Use of Flurbiprofen Ester in 4-Dimensional Hysterosalpingography: Does Flurbiprofen Ester Relieve Pain During an Infertility Evaluation?
1Department of Ultrasound, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, No. 107 Yanjiang Road West, Guangzhou 510120, China
2Department of Ultrasound, The First People’s Hospital of Kashi Prefectrue, No. 120 Yingbin Avenue, Kashi, Xinjiang 844000, China
aThese authors contributed equally to this work.
*Correspondence to: Prof. Na Di, Department of Ultrasound, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, No. 107 Yanjiang Road West, Guangzhou 510120, China. Tel.: +86-20-81332516, E-mail: dina3@mail.sysu.edu.cn; Prof. Baoming Luo, Department of Ultrasound, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, No. 107 Yanjiang Road West, Guangzhou 510120, China. Tel.: +86-20-81332516, E-mail: luobm@mail.sysu.edu.cn
Received: May 18 2024; Revised: June 22 2024; Accepted: July 16 2024; Published Online: July 24 2024
Cite this paper:
Tan L, Wu S, Ma A et al. Use of Flurbiprofen Ester in 4-Dimensional Hysterosalpingography: Does Flurbiprofen Ester Relieve Pain During an Infertility Evaluation? BIO Integration 2024; 5: 1–7.
DOI: 10.15212/bioi-2024-0026. Available at: https://bio-integration.org/
Download citation
© 2024 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
Objective: The purpose of this study was to determine the analgesic effect of a flurbiprofen ester injection via continuous intravenous drip during transvaginal 4-dimensional hysterosalpingography (TVS 4D-HyCoSy).
Methods: Two hundred thirty patients who underwent TVS 4D-HyCoSy for infertility from May 2018 to August 2021 at our hospital were selected. The participants were grouped based on tubal patency, flurbiprofen ester use, and uterine cannula diameter, as follows: bilateral tubal patency group; non-bilateral tubal patency group; atropine group; atropine + flurbiprofen ester group; coarse tube group; and fine tube group. The analgesic effect during TVS 4D-HyCoSy and pain relief were compared between groups using visual analog scoring (NRS). Additionally, the incidence of adverse effects was recorded and factors related to the influence of pain were analyzed.
Results: 1. Tubal patency reduced pain during ultrasound tubal examination, flurbiprofenate provided significant analgesia after ultrasound tubalography and reduced adverse effects (P < 0.001). 2. The tube diameter thickness had no effect on tubal ultrasonography procedure-related pain. 3. Multivariable analysis of pain relief during imaging suggested that the use of flurbiprofen for bilateral tubal patency had a significant positive effect on pain relief within 30 min after the examination with an AUC of 0.732 (95% CI: 0.665–0.798).
Conclusion: A flurbiprofen ester continuous intravenous drip had a good analgesic effect in patients with TVS 4D-HyCoSy. Specifically, the pain relief effect after examination was significant and reduced the incidence of adverse reactions during the contrast examination. Flurbiprofen ester can be administered independently and is worthy of clinical promotion and application.
Keywords
Analgesia, atropine, flurbiprofen ester, uterine tubal ultrasonography.
In recent years, the declining fertility rate has become a widespread concern in Chinese society and the infertility rate among women of childbearing age in China is increasing year-after-year. Indeed, tubal factors are the primary cause of female infertility, accounting for 30%–35% of all cases [1, 2]. Transvaginal hysterosalpingo-contrast sonography (TVS 4D-HyCoSy), which can dynamically display the size and shape of the uterine cavity, and demonstrate fallopian tube patency in real time, has been widely used for fertility assessment in infertile women. Our team began to study the clinical application of HyCoSy since 2010 and found that HyCoSy has high specificity (86.3%) and sensitivity (93.5%) for verifying tubal patency [3]. Domestic and international studies have confirmed the high specificity and sensitivity of HyCoSy for tubal and uterine cavity assessment [4–10]. However, during TVS 4D-HyCoSy intubation and contrast administration, patients often experience pain and discomfort, and even have adverse reactions, such as dizziness, nausea, vomiting, blurred vision, and shock. Moreover, it has been reported that pain during tubal imaging can cause tubal spasm, resulting in a higher false-positive rate and lower diagnostic accuracy [11]. Atropine is the currently used anti-spasmodic drug during HyCoSy and is often used by intramuscular injection 30 min before imaging, but studies have found that the effect of atropine in dilating the cervical canal is weak and the analgesic effect is not apparent [12]. Flurbiprofen ester belongs to a new type of NSAID that mainly consists of flurbiprofen and its encapsulated lipid microspheres, which is targeted compared to other NSAIDs and has been widely used for postsurgical analgesia [13], but no studies have been published related to the use of flurbiprofen ester during TVS 4D-HyCoSy. Therefore, this study was proposed to determine the analgesic effect of flurbiprofen ester injection via continuous drip during TVS 4D-HyCoSy.
Methods and materials
Patients
Two hundred thirty patients scheduled for TVS 4D-HyCoSy as part of an infertility evaluation from May 2018 to August 2021 at our hospital were selected and grouped according to tubal patency, use of flurbiprofen ester, and uterine cannula diameter, as follows: bilateral tubal patency group (Figure 1); non-bilateral tubal patency group (Figure 2); atropine group; atropine + flurbiprofen ester group; coarse tube group; and fine tube group. General statistics were based on tubal patency, i.e., bilateral (n = 139) and non-bilateral tubal patency (n = 91). General data, including age, years of infertility, history of dysmenorrhea, endometrial thickness at the time of examination, and tube diameter using a uterine cannula, were compared between the two groups. This study was approved by the Ethics Committee of the Medical Ethics Committee of our hospital, and informed consent was obtained from the patients.
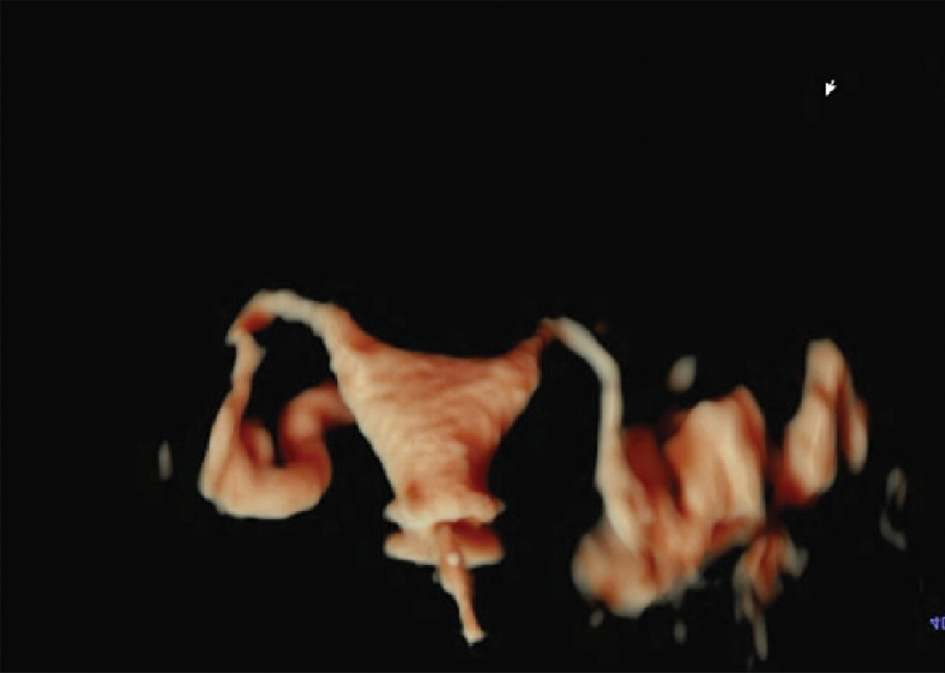
Figure 1 Bilateral tubal patency.
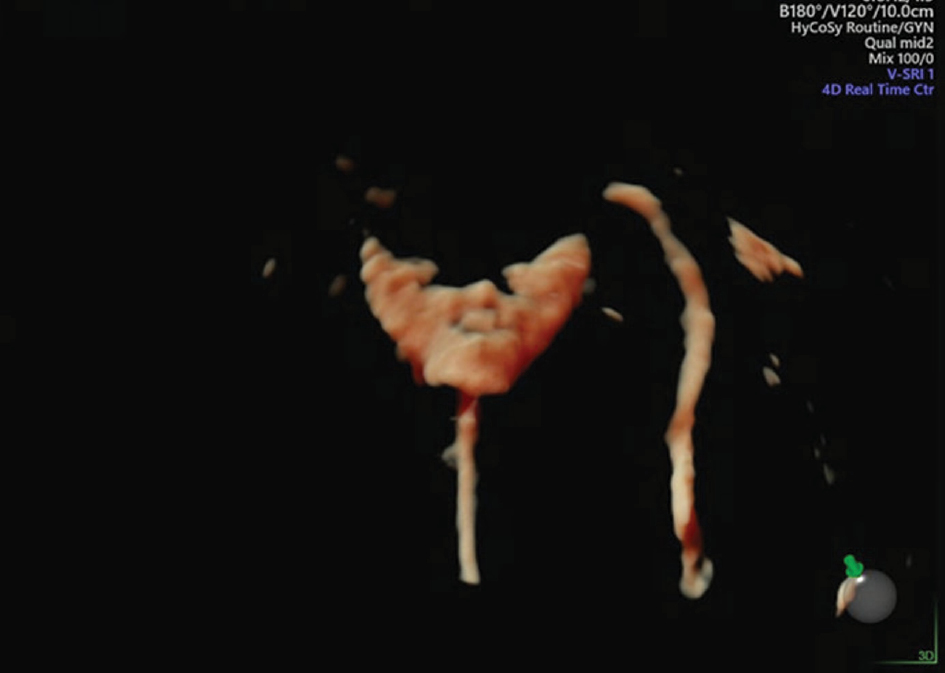
Figure 2 Non-bilateral tubal patency.
Inclusion and exclusion criteria
The inclusion criteria were as follows: ① healthy women who desire children without using contraception and are not pregnant; ② following treatment for tubal pregnancy; and ③ understood the study methodology and signed the informed consent form.
The exclusion criteria were as follows: ① peptic ulcers; ② severe hepatic, renal, and hematologic dysfunction; ③ severe heart failure and hypertension; ④ history of allergy to flurbiprofen ester, atropine, or contrast agent components; ⑤ aspirin-triggered asthma or a prior history of aspirin-triggered asthma; and ⑥ use of eloxacin, lomefloxacin, or norfloxacin.
Methodology
Preoperative preparation
Before undergoing TVS 4D-HyCoSy, a detailed medical history was obtained, a gynecologic examination was performed, and a routine white blood cell count and STD screening were performed. The patient was instructed to abstain from sexual intercourse after menstruation during the examination cycle. The examination was performed 5-10 d after menstruation or at the latest 20 d after menstruation if the patient has a long menstrual cycle. The bladder and rectum were emptied before the examination. In the atropine group, 0.5 mg of atropine sulfate (20180711; Henan Runhong Pharmaceutical Co., Ltd., Henan, China) was administered intramuscularly 30 min before TVS 4D-HyCoSy. In the combined atropine + flurbiprofen ester group, 0.5 mg of atropine sulfate (as above) was administered intramuscularly 30 min before TVS 4D-HyCoSy and a 20-mg flurbiprofen ester infusion was started 10 min before contrast cannulation. Flurbiprofen ester (3E171H; Hubei Noon Pharmaceutical Co., Ltd., Hubei, China) was added to 100 ml of saline for continuous intravenous infusion.
Inspection method
After moderate filling of the bladder, the patient was placed in the cystotomy position, disinfected, and draped with sterile towels. A coarse-tube 12-gauge Foley catheter was placed and 2 ml of 0.9% sodium chloride solution was injected into the balloon to occlude the endocervical opening. The balloon size was adjusted according to the patient’s height and uterine size to determine the passage tube to the endocervical opening without detachment or 1.5 ml of 0.9% sodium chloride solution with fine COOK hysterosalpingography tube parameters for those with no history of pregnancy and no history of hysterosalpingation. The contrast agent was selected from SonoVue (Bracco, Milan, Italy) and 5 ml of 0.9% NaCl solution was added to form a suspension. Two milliliters of the suspension was removed and 18 ml of 0.9% NaCl solution was added to dilute the contrast agent. Bilateral fallopian tubes and pelvic contrast coating were observed dynamically to assess the patency of the fallopian tubes. If the initial imaging of the fallopian tubes was poor or suggested obstruction, a second tubal imaging was performed after lavage treatment. Adverse reactions, such as pain, dizziness, and allergy were also recorded in patients.
Observed indicators and assessment methods
- To observe the patency of the uterine tubes in both groups
Tubal patency was divided into bilateral and non-bilateral patency (obstruction of one or both tubes). - Pain level scoring criteria
The imaging procedure was divided into three stages: placement of the scrotal speculum and balloon catheter (T1); injection of contrast medium/throughput (T2); and within 30 min after extubation (T3). Pain was assessed, observed, and recorded, as perceived by the patients. ① The pain level was evaluated by visual analog scoring (NRS) with a score of 0 representing no pain, 1–3 representing mild pain, 4–6 representing moderate pain, and 7–10 representing severe pain. Pain relief was defined as pain level after the examination compared to the time of examination (T3→T2), e.g., severe or moderate pain reduced to mild or no pain. ② Adverse reactions during intubation and the examination were recorded, including allergies, dizziness, nausea, vomiting, limb numbness, palpitations, flush, and blurred vision.
Statistical methods
SPSS 26.0 statistical software was used for data analysis and the measurement data conforming to a normal distribution are expressed as the mean ± standard deviation (x̅ + s). A t-test was used for comparisons between two groups. The counting data were expressed as percentage, and χ2 test was used for comparisons between groups. The difference was considered statistically significant at a P < 0.05 and stepwise forward binary logistic regression analysis was used for multivariable analysis.
Results
Comparison of general information
There were 139 cases with an age range of 21–43 years and a mean age of 31.68 ± 4.60 years in the bilateral tubal patency group. The mean years of infertility was 2.08 ± 1.67 years. The mean endometrium thickness at the time of examination was 7.49 ± 1.89 mm. There were 91 cases with an age range of 23–43 years and a mean age of 32.99 ± 4.91 years in the non-bilateral tubal patency group. The mean years of infertility was 2.94 ± 2.81 years, and the mean thickness of the endometrium at the time of examination was 7.82 ± 2.23 mm. A statistically significant difference in age and years of infertility between the two groups was detected (P = 0.042 and P = 0.009, respectively [t-test]), while no significant difference was detected with respect to endometrial thickness (P = 0.221). In the bilateral tubal patency group 65.5% (90/139) of patients had dysmenorrhea and 74.1% (103/139) used coarse tubes. In the non-bilateral tubal patency group 64.8% (59/91) had dysmenorrhea and 69.2% (63/91) used coarse tubes. No statistically significant difference was detected in the history of dysmenorrhea or the thickness of the intubated tube between the two groups (P = 0.922 and P = 0.420, respectively [χ2]).
Comparison of adverse reaction incidence
Among the 78 patients in the atropine group there were 21 with nausea and dizziness, 12 with facial flushing, 3 with blurred vision, and 2 with limb numbness, for an adverse reaction rate of 48.71%. Among the 152 patients in the atropine + flurbiprofen ester group there were 6 with nausea, for an adverse reaction rate of 3.9%. The incidence of adverse reactions in the atropine + flurbiprofen ester group was lower than the atropine group and the difference was statistically significant (P < 0.001).
Comparison of analgesic effects during TVS 4D-HyCoSy
A comparison of pain levels during the tubal imaging procedure in the bilateral tubal patency versus non-bilateral tubal patency groups with atropine alone versus the combination of atropine + flurbiprofen ester is shown in Table 1. The results showed that pain was significant in the non-bilateral tubal patency group during inspection (P < 0.0001). After inspection, pain was not significant in the atropine + flurbiprofenate group (P = 0.003).
Table 1 Comparison of Pain Levels During Tubal Imaging
| Pain Assessment Period | Pain Grading | Grouping | Percentage of Moderate-to-Severe Pain (%) | P |
|---|---|---|---|---|
| During intubation | II-III degree | Bilateral patency of the fallopian tubes | 54.7 (76/139) | 0.303 |
| Non-bilateral patent fallopian tubes | 61.5 (56/91) | |||
| Atropine | 56.4 (44/78) | 0.829 | ||
| Atropine + flurbiprofen ester | 57.9 (88/152) | |||
| During inspection | II-III degree | Bilateral patency of the fallopian tubes | 39.6 (55/139) | 0.000 |
| Non-bilateral patency of the fallopian tubes | 68.1 (62/91) | |||
| Atropine | 50.0 (39/78) | 1.000 | ||
| Atropine + flurbiprofen ester | 50.0 (76/152) | |||
| After inspection | II-III degree | Bilateral patency of the fallopian tubes | 12.9 (18/139) | 0.847 |
| Non-bilateral patency of the fallopian tubes | 12.1 (11/91) | |||
| Atropine | 21.8 (17/78) | 0.003 | ||
| Atropine + flurbiprofen ester | 7.9 (12/152) |
Comparison of pain relief between groups
- Pain relief in the atropine versus atropine + flurbiprofen ester groups are compared in Table 2. Atropine + flurbiprofenate relieved pain during tubal imaging in the bilateral and non-bilateral tubal groups (P = 0.001 and P = 0.001, respectively)
Table 2 Comparison of Pain Relief Between Atropine and Atropine + Flurbiprofen Ester in Tubal Imaging
Projects Grouping Number of Cases Relief No Relief P Bilateral tubal patency group
(N1 = 139 cases)Atropine 29 17 12 0.001 Atropine + flurbiprofen ester 62 58 4 Non-bilateral tubal patency group
(N2 = 91 cases)Atropine 49 22 29 0.001 Atropine + flurbiprofen ester 90 63 27 - Flurbiprofen ester use was compared in the thick tube group with pain relief in the thin tube group among patients with bilateral patent fallopian tubes (Table 3). The data suggested that atropine + flurbiprofenate relieved pain during tubalography in the bilateral tubal patency group in whom fine tubes were used (P = 0.001).
Table 3 Comparison of Pain Relief Between the Thick and Thin Tube Groups in Bilaterally Patent Fallopian Tubes
Grouping Coarse Tube Fine Tube No Relief Relief No Relief Relief Atropine 25 15 2 7 Atropine + flurbiprofen ester 21 42 6 21 P 0.278 0.001 - Flurbiprofen use was compared with pain relief in the thick versus thin tube group among patients in the non-bilateral tubal patency group, as shown in Table 4. The results indicated that in the non-bilateral tubal patency group, atropine + flurbiprofenate relieved pain during tubalography whether fine or coarse tubes were used (P < 0.001 and P < 0.001, respectively).
Table 4 Comparison of Pain Relief in Thick and Thin Tubes in the Non-bilateral Tubal Patency Group
Grouping Coarse Tube Fine Tube No Relief Relief No Relief Relief Atropine 8 14 4 3 Atropine + flurbiprofen ester 4 37 0 21 P 0.000 0.000
Multifactor logistic regression analysis for pain relief during tubal angiography
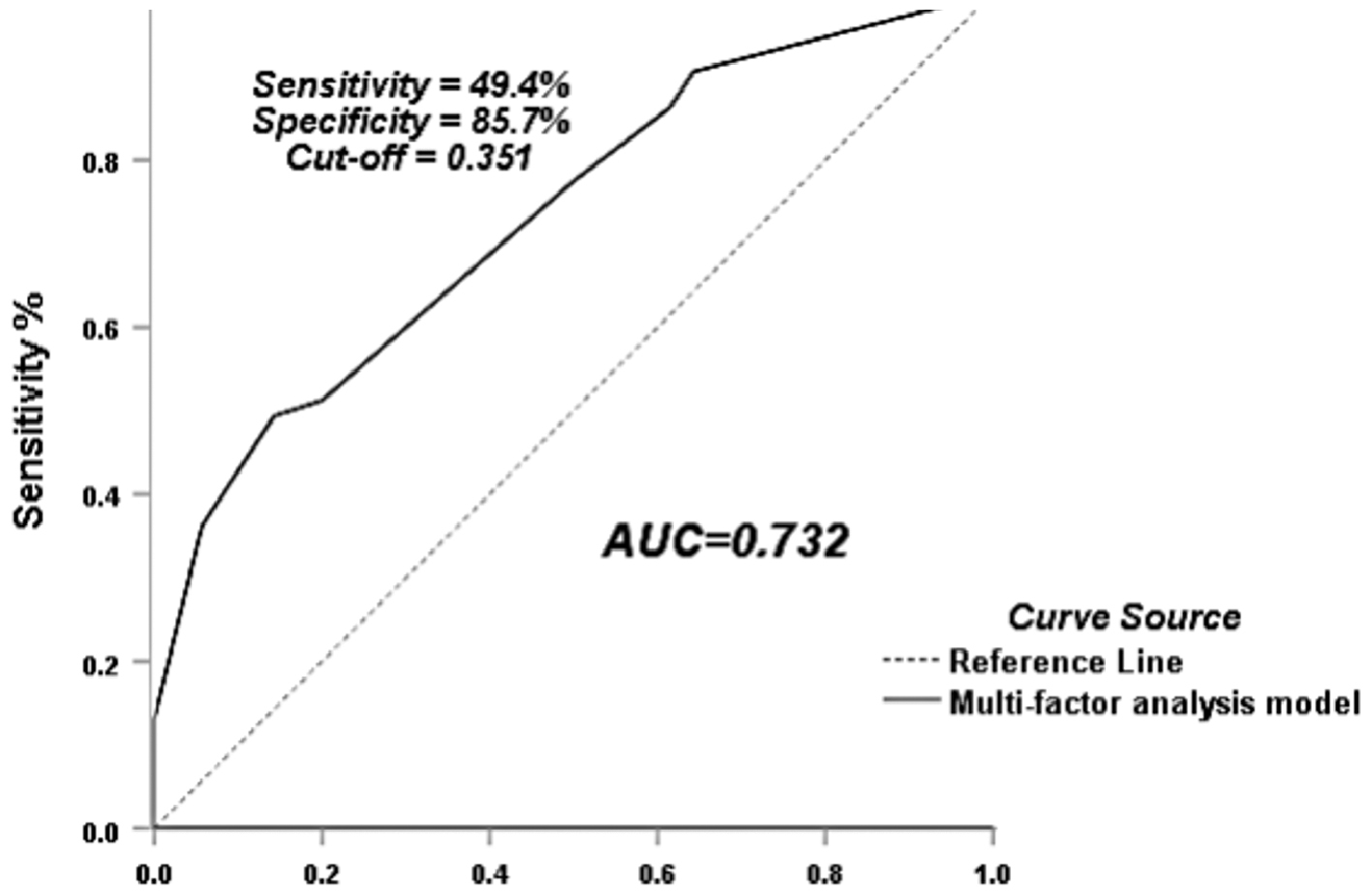
Patient age, years of infertility, history of dysmenorrhea, endometrial thickness, tube diameter at the site of insertion, tubal patency, and flurbiprofen continuous drip were analyzed for pain relief correlations. The statistically significant (P < 0.05) univariate indicators, including flurbiprofen continuous drip, thick tube, and bilateral tubal patency as independent variables and pain relief as the dependent variable were analyzed by binary logistic regression. The results suggested that flurbiprofen continuous drip and bilateral tubal patency had a significant positive relationship with pain relief, while tube diameter did not significantly affect pain relief (Table 5) and the ROC curve AUC of this model was 0.732 (95% CI: 0.665–0.798; Figure 3).
Table 5 Multi-factor Logistic Regression Analysis of Pain Relief During 4-Dimensional Hysterosalpingography
| Variables | B | Standard Error | Wald | P | OR (95% CI) |
|---|---|---|---|---|---|
| Coarse tube (vs. fine tube) | −0.703 | 0.381 | 3.395 | 0.065 | 0.495 (0.235-1.046) |
| Bilateral tubal patency (vs. non-bilateral tubal patency) | 1.134 | 0.345 | 10.824 | 0.001 | 3.107 (1.581-6.103) |
| Flurbiprofen esters (vs. flurbiprofen-free) | 1.356 | 0.317 | 18.277 | 0.000 | 3.881 (2.084-7.226) |
Figure 3 Flurbiprofen to relieve 4-dimensional hysterosalpingography pain ROC curve.
Discussion
The main clinical methods to determine tubal patency include laparoscopic fluid staining, X-HSG, and TVS 4D-HyCoSy, each with advantages and disadvantages. Laparoscopic fluid staining, which is the “gold standard,” technique is invasive [14] and has disadvantages, such as high invasiveness, high cost, and anesthesia risk, so laparoscopic fluid staining is usually used as a second-line verification or treatment after confirming obstruction by HSG. During X-HSG the patient is subjected to x-ray exposure, prolonged time to conception, and may be at risk for iodine allergy. HyCoSy is an ultrasound examination of the uterus and fallopian tubes to assess tubal patency with a transcervical contrast agent (air saline or microbubble contrast) [15]. HyCoSy has been shown to have a therapeutic role in addition to showing high accuracy in evaluating tubal patency [16, 17]. However, patients often exhibit varying degrees of pain and discomfort during the series of maneuvers during the HyCoSy examination which affects the accuracy of the results. Therefore, how to alleviate pain during and after the examination has become a focus of concern for clinicians and patients. Possible causes of pain from HyCoSy include psychological tension, excessive speed of contrast injection, size of the contrast catheter balloon, and the patient’s own diseases, such as uterine adhesions, cervical stenosis, and inflammation [18]. The pain level in most patients occurs from a combination of these factors. It has been suggested that HyCoSy triggers the most intense pain with cervical dilation [19]. In contrast, Guzel et al. [20] concluded that the most intense pain was caused by increased pressure in the uterine cavity during contrast injection.
Our study showed that preoperative injection of atropine was not effective in relieving patient pain, which is consistent with the literature [21]. Flurbiprofen ester has been widely used in surgical procedures, such as brain, upper abdominal, and gynecologic surgery, and significant analgesic effects have been achieved [13, 22, 23]. In the present study we attempted to introduce flurbiprofen ester during uterine tubal ultrasonography. Flurbiprofen ester is a non-steroidal analgesic with significant effects for various types of pain and targeted effects on inflammation and surgical sites [21, 23]. After entering the site of action through the carrier lipid microspheres, flurbiprofen ester was released by the carrier and rapidly hydrolyzed by the action of carboxyl lipase to produce flurbiprofen, which has a significant inhibitory effect on prostaglandin synthesis and in turn produces analgesic effects. The drug has a high safety factor, rapid onset of action, and long-lasting analgesic effect [21, 24].
In this study we showed that the addition of a flurbiprofen ester continuous intravenous drip to the routine preoperative injection of atropine had an effect on analgesia during ultrasound tubography, which was mainly reflected after the examination (P = 0.003). The flurbiprofen ester continuous intravenous drip was not significant for analgesia during intubation and examination, likely for the following reasons: 1. the greatest pain occurred during intubation and the TVS 4D-HyCoSy examination; 2. There was a high level of patient tension during intubation and the TVS 4D-HyCoSy examination, as well as pain-inducing factors, such as cervical dilation and increased pressure in the uterine cavity during contrast injection; and 3. after the examination the catheter was removed, the patient’s tension was relaxed, and flurbiprofen ester was more likely to have an objective role. We also found that at the time of examination, patency of the fallopian tube had a significant positive significance for analgesia (P = 0.000), and at the time of catheterization and after the examination, patency of the fallopian tube had no significant effect on analgesia, which suggests that patency of the fallopian tube may be one of the main causes of pain during HyCoSy. Flurbiprofen ester under continuous intravenous drip conditions was effective in relieving pain within 30 min after imaging, regardless of the patency of the fallopian tube. In conclusion, the patency of the fallopian tube affects pain during the examination and a flurbiprofen ester continuous intravenous infusion is effective in relieving pain.
During HyCoSy a flurbiprofen ester continuous intravenous infusion had a significant positive effect on pain relief during ultrasound tubography. With the use of thick tubes, flurbiprofen ester provided insignificant pain relief in those with bilateral tubal patency and significant pain relief in those with non-bilateral tubal patency, suggesting that tubal patency correlates with pain relief during the imaging procedure. With the use of fine tubes, flurbiprofen ester is effective in relieving pain regardless of the patency of the tubes. One possible reason to explain this phenomenon is that women with thick tubes, all of whom had been pregnant or delivered and had a history of hysterectomy, may be less nervous and more tolerant of pain. Second, those with bilateral patent fallopian tubes and with thin tubes who had no history of pregnancy and had not undergone a hysterectomy may be more nervous and less tolerant of pain. In order to further verify the relationship between pain relief during tubal imaging and the patency of the tubes, the effect of flurbiprofenac, and the diameter of the cannula, we performed a multivariable logistic regression analysis of pain relief during tubal imaging. The results showed that the patency of the tubes and the effect of flurbiprofenac were associated with pain relief during tubal imaging and were not related to the diameter of the cannula.
Moreover, our study also showed that the incidence of adverse reactions (excluding pain and dry mouth) was significantly lower in the flurbiprofen ester group than the atropine alone group. In addition, the psychological burden of the patients was relieved during the imaging process.
This study had several limitations. First, this study was single-center data and the sample size was not large enough. Second, this study was a retrospective study. This study needs to be validated by more center samples at a later stage.
Conclusion
A flurbiprofen ester continuous drip has a good analgesic effect in patients undergoing TVS 4D-HyCoSy. Specifically, the pain relief effect was significant after the examination and reduced the incidence of adverse reactions. A flurbiprofen ester continuous drip can be administered independently during a contrast examination and is worthy of clinical promotion and application.
Acknowledgements
This work was supported in part by National Natural Science Foundation of China (Grant Nos. 82060320 and 82260348) and the Natural Science Foundation of Xinjiang Uygur Autonomous Region (Grant No.2 021D01C009). We thank the study participants.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Sun Yat-sen Memorial Hospital of Sun Yat-sen University (SYSKY-2022-479-01).
Conflict of interest
The authors have no potential conflicts of interest to declare.
Author contributions
All authors contributed to the design and implementation of the study. LT and SJW: writing—original draft, collected, and analyzed data; ALM and SML: writing—review and editing, collected, and analyzed data; SLZ: writing—review and editing; SZ and PXC: supervision, writing—review and editing; ND and BML: conceptualization, supervision, writing—review and editing. All authors read and approved the final manuscript.
References
- Pournourali M, Tarang A, Haghighi SF, Yousefi M, Bahadori MH. Polymorphism variant of MnSOD A16V and risk of female infertility in northern Iran. Taiwan J Obstet Gynecol 2016;55(6):801-3. [PMID: 28040123 DOI: 10.1016/j.tjog.2016.06.018]
- Steinkeler JA, Woodfield CA, Lazarus E, Hillstrom MM. Female infertility: a systematic approach to radiologic imaging and diagnosis. Radiographics 2009;29(5):1353-70. [PMID: 19755600 DOI: 10.1148/rg.295095047]
- Zhou L, Zhang X, Chen X, Liao L, Pan R, et al. Value of three-dimensional hysterosalpingo-contrast sonography with SonoVue in the assessment of tubal patency. Ultrasound Obstet Gynecol 2012;40(1):93-8. [PMID: 22223543 DOI: 10.1002/uog.11085]
- Lim CP, Hasafa Z, Bhattacharya S, Maheshwari A. Should a hysterosalpingogram be a first-line investigation to diagnose female tubal subfertility in the modern subfertility workup? Hum Reprod 2011;26(5):967-71. [PMID: 21357604 DOI: 10.1093/humrep/der046]
- Luciano DE, Exacoustos C, Johns DA, Luciano AA. Can hysterosalpingo-contrast sonography replace hysterosalpingography in confirming tubal blockage after hysteroscopic sterilization and in the evaluation of the uterus and tubes in infertile patients? Am J Obstet Gynecol 2011;204(1):79.e1-5. [PMID: 21187197 DOI: 10.1016/j.ajog.2010.08.065]
- Luciano DE, Exacoustos C, Luciano AA. Contrast ultrasonography for tubal patency. J Minim Invasive Gynecol 2014;21(6):994-8. [PMID: 24910933 DOI: 10.1016/j.jmig.2014.05.017]
- Graziano A, Lo Monte G, Soave I, Caserta D, Moscarini M, et al. Sonohysterosalpingography: a suitable choice in infertility workup. J Med Ultrason 2013;40(3):225-9. [PMID: 27277240 DOI: 10.1007/s10396-012-0417-0]
- Moro F, Tropea A, Selvaggi L, Scarinci E, Lanzone A, et al. Hysterosalpingo-contrast-sonography (HyCoSy) in the assessment of tubal patency in endometriosis patients. Eur J Obstet Gynecol Reprod Biol 2015;186:22-5. [PMID: 25597884 DOI: 10.1016/j.ejogrb.2014.12.013]
- Calles-Sastre L, Engels-Calvo V, Ríos-Vallejo M, Serrano-González L, García-Espantaleón M, et al. Prospective study of concordance between hysterosalpingo-contrast sonography and hysteroscopy for evaluation of the uterine cavity in patients undergoing infertility studies. J Ultrasound Med 2018;37(6):1431-7. [PMID: 29143353 DOI: 10.1002/jum.14483]
- Ludwin I, Ludwin A, Wiechec M, Nocun A, Banas T, et al. Accuracy of hysterosalpingo-foam sonography in comparison to hysterosalpingo-contrast sonography with air/saline and to laparoscopy with dye. Hum Reprod 2017;32(4):758-69. [PMID: 28184447 DOI: 10.1093/humrep/dex013]
- Handelzalts JE, Levy S, Peled Y, Binyamin L, Wiznitzer A, et al. Information seeking and perceptions of anxiety and pain among women undergoing hysterosalpingography. Eur J Obstet Gynecol Reprod Biol 2016;202:41-4. [PMID: 27160813 DOI: 10.1016/j.ejogrb.2016.04.037]
- Zhang N, Liu Y, He Y, Shi J, Zhou M, et al. Transvaginal four-dimensional hysterosalpingo-contrast sonography: pain perception and factors influencing pain severity. J Obstet Gynaecol Res 2021;47(1):302-10. [PMID: 33107172 DOI: 10.1111/jog.14538]
- Zhang L, Zhu J, Xu L, Zhang X, Wang H, et al. Efficacy and safety of flurbiprofen axetil in the prevention of pain on propofol injection: a systematic review and meta-analysis. Med Sci Monit 2014;20:995-1002. [PMID: 24935068 DOI: 10.12659/msm.890102]
- Watrowski R, Babbel B, Jäger C. Uterine rupture after balloon inflation of the intrauterine Foley catheter during laparoscopic chromopertubation. Wien Klin Wochenschr 2016;128(15-16):599-601. [PMID: 27370269 DOI: 10.1007/s00508-016-1031-8]
- Dishuck CF, Perchik JD, Porter KK, Gunn DD. Advanced imaging in female infertility. Curr Urol Rep 2019;20(11):77. [PMID: 31734736 DOI: 10.1007/s11934-019-0942-0]
- Socolov D, Boian I, Boiculese L, Tamba B, Anghelache-Lupascu I, et al. Comparison of the pain experienced by infertile women undergoing hysterosalpingo contrast sonography or radiographic hysterosalpingography. Int J Gynaecol Obstet 2010;111(3):256-9. [PMID: 20850745 DOI: 10.1016/j.ijgo.2010.07.018]
- Lindborg L, Thorburn J, Bergh C, Strandell A. Influence of HyCoSy on spontaneous pregnancy: a randomized controlled trial. Hum Reprod 2009;24(5):1075-9. [PMID: 19164305 DOI: 10.1093/humrep/den485]
- Li CW, Shen M, Guo XZ, Peng LW, Jiang KM, et al. Clinical observation of flurbiprofen ester to relieve pain in hysterosalpingography. Int J Med Health 2021;27(08):1197-9. [DOI: 10.3760/cma.j.issn.1007-1245.2021.08.022]
- Gupta N, Ghosh B, Mittal S. Comparison of oral naproxen and intrauterine lignocaine instillation for pain relief during hysterosalpingography. Int J Gynaecol Obstet 2008;102(3):284-6. [PMID: 18603250 DOI: 10.1016/j.ijgo.2008.04.013]
- Guzel AI, Kuyumcuoglu U, Erdemoğlu M. The effect of flurbiprofen as prophylactic analgesic before hysterosalpingography. J Int Med Res 2010;38(5):1780-4. [PMID: 21309493 DOI: 10.1177/147323001003800524]
- Wang RD, Sheng XR, Guan WX, Wang M, Peng C, et al. Flurbiprofen axetil for postoperative analgesia in upper abdominal surgery: a randomized, parallel controlled, double-blind, multicenter clinical study. Surgery Today 2020;50(7):749-56. [PMID: 31925579 DOI: 10.1007/s00595-019-01951-1]
- Kotera A. Efficacy of flurbiprofen axetil for preventing postanesthetic shivering in patients undergoing gynecologic laparotomy surgeries. JA Clin Rep 2020;6(1):96. [PMID: 33289050 DOI: 10.1186/s40981-020-00403-x]
- Hao J, Wang K, Shao Y, Cheng X, Yan Z. Intravenous flurbiprofen axetil to relieve cancer-related multiple breakthrough pain: a clinical study. J Palliat Med 2013;16(2):190-2. [PMID: 23391401 DOI: 10.1089/jpm.2012.0353]
- Zhao X, Ji L. Flurbiprofen axetil: analgesic effect and adverse reaction. Pak J Pharm Sci 2018;31(3(Special)):1163-7. [PMID: 29735468]