Comparative Study of Spray-Drying and Freeze-Drying Techniques for Increasing Fenofibrate’s Solubility and Dissolution Rate
1MGV’s SPH College of Pharmacy, Malegaon, Nashik 423105, Maharashtra, India
2Department of Oil Technology, University Institute of Chemical Technology, Kavayitri Bahinabai Chaudhari North Maharashtra University, Jalgaon 425001, Maharashtra, India
3Dr. Shivajirao Kadam College of Pharmacy, Kasabe Digraj, Sangli 416305, Maharashtra, India
4Department Quality Assurance, Raosaheb Patil Danve College of Pharmacy, Badnapur, Jalna, Maharashtra 431202, India
*Correspondence to: Dr. Aarti P. Nikam, Assistant Professor, MGV’s SPH College of Pharmacy, Malegaon, Nashik 423105, Maharashtra, India. E-mail: artipawar.pharma@gmail.com
Received: 28 March 2024; Revised: 28 April 2024; Accepted: 13 May 2024; Published Online: 22 July 2024
Cite this paper:
Nikam AP, Meshram PD, Vanjari AV et al. Comparative Study of Spray-Drying and Freeze-Drying Techniques for Increasing Fenofibrate’s Solubility and Dissolution Rate. BIO Integration 2024; 5: 1–11.
DOI: 10.15212/bioi-2024-0010. Available at: https://bio-integration.org/
Download citation
© 2024 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
Background: Fenofibrate (FF) is a BCS class II compound whose poor solubility poses challenges in drug delivery and bioavailability. Solid self-micro emulsifying drug delivery systems (S-SMEDDS) have emerged as a promising solution to address these issues. These systems are aimed at enhancing the solubility and dissolution rates of poorly soluble drugs, such as FF, by formulating them into solid dosage forms.
Methods: FF solubility was investigated in various oils, surfactants, and co-surfactants to identify the most suitable components for formulating S-SMEDDS. The preparation of S-SMEDDS was carefully evaluated according to parameters including drug content, morphological characteristics, and structural features. Two methods, freeze-drying, and spray-drying, were compared for their efficacy in producing S-SMEDDS. Additionally, in vitro dissolution studies were conducted to assess the dissolution rates of FF-loaded S-SMEDDS tablets compared with conventional tablets.
Results: Among the oils tested, oleic oil achieved the highest FF solubility, whereas Tween 80 and Transcutol HP were identified as the optimal surfactant and co-surfactant, respectively. The preparation method significantly influenced the properties of S-SMEDDS. Freeze-drying outperformed the other methods by enhancing dissolution rates, primarily through increased surface area. Moreover, the solid-state characteristics of S-SMEDDS were dependent on the polymer concentration and processing method. In vitro dissolution studies demonstrated that FF-loaded S-SMEDDS tablets exhibited faster drug release than conventional tablets, owing to the inclusion of the super disintegrating agent CCS and the S-SMEDDS component. Freeze-drying was superior to spray-drying in enhancing dissolution, albeit with potentially higher production costs.
Conclusions: The study highlights the potential of S-SMEDDS to overcome the solubility and bioavailability challenges associated with FF. Freeze-drying emerged as the preferred method for producing S-SMEDDS, because of its superior dissolution enhancement capabilities, despite potentially higher production costs, whereas spray-dried S-SMEDDS offers economic and environmental benefits, but may achieve lower dissolution rates. Overall, our findings underscore the importance of formulation strategy in enhancing the efficacy of poorly soluble drugs such as FF.
Keywords
BCS-II, fenofibrate, freeze drying, spray drying.
Introduction
Advancements in biochemical understanding, combinatorial chemistry, and high-throughput selection have led to the development of NCE with poor water solubility, thus posing challenges in oral drug delivery [1–3]. Using amorphous drug forms increases dissolution rates, thereby overcoming solubility limitations [4–6]. However, amorphous systems are inherently unstable because of their elevated free energy, thus substantially hindering their inclusion in commercially available drug products [7, 8].
Lipid-based drug carriers play a major role in increasing drug molecule solubility, dissolution, and bioavailability within the gastrointestinal tract. SMEDDS formulations use bile salt as well as lipolytic agents to create a solubilized phase, thus aiding in drug release during digestion. After dilution in the aqueous environment, the formulation transitions to small oil-in-water micro-emulsions, thereby enhancing drug delivery [9]. The mechanical churning that naturally occurs in the stomach and intestines during digestion leads to emulsion formation. Beyond enhancing solubilization, the inclusion of fat in these formulations increases drug bioavailability by influencing absorption [10, 11].
Fenofibrate (FF), a BCS class II lipid-lowering medication, poses challenges of low solubility and limited bioavailability [12–15]. Various approaches, including cyclodextrin complexation and solid dispersion, have been explored to address these limitations. SMEDDS and SEDDS formulations have gained attention for enhancing FF’s bioavailability and dissolution [16–21].
Solid self-micro emulsifying drug delivery systems (S-SMEDDS) offer various advantages, such as cost-effective manufacturing; straightforward process control; high stability, consistency; and enhanced patient adherence, solubility and bioavailability. In addition, they can be used for generating nanoparticles, microspheres, and easily flowable powders [22]. Hence, producing solid-state formulations of L-SMEDDS or converting formulations into solid dosage forms are recommended to leverage the combined benefits of SMEDDS. Spray-drying and lyophilization are widely used methods to transform L-SMEDDS into S-SMEDDS. These methods can create dry micro-emulsions containing submicrometer particles, such as microspheres and micro-particles. This technique has recently received substantial interest to effectively solidify liquid SMEDDS. The spray-drying technique is favored for converting liquid SMEDDS in industrial applications. Rapid solvent evaporation increases viscosity, and entraps oil droplets with drug molecules in a carrier matrix [23–25].
Limited research has examined conversion of FF-SMEDDS into solid forms through spray-drying and freeze-drying approaches. The study was aimed at developing solid SMEDDS through these techniques for tablet dosage forms, to increase patient adherence as well as product stability.
Materials and methods
Material
FF was sourced from Wockhardt Ltd, Aurangabad. Ingredients including Kolliphor RH 40, Cremophore RH 40, PEG 300 and 400, castor oil, oleic acid, Tween 80, and Transcutol HP were obtained from BASF, Mumbai. Maltodextrin was obtained from Nutrichem Products, Mumbai, whereas MCC CCS, talc, and magnesium stearate were obtained from Maple Biotech, Pune.
Methods
Solubility profiling of FF
FF solubility was evaluated in various media by mixing of 500 mg of FF with the chosen vehicles, vortexing for 5 minutes, and incubating for 3 days at 25°C with continuous shaking. After centrifugation at 12,000 RPM for 20 minutes, the clear supernatant was collected and diluted with acetonitrile. UV spectroscopy was performed to measure the absorbance at 286 nm [18].
Preparation of SMEDDS
For preparation of liquid SMEDDS, FF (50 mg) was dissolved in a specific mixture of oleic acid, Tween 80, and Transcutol HP in a volume ratio of 43:28.5:28.5, respectively, and the mixture was stirred until clear. Dextran (1.0 g) was dissolved in water, and SMEDDS was subsequently added and mixed for 15 minutes. The resulting suspension was spray-dried with specified parameters. Lyophilization involved freezing the L-SMEDDS with 1% trehalose as a cryoprotectant and 72-hour lyophilization. The dried powder was subsequently characterized [12, 23, 24].
Drug content
Approximately 100 ml methanol was used to dissolve the S-SMEDDS. Subsequently, the solution was filtered through Whatman filter paper to determine the drug content. Analysis of the filtrates was conducted with UV spectroscopy at 286 nm.
Morphological analysis (SEM)
The morphological analyses, in addition to features of the S-SMEDDS, were examined with SEM conducted a voltage of 1.0 kV with a distance setting of 8.6–8.7 mm.
XRD study
XRD (Shimadzu XRD-7000, Japan) was used to determine whether the S-SMEDDS and pure FF were amorphous or crystalline.
Thermal investigation: DSC examination
Thermal analysis of pure FF as well as spray-dried S-SMEDDS loaded with FF was conducted with a DSC-Hitachi 9020 instrument. Samples weighing approximately 1–3 mg were positioned on a heated aluminum pan under an N2 flow rate of 50 ml/min, with a heating rate set at 100°C/min.
Saturation solubility study
The study of pure FF and its formulation in DW was conducted through addition of excess amount of drug in 10 ml of DW, and stirring for 24 hours at 37°C. The resulting solutions were sifted and investigated with UV spectroscopy at 286 nm [26].
Preparation of S-SMEDDS tablets
Tablets were formed through direct compression. A 100 mg S-SMEDDS complex was mixed with compressible diluents in a plastic container. Croscarmellose sodium, magnesium stearate, and talc were added, passed through a #60 sieve, and blended. Tablets were compressed with 8 mm punches on an automatic tablet punching machine (formula composition details in Tables 1 and 2) [27].
Table 1 Tablet Preparation Batches of FF-S-SMEDDS (Spray-Drying)
| Excipients | Amount (mg/tab) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | |
| FF eq. to 100 mg | 170 | 170 | 170 | 170 | 170 | 170 | 170 | 170 | 170 |
| MCC | 75 | 73.75 | 72.5 | 71.25 | 70 | 68.75 | 67.5 | 66.25 | 65 |
| Croscarmellose sodium | 1.25 | 2.5 | 3.75 | 5 | 6.25 | 7.5 | 8.75 | 10 | 11.25 |
| Talc | 2.5 | ||||||||
| Magnesium stearate | 1.25 | ||||||||
| Total weight | 250 | ||||||||
Table 2 Tablet Preparation Batches From FF-S-SMEDDS (Freeze-Drying)
| Ingredients | Quantity (mg/tablet) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | |
| FF (equivalent to 100 mg) | 150 | 150 | 150 | 150 | 150 | 150 | 150 | 150 | 150 |
| MCC | 95 | 93.75 | 92.5 | 91.25 | 90 | 88.75 | 87.5 | 86.25 | 85 |
| Croscarmellose sodium | 1.25 | 2.5 | 3.75 | 5 | 6.25 | 7.5 | 8.75 | 10 | 11.25 |
| Talc | 2.5 | ||||||||
| Magnesium stearate | 1.25 | ||||||||
| Total weight | 250 | ||||||||
In vitro dissolution investigations
Dissolution was assessed with a type II apparatus in 900 ml pH 6.8 PBS buffer at 37 ± 0.5°C with a rotational speed of 25 rpm. Samples (5 ml) were collected at stated intermissions, and an equivalent amount of buffer was replenished to maintain sink conditions. Analysis was conducted with a UV spectrophotometer at 286 nm [27].
Results
Solubility study of FF
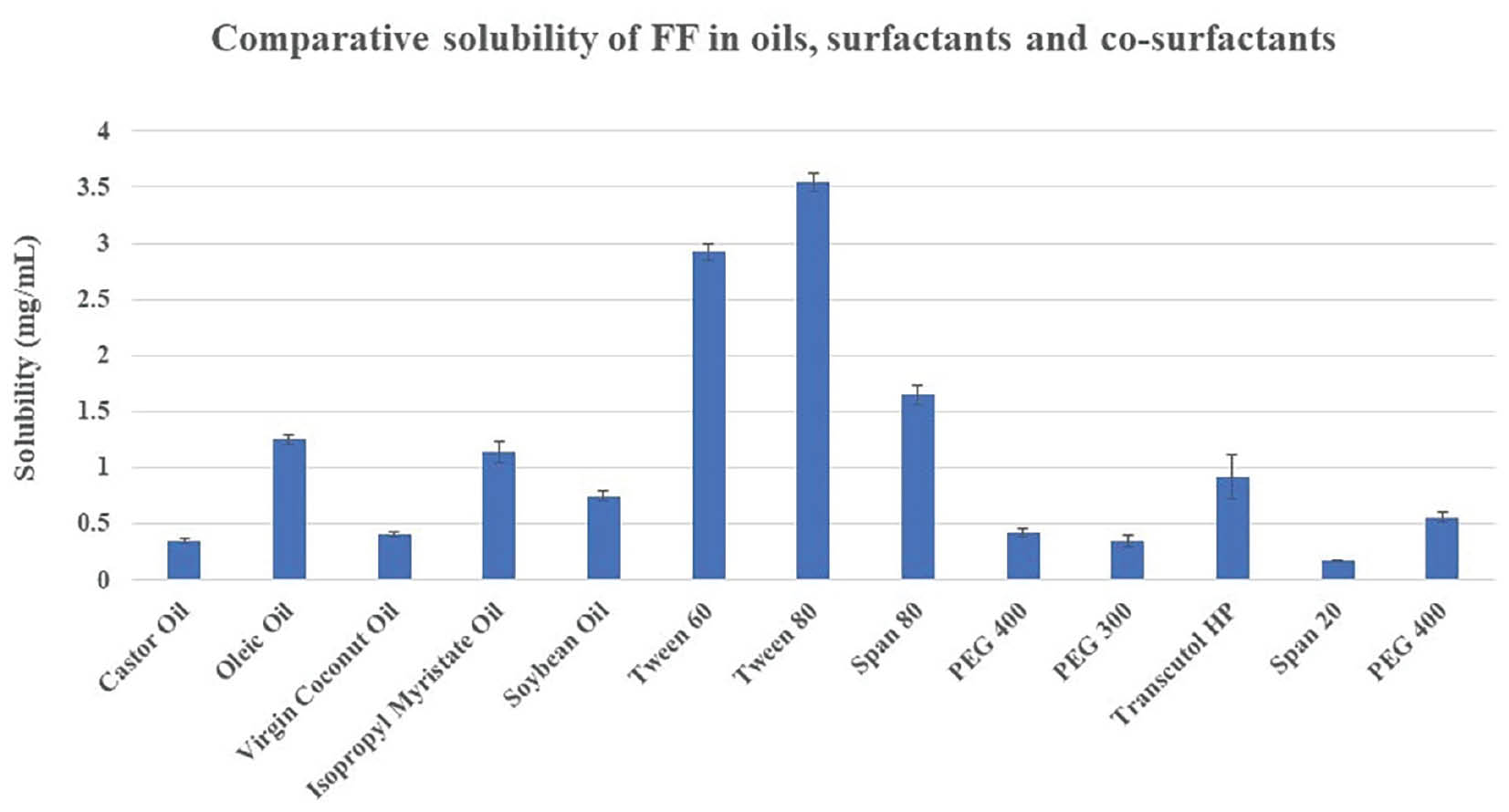
Oleic oil resulted in maximum FF solubility among oils, at 1.25 ± 0.32 mg/ml. FF displayed maximum solubility in Tween 80, among surfactants, at 3.54 ± 0.43 mg/ml. Transcutol HP exhibited the highest FF solubility among co-surfactants, at 0.92 ± 0.15 mg/ml. Proportional solubility statistics of FF in altered oils, surfactants, and co-surfactants are shown in Figure 1 and Table 3.
Figure 1 Comparative solubility of FF.
Table 3 Solubility of FF
| Sr. No. | Medium | Solubility (mg/ml) |
|---|---|---|
| Oils | ||
| 1. | Castor oil | 0.35 ± 0.20 |
| 2. | Oleic oil | 1.25 ± 0.22 |
| 3. | Virgin coconut oil | 0.41 ± 0.65 |
| 4. | Isopropyl myristate oil | 1.14 ± 0.03 |
| 5. | Soybean oil | 0.75 ± 0.66 |
| Surfactants | ||
| 6. | Tween 60 | 2.92 ± 0.21 |
| 7. | Tween 80 | 3.54 ± 0.43 |
| 8. | Span 80 | 1.65 ± 0.65 |
| Co-surfactants | ||
| 9. | PEG 400 | 0.42 ± 0.65 |
| 10. | PEG 300 | 0.35 ± 0.12 |
| 11. | Transcutol HP | 0.92 ± 0.15 |
| 12. | Span 20 | 0.17 ± 0.76 |
| 13. | PEG 400 | 0.56 ± 0.22 |
Evaluation of S-SMEDDS
Drug content
The FF content of S-SMEDDS was assessed and found within acceptable range of 96–110% w/w, as indicated in Table 4.
Table 4 Percentage Drug Content of SMEDDS Formulations
| Sr. No. | Freeze-Drying Drug Content (%w/w) |
Spray-Drying Drug Content (%w/w) |
|---|---|---|
| 1. | 100.37 ± 0.86 | 99.37 ± 0.68 |
| 2. | 99.67 ± 2.61 | 98.67 ± 2.41 |
| 3. | 98.63 ± 1.85 | 97.96 ± 1.47 |
| 4. | 101.53 ± 2.00 | 100.21 ± 1.69 |
SEM analysis
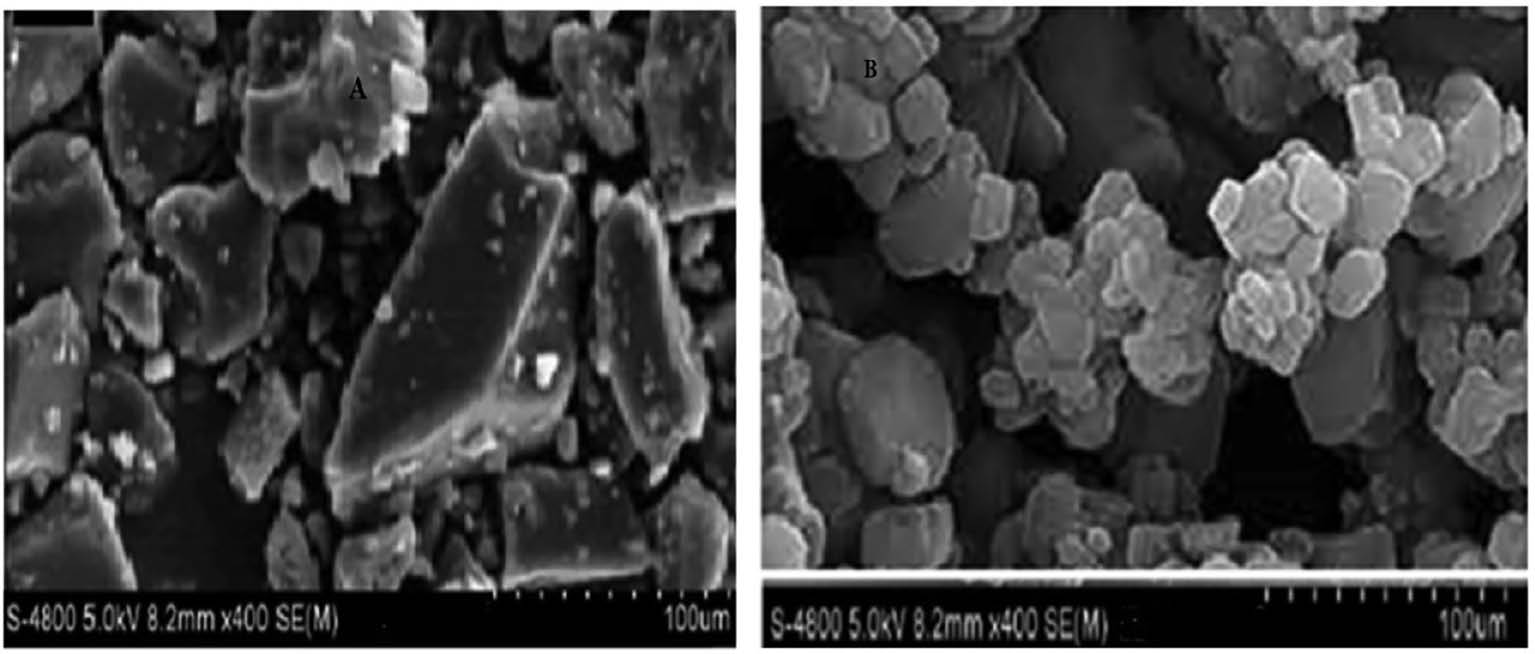
Pure FF and prepared FF loaded SMEDDS were subjected to SEM analysis to validate their morphological characteristics and elucidate their structural features. The SEM images revealed that the optimized FF-loaded SMEDDS had smooth surfaces with a mixture of round, oval, and rectangular particles. This technique effectively revealed the surface structure and morphology of both pure FF in addition to the Formulated FF-loaded SMEDDS. The SEM images in Figures 2 and 3 depict the surface characteristics of pure FF as well as FF-S-SMEDDS through two methods for comparison.
Figure 2 SEM images of pure FF and FF-S-SMEDDS prepared by spray-drying.
Figure 3 SEM images of pure FF and FF-S-SMEDDS prepared by freeze-drying.
X-ray diffraction
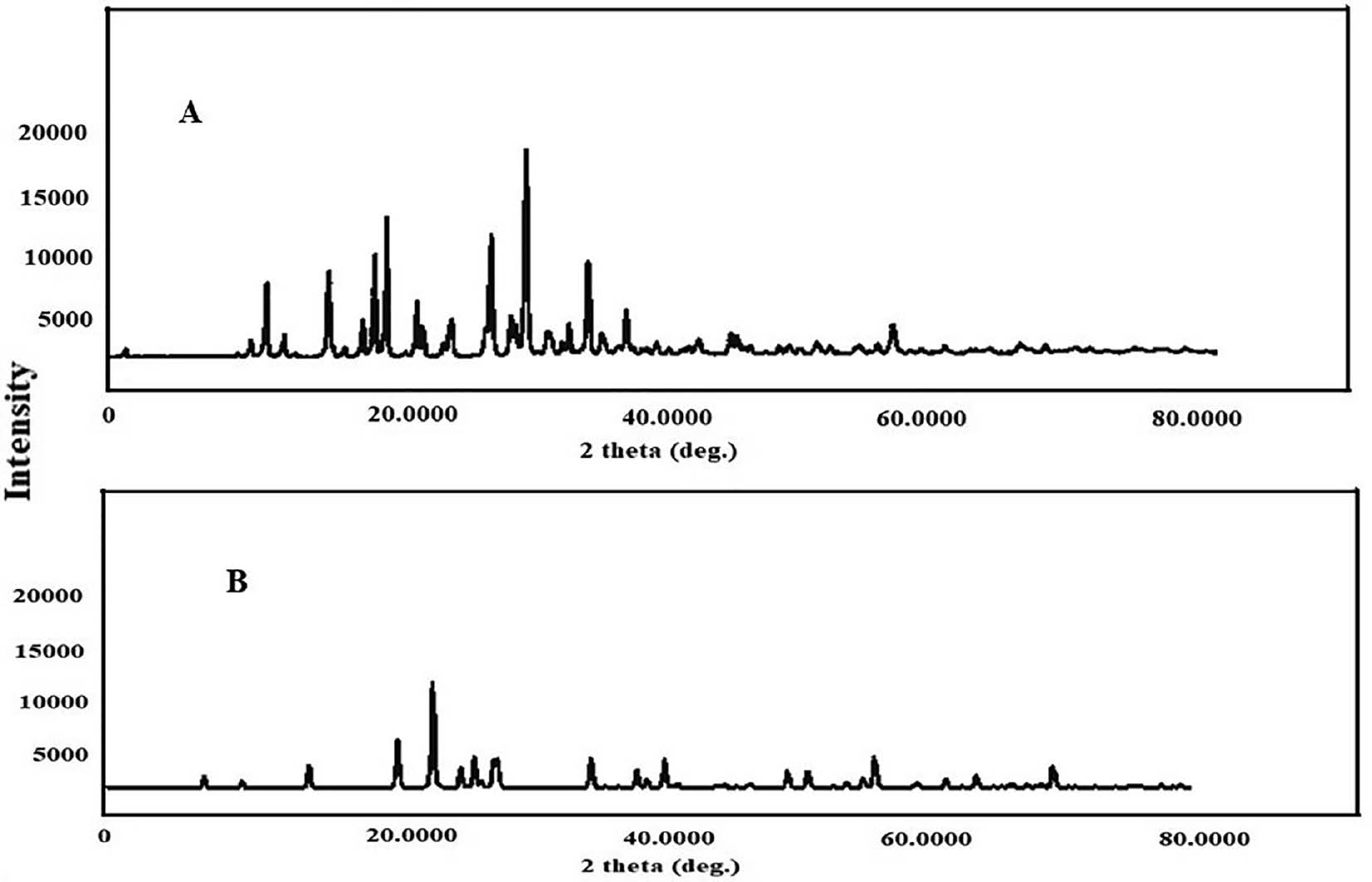
XRD analysis is valuable for examining the nature of a substance, regardless of whether it is in crystalline or amorphous form. For pure FF, the X-ray diffraction pattern revealed multiple distinct, intense peaks at specific 2θ values, such as 14.3°, 16.1°, and 22.2°, thus indicating its crystalline nature. However, when FF was loaded into S-SMEDDS, the peak intensities were markedly lower than those for pure FF. This finding suggested that FF in the SMEDDS formulation had transitioned into an amorphous state. This significant decrease in peak concentrations provided insight into the substantial increase in bioavailability and dissolution rates observed with the FF-SMEDDS formulation.
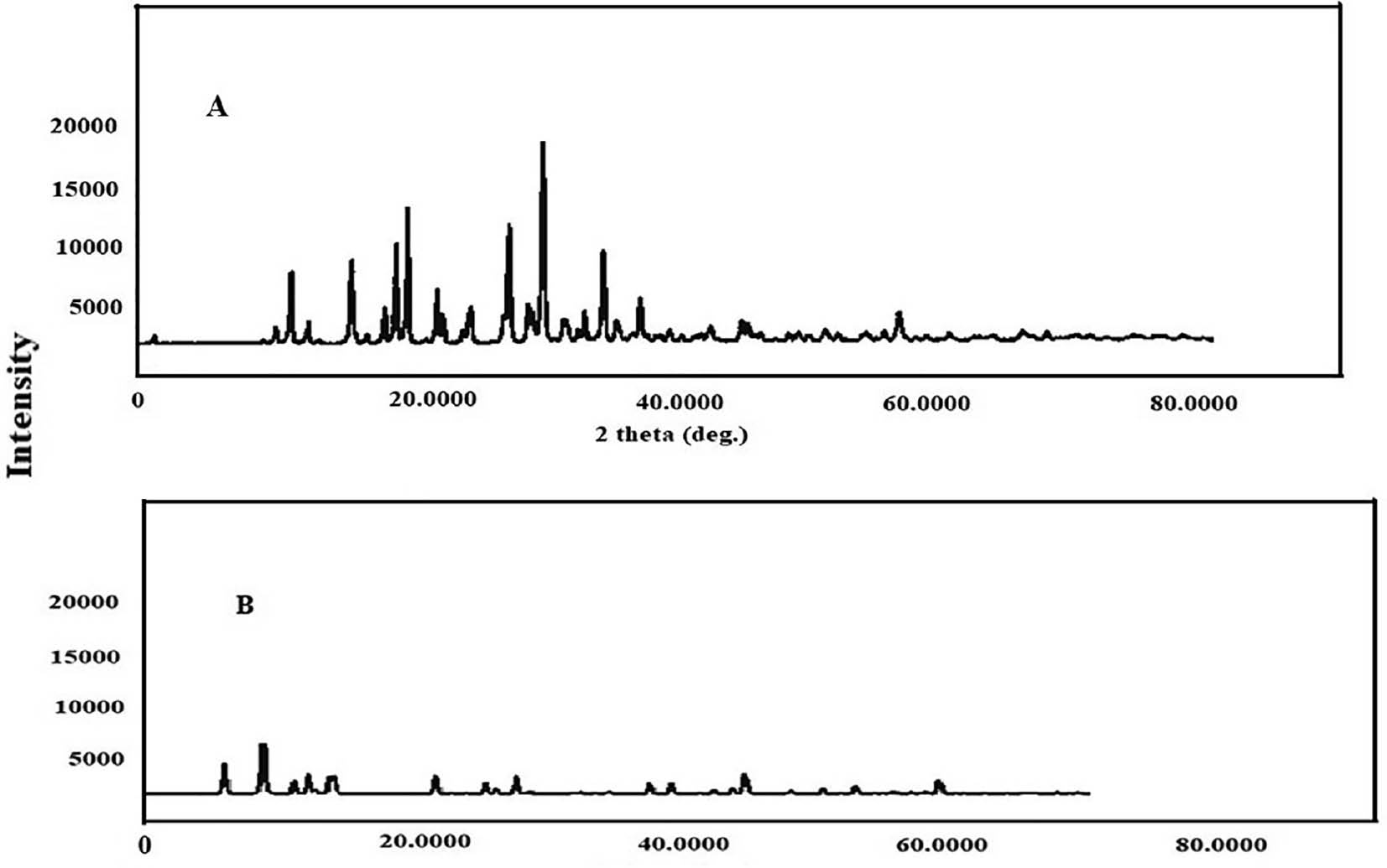
The graph of freeze-dried S-SMEDDS showed decreased peak intensities across various angles, thus indicating its amorphous nature. This decrease in peak intensities was associated with enhanced dissolution rates and increased bioavailability of FF in the SMEDDS. Figures 4 and 5 show the XRD results.
Figure 4 XRD graphs of pure FF and FF-S-SMEDDS prepared by spray-drying.
Figure 5 XRD graphs of pure FF and FF-S-SMEDDS prepared by freeze-drying.
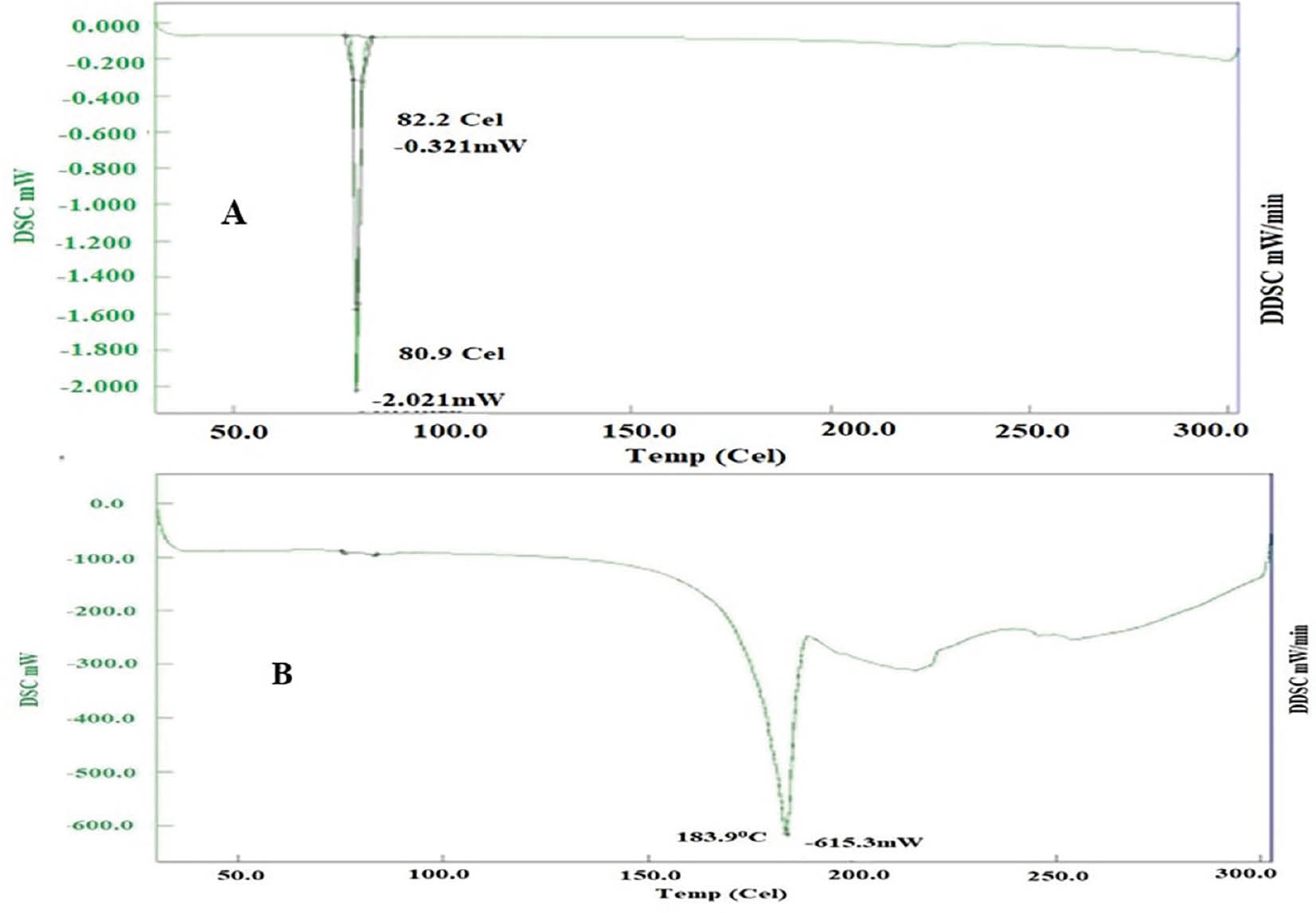
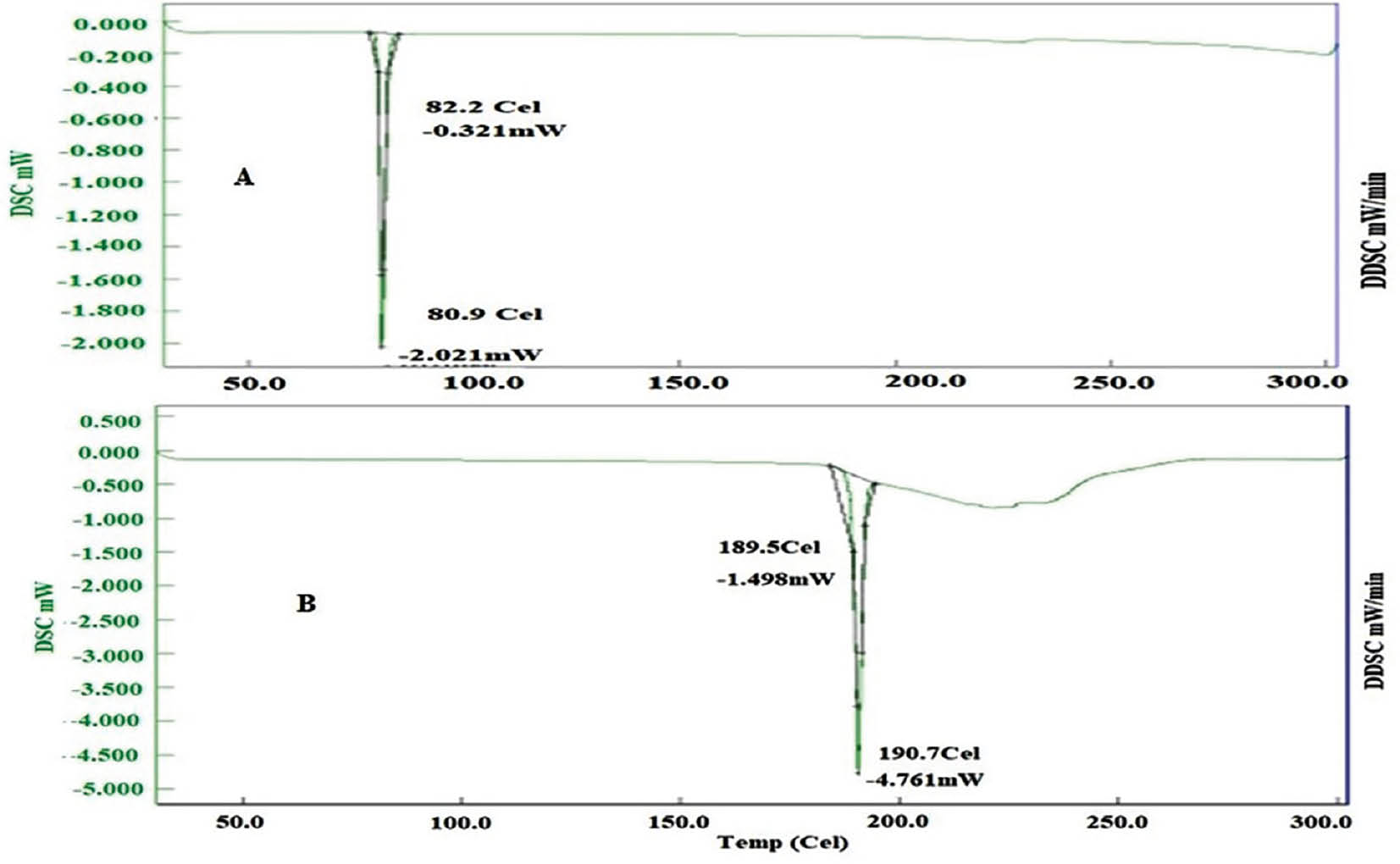
DSC study
Pure FF displayed an endothermic peak at 80.9°C, matching its known melting point. In contrast, spray-dried S-SMEDDS with FF showed an endothermic peak at 183.9°C, thus indicating successful FF encapsulation within the formulation. Similarly, the thermal analysis of pure FF, as illustrated in Figures 6 and 7, exhibited a well-defined endothermic peak at 80.9°C, aligning with FF’s melting point. However, when FF was incorporated into freeze-dried S-SMEDDS, a slightly higher endothermic peak at 190.7°C was observed. This slight alteration in the endothermic peak further demonstrated the effective encapsulation of FF within the formulation.
Figure 6 DSC thermogram of FF-API and FF-loaded SEMDDS prepared by spray-drying.
Figure 7 DSC thermogram of FF-API and FF-loaded SEMDDS prepared by freeze-drying.
Saturation solubility study
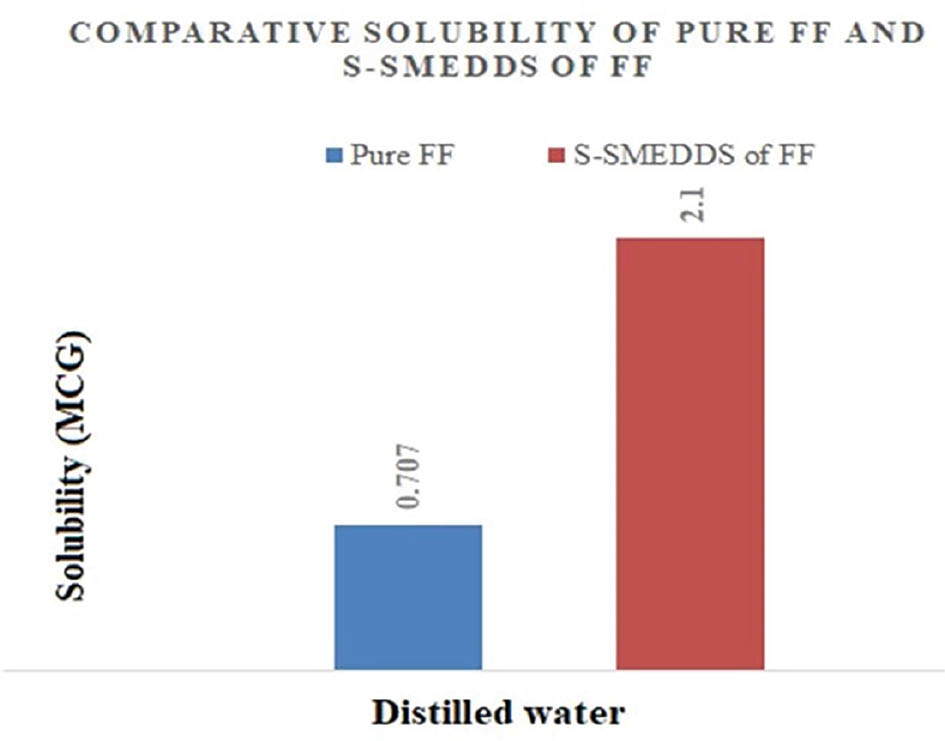
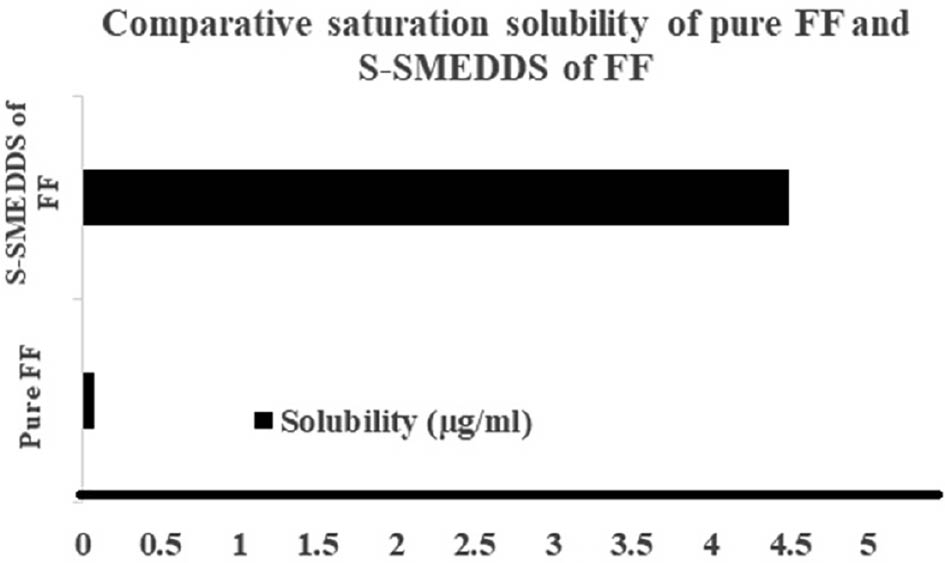
FF has a low aqueous solubility of 0.707 μg/ml. In contrast, the S-SMEDDS loaded with FF demonstrated a significant solubility enhancement, reaching 2.1 μg/ml with spray-drying and 4.5 μg/ml with freeze-drying. These enhancements, illustrated in Figures 8 and 9, were attributed to the amorphous nature of the formulations, which achieved nearly 3-fold and 58-fold increases in solubility, respectively.
Figure 8 Saturation solubility of samples prepared by spray-drying.
Figure 9 Saturation solubility of samples prepared by freeze-drying.
In vitro dissolution study of tablets prepared from FF-loaded S-SMEDDS by spray-drying
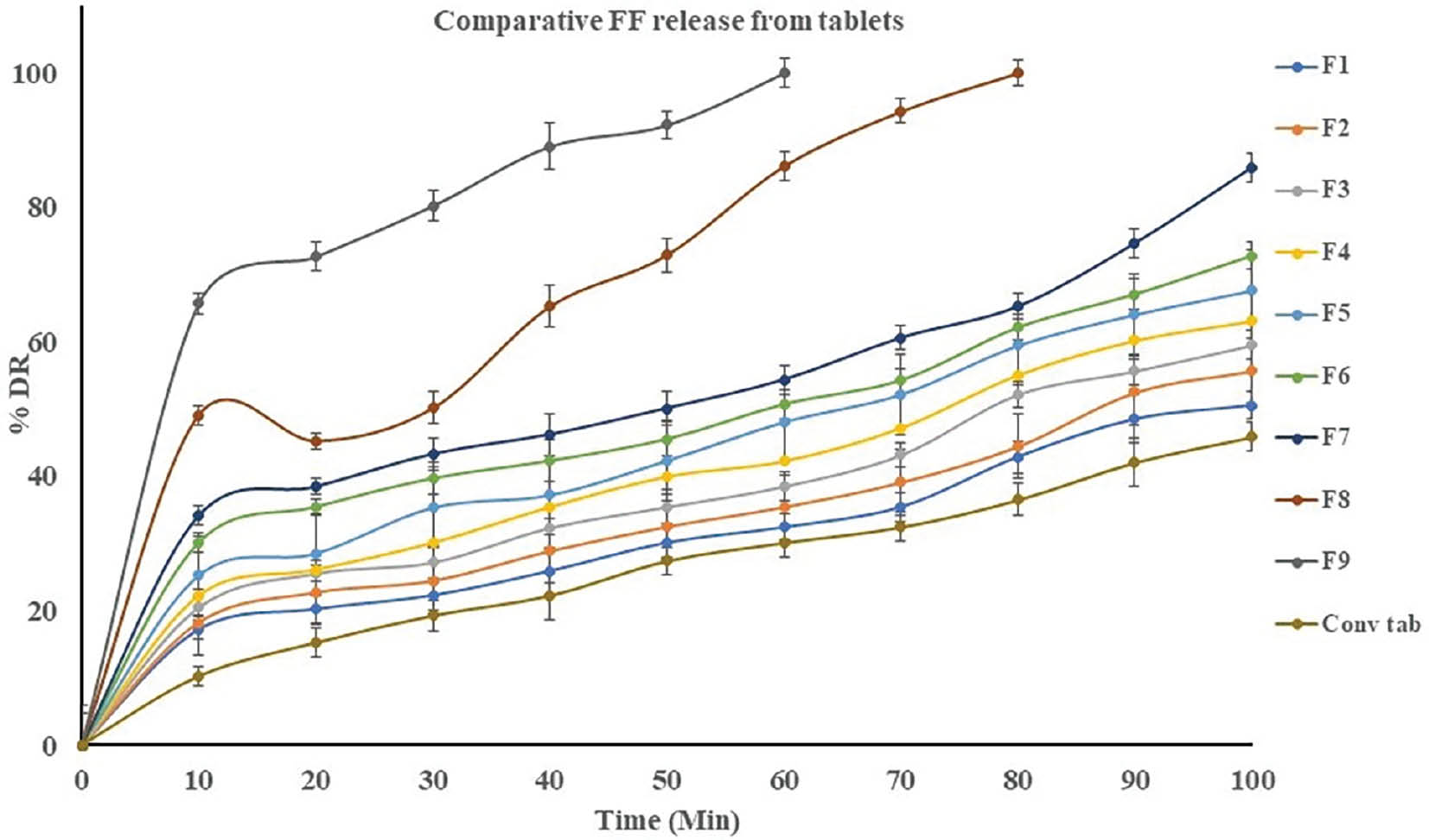
A dissolution study conducted in pH 6.8 PBS buffer revealed that S-SMEDDS tablets exhibited a notably faster drug release profile than conventional tablets. Among the different formulations, the F9 batch displayed an exceptionally rapid dissolution profile in 60 minutes. The immediate dissolution was attributed to two key factors: the existence of S-SMEDDS loaded with FF and the inclusion of CCS, an effective super disintegrating agent that was crucial for the rapid disintegration of the tablet on contact with the release medium. Simultaneously, the incorporation of S-SMEDDS in the tablet was critical in enhancing FF solubility in the pH 6.8 PBS buffer. This increased solubility resulted in rapid release of FF in the buffer. A side-by-side comparison of tablet dissolution profiles is provided in Table 5 and Figure 10.
Table 5 Cumulative Percentage Drug Release from Tablets Compressed with Spray Dried Product
| Time (min) | Batch | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | Conv. Tab | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 17.2 | 18.27 | 20.47 | 22.27 | 25.25 | 30.12 | 34.11 | 49.11 | 65.71 | 10.25 |
| 20 | 20.22 | 22.71 | 25.51 | 26.11 | 28.51 | 35.42 | 38.39 | 45.26 | 72.67 | 15.24 |
| 30 | 22.27 | 24.46 | 27.18 | 30.12 | 35.34 | 39.67 | 43.27 | 50.12 | 80.25 | 19.27 |
| 40 | 25.9 | 28.89 | 32.34 | 35.45 | 37.21 | 42.28 | 46.19 | 65.25 | 89.09 | 22.25 |
| 50 | 30.12 | 32.51 | 35.44 | 39.98 | 42.34 | 45.51 | 50.09 | 72.89 | 92.28 | 27.41 |
| 60 | 32.41 | 35.44 | 38.51 | 42.27 | 48.11 | 50.71 | 54.34 | 86.11 | 100 | 30.12 |
| 70 | 35.44 | 39.11 | 43.21 | 47.23 | 52.21 | 54.28 | 60.56 | 94.28 | – | 32.45 |
| 80 | 42.9 | 44.47 | 52.21 | 55.11 | 59.51 | 62.13 | 65.21 | 100 | – | 36.52 |
| 90 | 48.54 | 52.51 | 55.71 | 60.24 | 64.11 | 67.09 | 74.65 | – | – | 42.11 |
| 100 | 50.55 | 55.7 | 59.56 | 63.11 | 67.67 | 72.81 | 85.9 | – | – | 45.9 |
Figure 10 Comparative cumulative percentage drug release from tablets compressed with spray dried product.
In vitro dissolution study of tablets prepared from FF-loaded S-SMEDDS by freeze-drying
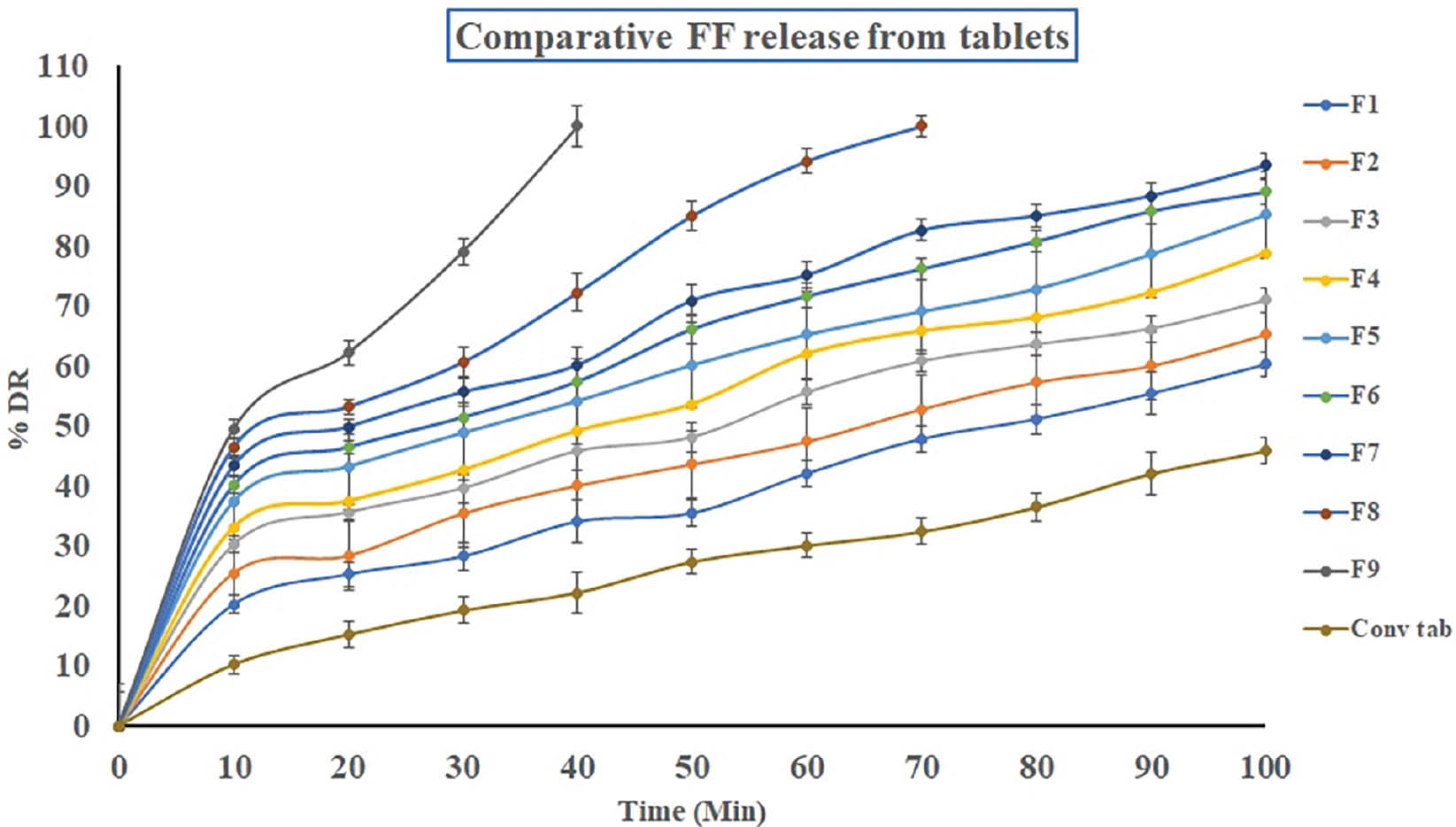
Tablets comprising lyophilized S-SMEDDS showed significantly faster FF release than conventional tablets. The F9 formulation exhibited exceptional instant release, with 100% of the FF released in 40 minutes. The accelerated dissolution was attributed to the presence of both CCS and S-SMEDDS facilitating rapid disintegration and enhancing FF solubility in pH 6.8 PBS buffer. Detailed dissolution profiles are shown in Table 6 and Figure 11.
Table 6 Cumulative Percentage Drug Release from Tablets Compressed with Freeze Dried Product
| Time (min) | Batch | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | Conv. Tab | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 20.25 | 25.51 | 30.24 | 33.11 | 37.42 | 40.16 | 43.34 | 46.46 | 49.51 | 10.25 |
| 20 | 25.3 | 28.42 | 35.67 | 37.51 | 43.26 | 46.51 | 49.9 | 53.21 | 62.23 | 15.24 |
| 30 | 28.29 | 35.46 | 39.65 | 42.67 | 48.9 | 51.51 | 55.71 | 60.67 | 79.11 | 19.27 |
| 40 | 34.11 | 40.11 | 45.87 | 49.26 | 54.2 | 57.47 | 60.21 | 72.26 | 100 | 22.25 |
| 50 | 35.51 | 43.67 | 48.21 | 53.71 | 60.2 | 66.2 | 70.97 | 85.11 | 27.41 | |
| 60 | 42.11 | 47.45 | 55.67 | 62.11 | 65.26 | 71.71 | 75.2 | 94.2 | 30.12 | |
| 70 | 47.9 | 52.89 | 60.9 | 65.89 | 69.11 | 76.24 | 82.67 | 100 | 32.45 | |
| 80 | 51.18 | 57.41 | 63.71 | 68.12 | 72.87 | 80.82 | 85.11 | 36.52 | ||
| 90 | 55.46 | 60.11 | 66.29 | 72.27 | 78.65 | 85.9 | 88.46 | 42.11 | ||
| 100 | 60.31 | 65.34 | 71 | 78.9 | 85.23 | 89.11 | 93.45 | 45.9 | ||
Figure 11 Comparative cumulative percentage drug release from tablets compressed with freeze dried product.
Discussion
FF, a BCS class II drug with low aqueous solubility, poses formulation challenges for effective drug delivery [14, 15]. In this study, various formulations of S-SMEDDS were explored to enhance the solubility and dissolution rate of FF, and increase its bioavailability. Solubility studies of FF in different oils, surfactants, and co-surfactants revealed significant variations in solubility across the tested media. Notably, oleic oil, Tween 80, and Transcutol HP exhibited the highest solubility among oils, surfactants, and co-surfactants, respectively. These findings may aid in selecting appropriate components for the formulation of S-SMEDDS that directly affect drug solubility and subsequent dissolution behavior.
The evaluation of S-SMEDDS formulations, conducted through drug content analysis, SEM imaging, XRD, DSC, and saturation solubility studies, provided comprehensive insights into the morphological characteristics and solid-state properties of FF-loaded S-SMEDDS. SEM analysis revealed the morphology of FF-loaded S-SMEDDS, which displayed smooth surfaces with various particle shapes, thus indicating successful encapsulation of FF within the formulation. XRD analysis confirmed the transition of FF from a crystalline state to an amorphous state within the S-SMEDDS, thereby enhancing the dissolution rates and bioavailability.
DSC studies further supported the successful encapsulation of FF within the S-SMEDDS formulations, as evidenced by altered endothermic peak temperatures with respect to those for pure FF, thus indicating changes in the crystalline structure. Saturation solubility studies demonstrated substantially greater solubility of FF-loaded S-SMEDDS than pure FF; spray-dried and freeze-dried formulations exhibited approximately 3-fold and 58-fold enhancements, respectively. These enhancements were recognized near the amorphous nature of the formulations and significantly improved FF solubility.
In vitro dissolution studies of tablets prepared from FF-loaded S-SMEDDS revealed significantly faster drug release profiles than observed for conventional tablets. The rapid dissolution was attributed to the incidence of S-SMEDDS and the inclusion of super-disintegrating agents, which facilitated rapid disintegration and enhanced FF solubility.
Freeze-drying is favored over spray-drying for preparing FF-loaded S-SMEDDS for several reasons. SEM analysis showed smoother surfaces and successful encapsulation of FF, thereby indicating better preservation of structural integrity. XRD analysis revealed a more pronounced transition of FF into an amorphous state with freeze-dried than spray-dried formulations; this state was crucial for enhancing dissolution rates. Additionally, freeze-dried formulations exhibited 58-fold greater solubility than spray-dried formulations, owing to superior amorphous state preservation. Hence, freeze-drying enables enhanced amorphous state preservation and solubility, and therefore is best suited for preparation of FF-loaded S-SMEDDS.
This research should be applicable to other BCS class II drugs beyond FF. The findings regarding formulation design, solubility enhancement, and dissolution kinetics can be generalized to similar drugs with low aqueous solubility [28]. However, specific adjustments might be necessary according to the physicochemical properties of the particular drug molecule. Overall, the principles and methods outlined in this study provide a useful framework for increasing the bioavailability of other BCS class II drugs.
The significant enhancement in solubility and dissolution of FF was attributed to several key factors in the techniques applied. First, the careful selection of optimal components, including specific oils, surfactants (e.g., Tween 80), and co-surfactants (e.g., Transcutol HP), were critical in enhancing FF solubility. Second, the formulation design, notably the development of S-SMEDDS, facilitated efficient FF incorporation, thus increasing FF solubility and dissolution. Additionally, both freeze-drying and spray-drying techniques effectively encapsulated FF within the S-SMEDDS, thereby preserving drug integrity and enhancing bioavailability. X-ray diffraction analysis confirmed FF’s transition from a crystalline to an amorphous state within the S-SMEDDS, thereby further increasing dissolution rates. Notably, freeze-dried formulations exhibited superior dissolution rates, owing to the increased surface area. In summary, the combined effects of optimal components, formulation design, encapsulation techniques, and promotion of an amorphous state together markedly enhanced FF solubility and dissolution.
This study was aimed at overcoming the challenges associated with FF. By exploring various S-SMEDD formulations, we sought to enhance FF’s solubility and dissolution rate, and ultimately increase its bioavailability. Initial solubility investigations across various oils, surfactants, and co-surfactants were conducted to select the optimal components for S-SMEDDS formulation. Subsequent evaluations, including SEM imaging, XRD, DSC, and saturation solubility studies, provided insights into the morphology and solid-state properties of FF-loaded S-SMEDDS. The analyses confirmed successful FF encapsulation and transition to an amorphous state within S-SMEDDS, thus increasing dissolution rates and bioavailability. Both spray-dried and freeze-dried formulations exhibited significant enhancements in FF solubility and consequently have potential for enhancing drug delivery. Finally, in vitro dissolution studies indicated faster drug release profiles for tablets prepared from FF-loaded S-SMEDDS than conventional tablets. Therefore, S-SMEDDS holds promise for improving FF delivery, and this study lays the groundwork for similar delivery of BCS class II drugs.
Overall, the outcomes from these investigations underscore the importance of formulation design in augmenting the solubility and dissolution kinetics of poorly soluble drugs such as FF. The preparation process significantly influences the performance of S-SMEDDS: freeze-dried formulations exhibit higher dissolution rates because of increased surface area. However, economic and environmental considerations must also be considered, and spray-dried formulations offer potential advantages in terms of cost-effectiveness and sustainability.
Conclusion
The method used to prepare S-SMEDDS significantly affects FF dissolution performance, including saturation solubility, surface area, and drug-polymer interactions. Freeze-dried S-SMEDDS showed higher dissolution rates than spray-dried S-SMEDDS, owing to increased surface area. The solid-state characteristics depended on the polymer concentration and processing method. Whereas freeze-drying enhanced dissolution, production costs must additionally be considered. Alternatively using aqueous solvent mixtures in spray-dried S-SMEDDS would offer economic and environmental benefits.
Conflicts of interest
None.
References
- Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods 2000;44(1):235-49. [PMID: 11274893 DOI: 10.1016/S1056-8719(00)00107-6]
- Narala S, Komanduri N, Nyavanandi D, Youssef AA, Mandati P, et al. Hard gelatin capsules containing hot melt extruded solid crystal suspension of carbamazepine for improving dissolution: preparation and in vitro evaluation. J Drug Deliv Sci Technol 2023;82:104384. [PMID: 37124158 DOI: 10.1016/j.jddst.2023.104384]
- Corrigan OI, Timoney RF. The influence of polyvinylpyrrolidone on the dissolution properties of hydroflumethiazide. J Pharm Pharmacol 1975;27(10):759-64. [PMID: 241789 DOI: 10.1111/j.2042-7158.1975.tb09396.x]
- Zhang J, Huang J, Huang K, Zhang J, Li Z, et al. Egg white coated alginate nanoparticles with electron sprayer for potential anticancer application. Int J Pharm 2019;564:188-96. [PMID: 30999047 DOI: 10.1016/j.ijpharm.2019.04.045]
- Mosharraf M, Nyström C. The effect of dry mixing on the apparent solubility of hydrophobic, sparingly soluble drugs. Eur J Pharm Sci 1999;9(2):145-56. [PMID: 10620727 DOI: 10.1016/S0928-0987(99)00043-3]
- Panchagnula R, Bhardwaj V. Effect of amorphous content on dissolution characteristics of rifampicin. Drug Dev Ind Pharm 2008;34(6):642-9. [PMID: 18568915 DOI: 10.1080/03639040701842451]
- Wicaksono Y, Rosidi VA, Saragih SY, Fauziah LS, Setyawan D. Preparation of spray dried coamorphous solids to improve the solubility and dissolution rate of atorvastatin calcium. J Teknol 2021;83(2):77-83. [DOI: 10.11113/jurnalteknologi.v83.14706]
- Qiu Y, Chen Y, Zhang GG, Yu L, Mantri RV, editors. Developing solid oral dosage forms: pharmaceutical theory and practice. Elsevier Academic Press, London, UK; 2016. pp. 25-60.
- Patel AR, Vavia PR. Preparation and in vivo evaluation of SMEDDS (self-microemulsifying drug delivery system) containing fenofibrate. AAPS J 2007;9:E344-52. [PMID: 18170981 DOI: 10.1208/aapsj0903041]
- Shah NH, Carvajal MT, Patel CI, Infeld MH, Malick AW. Self-emulsifying drug delivery systems (SEDDS) with polyglycolyzed glycerides for improving in vitro dissolution and oral absorption of lipophilic drugs. Int J Pharm 1994;106(1):15-23. [DOI: 10.1016/0378-5173(94)90271-2]
- Nyavanandi D, Mandati P, Narala S, Alzahrani A, Kolimi P, et al. Twin screw melt granulation: a single step approach for developing self-emulsifying drug delivery system for lipophilic drugs. Pharmaceutics 2023;15(9):2267. [DOI: 10.3390/pharmaceutics15092267]
- Zhu JX, Tang D, Feng L, Zheng ZG, Wang RS, et al. Development of self-microemulsifying drug delivery system for oral bioavailability enhancement of berberine hydrochloride. Drug Dev Ind Pharm 2013;39(3):499-506. [PMID: 22563917 DOI: 10.3109/03639045.2012.683875]
- Kasim NA, Whitehouse M, Ramachandran C, Bermejo M, Lennernäs H, et al. Molecular properties of WHO essential drugs and provisional biopharmaceutical classification. Mol Pharm 2004;1(1):85-96. [PMID: 15832504 DOI: 10.1021/mp034006h]
- Patel AR, Vavia PR. Effect of hydrophilic polymer on solubilization of fenofibrate by cyclodextrin complexation. J Incl Phenom Macrocycl Chem 2006;56(1):247-51. [DOI: 10.1007/s10847-006-9091-4]
- Law D, Wang W, Schmitt EA, Qiu Y, Krill SL, et al. Properties of rapidly dissolving eutectic mixtures of poly(ethylene glycol) and fenofibrate: the eutectic microstructure. J Pharm Sci 2003;92(3):505-15. [PMID: 12587112 DOI: 10.1002/jps.10324]
- Curtet B, Teillaud E, Renault P, inventors. Fournier innovation et Synergie SA, assignee. Novel dosage form of fenofibrate. United States patent US 4,895,726. 23 Jan 1990.
- Shah AV, Desai HH, Thool P, Dalrymple D, Serajuddin AT. Development of self-microemulsifying drug delivery system for oral delivery of poorly water-soluble nutraceuticals. Drug Dev Ind Pharm 2018;44(6):895-901. [PMID: 29254385 DOI: 10.1080/03639045.2017.1419365]
- Xiumin LI, Man GE, Minzi LU, Yinghua J, Dongqin Q. The in vitro and in vivo evaluation of fenofibrate with a self-microemulsifying formulation. Curr Drug Deliv 2015;12(3):308-13. [PMID: 260545347]
- Kanaujia P, Ng WK, Tan RB. Solid self-emulsifying drug delivery system (S-SEDDS) for improved dissolution rate of fenofibrate. J Microencapsul 2014;31(3):293-8. [PMID: 24156747 DOI: 10.3109/02652048.2013.843601]
- Manohari PJ, Kunchitapatham J, Seshadri VC, Muthusamy C. Development of self-micro emulsifying drug delivery system: application to pimozide delivery. Der Pharmacia Sinica 2013;4(6):48–58.
- Shah AV, Serajuddin AT. Development of solid self-emulsifying drug delivery system (SEDDS) I: use of poloxamer 188 as both solidifying and emulsifying agent for lipids. Pharm Res 2012;29:2817-32. [PMID: 22371051 DOI: 10.1007/s11095-012-0704-x]
- Li P, Hynes SR, Haefele TF, Pudipeddi M, Royce AE, et al. Development of clinical dosage forms for a poorly water-soluble drug II: formulation and characterization of a novel solid microemulsion preconcentrate system for oral delivery of a poorly water-soluble drug. J Pharm Sci 2009;98(5):1750-64. [PMID: 18781639 DOI: 10.1002/jps.21547]
- Jannin V, Musakhanian J, Marchaud D. Approaches for the development of solid and semi-solid lipid-based formulations. Adv Drug Deliv Rev 2008;60(6):734-46. [PMID: 18045728 DOI: 10.1016/j.addr.2007.09.006]
- Čerpnjak K, Zvonar A, Vrečer F, Gašperlin M. Characterization of naproxen-loaded solid SMEDDSs prepared by spray drying: the effect of the polysaccharide carrier and naproxen concentration. Int J Pharm 2015;485(1-2):215-28. [PMID: 25772420 DOI: 10.1016/j.ijpharm.2015.03.015]
- Paudel A, Worku ZA, Meeus J, Guns S, Van den Mooter G. Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: formulation and process considerations. Int J Pharm 2013;453(1):253-84. [PMID: 22820134 DOI: 10.1016/j.ijpharm.2012.07.015]
- Yousaf AM, Kim DW, Oh YK, Yong CS, Kim JO, et al. Enhanced oral bioavailability of fenofibrate using polymeric nanoparticulated systems: physicochemical characterization and in vivo investigation. Int J Nanomedicine 2015;10:1819-30. [PMID: 25784807 DOI: 10.2147/IJN.S78895]
- Butreddy A, Sarabu S, Almutairi M, Ajjarapu S, Kolimi P, et al. Hot-melt extruded hydroxypropyl methylcellulose acetate succinate based amorphous solid dispersions: impact of polymeric combinations on supersaturation kinetics and dissolution performance. Int J Pharm 2022;615:121471. [PMID: 35041915 DOI: 10.1016/j.ijpharm.2022.121471]
- De Mohac LM, Raimi-Abraham B, Caruana R, Gaetano G, Licciardi M. Multicomponent solid dispersion a new generation of solid dispersion produced by spray-drying. J Drug Deliv Sci Technol 2020;57:101750. [DOI: 10.1016/j.jddst.2020.101750]