Capparis thonningii prevent scopolamine-induced memory deficit in mice. Pharm Biol 2013;51(1):49-57. [PMID: 22979904 DOI: 10.3109/13880209.2012.704518]
- Kpemissi M, Eklu-Gadegbeku K, Veerapur VP, Negru M, Taulescu M, et al. Nephroprotective activity of Combretum micranthum G. Don in cisplatin induced nephrotoxicity in rats: in-vitro, in-vivo and in-silico experiments. Biomed Pharmacother 2019;116:108961. [PMID: 31146106 DOI: 10.1016/j.biopha.2019.108961]
- Forid MS, Rahman MA, Aluwi MFFM, Uddin MN, Roy TG, et al. Pharmacoinformatics and UPLC-QTOF/ESI-MS-based phytochemical screening of Combretum indicum against oxidative stress and alloxan-induced diabetes in long-evans rats. Molecules 2021;26(15):4634. [PMID: 34361788 DOI: 10.3390/molecules26154634]
- Viau CM, Moura DJ, Pflüger P, Facundo VA, Saffi J. Structural aspects of antioxidant and genotoxic activities of two flavonoids obtained from ethanolic extract of Combretum leprosum. Evid Based Complement Alternat Med 2016;2016:9849134. [PMID: 27478483 DOI: 10.1155/2016/9849134]