Can Nanocrystals Help Create Our Dream Cosmetics?
1Department of Biology, Faculty of Science and Technology, Universitas Airlangga, Jl. Ir. H. Soekarno, Mulyorejo Surabaya 60115, Indonesia
*Correspondence to: Ummi Zubaidah, Department of Biology, Faculty of Science and Technology, Universitas Airlangga, Jl. Ir. H. Soekarno, Mulyorejo Surabaya 60115, Indonesia, Tel: +62-0311-5936501; Fax: +62-0311-5936502, E-mail: ummi.zubaidah-2022@fst.unair.ac.id
Received: April 6 2024; Revised: May 2 2024; Accepted: May 21 2024; Published Online: July 9 2024
Cite this paper:
Zubaidah U. Can Nanocrystals Help Create Our Dream Cosmetics? BIO Integration 2024; 5: 1–3.
DOI: 10.15212/bioi-2024-0014. Available at: https://bio-integration.org/
Download citation
© 2024 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
Cosmetics have evolved beyond mere skin beautifying agents. Cosmetics are now combined with active ingredients to support skin health. This evolution underpins the emergence of dream cosmetics, where innovation is continuously pursued. Indeed, nanotechnology has responded in force to the notion of dream cosmetics. Nanocrystals are expected to overcome the barriers posed by issues, such as poor solubility of bioactive agents and skin penetration. Moreover, nanocrystals exhibit multiple features, making dream cosmetics achievable. However, the clinical translation of nanocrystals is hampered by concern of negative host responses associated with long-term use. Accordingly, screening by integrated omics is needed because because multiple omics offer comprehensive nanotoxicity evaluation by identifying new molecular pathways and toxicity markers.
Keywords
Cosmetics, nanocrystal, omics, solubility, toxicity.
Everyone needs cosmetics, which has facilitated the continuous evolution of cosmetics across time and space. The use of cosmetics dates back to 10,000 BCE when Egyptians used cosmetics to enhance beauty, elevate social status, and for spiritual purposes. Recent developments have made cosmetics more accessible to people in all social strata and genders [1]. With the rise in digitalization, consumers are increasingly conscious about beauty, essentially making cosmetics a necessity, while advances in cosmetic manufacturing technology have ensured that products meet consumer needs. Cosmetics have become a significant economic product with the worldwide cosmetic market valued at $380.2 billion in 2019 and predicted to reach $463.5 billion by 2027 [2, 3]. There are many types of cosmetics with specific functions. Some cosmetic products have a single function (e.g., cleansing, cetaphil gentle skin cleanser; brightening, whitelab brightening day cream; moisturizing, olay regenerist retinol 24 night moisturizer; hydrating, wardah aloe multifunction gel; protective, biore UV aqua rich watery essence; brightening and hydrating agent, gush beauty glow getter moisturizer; cleansing, exfoliating, and brightening agent, ras luxury oils polish brightening & exfoliating face wash cleanser; nourishing and brightening, D’you hustle serum), while other types of cosmetics have double or even multiple functions. Dozens of cosmetics innovations are ongoing to meet consumer needs and satisfaction. Specifically, cosmeceuticals are a combination of cosmetic and therapeutic formulations with active ingredients that improve and maintain skin health after topical treatment [4]. Additionally, nanotechnology has been introduced to cosmetic formulations, offering better performance. The idea of reducing cosmetic materials into a nanoscale (i.e., one billionth of a meter) enhance skin penetration, provide a long-lasting effect, offer better UV protection, and improve finish quality. These advances are expected to benefit the business market [2]. As a result of the connection with the business market, the concept of dream cosmetics begins to resonate.
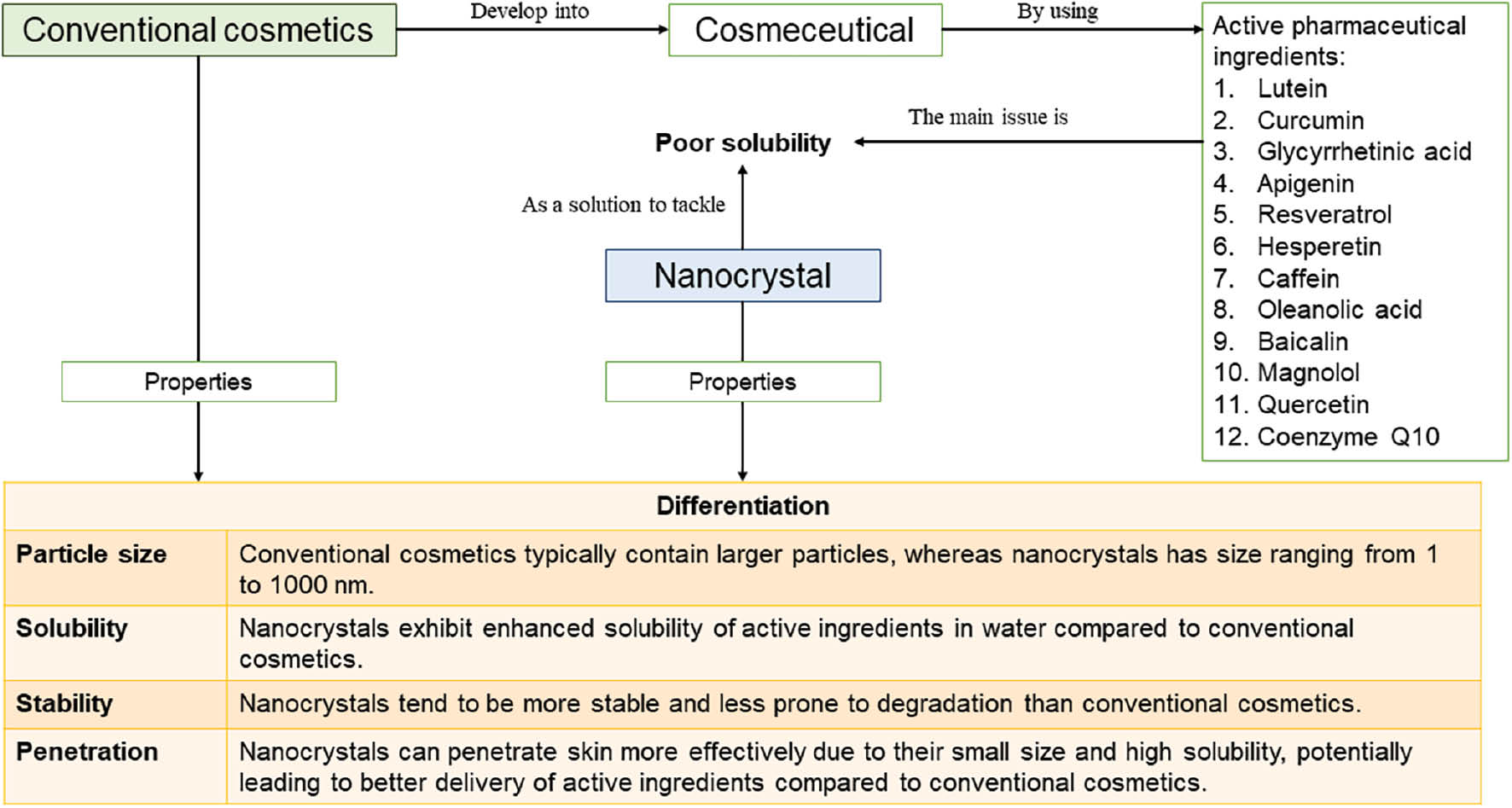
The term, dream cosmetic, defines agents that exhibit multiple enhanced bioactivities, especially antioxidant and anti-aging activities. Unfortunately, active ingredients used in cosmeceuticals, especially those from natural products, have low solubility in water, which leads to low bioavailability, skin penetration, and bioactivity [4]. This limitation diminishes the dream cosmetic formulation. In this case, nanocrystals, as one of the nanotechnology-based delivery systems, may address the problem by enhancing the water solubility of active ingredients (see Figure 1). Nanocrystals are a type of nanoparticle composed of thousands of molecules joined together by surfactants without any polymers in a fixed pattern to form a group ranging in size from 1–1000 nm. Nanocrystals have a straightforward structure that enhances skin penetration against the stratum corneum barrier, making the nanocrystal more valuable in addition to improving solubility. Furthermore, preparation of nanocrystals is also simpler than other formulations [5].
Figure 1 Nanocrystal-based cosmetics offer better performance over conventional cosmetics because solubility of active pharmaceutical ingredients is improved.
The combination of media milling and homogenization techniques can produce nanocrystals, while the introduction into topical formulations, such as creams, lotions, and liposomal dispersions, has been done repeatedly [5, 6]. Nanocrystals are reported to enhance the bioactivity performance of several active ingredients, such as flavonoids, lutein, beta-carotene, and coenzyme Q10, by improving water solubility [4]. The first marketed products include Juvedical® Age-decoder Cream, which is made from rutin by Juvena (St. Margrethen, Switzerland), and Cellular Serum Platinum Rare, which is made from hesperidin by La Prairie (Montreux, Switzerland). Both formulations bear antioxidant, chelating, and anti-aging properties [5], and both are followed by formulations of other nanocrystal-related cosmetics [7–15]. Table 1 provides further examples of bioactive compounds made into nanocrystal. Accordingly, nanocrystals enable cosmetic production with multiple performances.
Table 1 Record of Nanocrystal Application in Biomedical Research
| Nanocrystal-Containing Active Pharmaceutical Ingredient | Benefit of Formulation | Reference |
|---|---|---|
| Quercetin | Enhanced in vitro antioxidant activity | [7] |
| Apigenin | Rapid dissolution rate | [8] |
| Baicalein | Improved stability | [9] |
| Rutin | High dissolution and bioavailability | [10] |
| High ex vitro skin permeability (3–5-fold) and antioxidant activity (60%) | [11] | |
| Lutein | Better skin penetration (60%) | [12] |
| Hesperetin | Increased release rate (1.79-fold), and anti-inflammatory and antioxidant enzyme activities | [13] |
| Improved skin penetration and stability of active product | [14] | |
| Hesperidin | With long-lasting antioxidant activity and stability enhancement | [15] |
In addition to the benefits of nanocrystals for cosmetics, nanocrystal prominence remains confined largely to research with clinical application still relatively limited. Not only are there technological bottlenecks and rapid expansion of the market scale but legal approval is lagging and concern of toxicity with long-term use has hampered nanocrystal application [5]. Once again, those issues cast doubt on the use of nanocrystals in formulating dream cosmetics. The alteration in physicochemical properties due to nanosize leads to toxicity and warrants thorough study.
To effectively address issues of toxicity, integrated omics studies have emerged as a prudent strategy. Omics represent a holistic assessment of biological molecules that shed light on the diverse roles within biological processes. Omics encompass genomics, epigenomics, miRNomics, transcriptomics, proteomics, phosphoproteomics, and metabolomics. In contrast, integrated or multi-omics methodologies integrate single omics approaches. Compared to singular omics analyses, integrated omics offer a more nuanced and comprehensive understanding of nanotoxicity, identifying novel molecular pathways and key markers of toxicity with heightened sensitivity. This integrative approach facilitates the seamless flow of information across omics layers, which bridges the divide between genotype and phenotype in the context of nanotoxicity. Numerous studies have corroborated the efficacy of this approach [16–18], underscoring the manifold benefits. Therefore, utilization of integrated omics to assess nanocrystal toxicity is imperative. Furthermore, in the endeavor to advance nanocrystal cosmetics into nanocrystal-based cosmeceuticals, integrated omics unveil intricate toxicological mechanisms and cellular responses that conventional nanotoxicity evaluations may not detect. Moreover, in the pursuit of elevating nanocrystal-based cosmetics to nanocrystal-based cosmeceuticals, this approach provides nuanced insight into the temporal aspects of nanotoxicity, encompassing both early acute reactions and delayed responses to nanocrystals. Ultimately, integrated omics may rapidly pave the way for transforming the concept of dream cosmetics into a reality.
In conclusion, nanocrystals can contribute to formulating dream cosmetics. These products offer dual benefits, combining skin beautification with health maintenance, without concerns about the solubility of active ingredients. Toxicity-related issues associated with nanocrystals can be addressed through comprehensive toxicity screening utilizing integrated omic studies.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Nayak M, Ligade VS. History of cosmetic in Egypt, India, and China. J Cosmet Sci 2021;72(4):432-41. [PMID: 35262483]
- Salvioni L, Morelli L, Ochoa E, Labra M, Fiandra L, et al. The emerging role of nanotechnology in skincare. Adv Colloid Interface Sci 2021;293:102437. [PMID: 34023566 DOI: 10.1016/j.cis.2021.102437]
- Allied Market Research. Cosmetics market: global opportunity analysis and industry forecast, 2021-2027. 2021; p. 338.
- Gupta V, Mohapatra S, Mishra H, Farooq U, Kumar K, et al. Nanotechnology in cosmetics and cosmeceuticals—a review of latest advancements. Gels 2022;8(3):173. [PMID: 35323286 DOI: 10.3390/gels8030173]
- Liu Y, Zhao J, Chen J, Miao X. Nanocrystals in cosmetics and cosmeceuticals by topical delivery. Colloids Surf B Biointerfaces 2023;227:113385. [PMID: 37270904 DOI: 10.1016/j.colsurfb.2023.113385]
- Paredes AJ, McKenna PE, Ramöller IK, Naser YA, Volpe-Zanutto F, et al. Microarray patches: poking a hole in the challenges faced when delivering poorly soluble drugs. Adv Funct Mater 2021;31(1):2005792. [DOI: 10.1002/adfm.202005792]
- Sahoo NG, Kakran M, Shaal LA, Li L, Müller RH, et al. Preparation and characterization of quercetin nanocrystals. J Pharm Sci 2011;100(6):2379-90.
- Al Shaal L, Shegokar R, Müller RH. Production and characterization of antioxidant apigenin nanocrystals as a novel UV skin protective formulation. Int J Pharm 2011;420(1):133-40. [PMID: 21871547 DOI: 10.1016/j.ijpharm.2011.08.018]
- Zhang J, Lv H, Jiang K, Gao Y. Enhanced bioavailability after oral and pulmonary administration of baicalein nanocrystal. Int J Pharm 2011;420(1):180-8. [PMID: 21878378 DOI: 10.1016/j.ijpharm.2011.08.023]
- Mauludin R, Müller RH, Keck CM. Development of an oral rutin nanocrystal formulation. Int J Pharm 2009;370(1-2):202-9. [PMID: 19114097 DOI: 10.1016/j.ijpharm.2008.11.029]
- Pyo S, Meinke M, Keck C, Müller R. Rutin—increased antioxidant activity and skin penetration by nanocrystal technology (smartCrystals). Cosmetics 2016;3(1):9. [DOI: 10.3390/cosmetics3010009]
- Mitri K, Shegokar R, Gohla S, Anselmi C, Müller RH. Lutein nanocrystals as antioxidant formulation for oral and dermal delivery. Int J Pharm 2011;420(1):141-6. [PMID: 21884768 DOI: 10.1016/j.ijpharm.2011.08.026]
- Shete G, Pawar YB, Thanki K, Jain S, Bansal AK. Oral bioavailability and pharmacodynamic activity of hesperetin nanocrystals generated using a novel bottom-up technology. Mol Pharm 2015;12(4):1158-70. [PMID: 25785392 DOI: 10.1021/mp5008647]
- Romero GB, Chen R, Keck CM, Müller RH. Industrial concentrates of dermal hesperidin smartCrystals® – production, characterization & long-term stability. Int J Pharm 2015;482(1-2):54-60. [PMID: 25448556 DOI: 10.1016/j.ijpharm.2014.11.039]
- Varghese JJ, Mallya R, Varghese J. Formulation development and evaluation of antioxidant potential of hesperidin nanocrystals. World J Pharm Res 2015;4(8):1149-70.
- Shin TH, Ketebo AA, Lee DY, Lee S, Kang SH, et al. Decrease in membrane fluidity and traction force induced by silica-coated magnetic nanoparticles. J Nanobiotechnology 2021;19(1):21. [PMID: 33430909 DOI: 10.1186/s12951-020-00765-5]
- Shim W, Paik MJ, Nguyen DT, Lee JK, Lee Y, et al. Analysis of changes in gene expression and metabolic profiles induced by silica-coated magnetic nanoparticles. ACS Nano 2012;6(9):7665-80. [PMID: 22830605 DOI: 10.1021/nn301113f]
- Shin TH, Lee DY, Lee HS, Park HJ, Jin MS, et al. Integration of metabolomics and transcriptomics in nanotoxicity studies. BMB Rep 2018;51(1):14-20. [PMID: 29301609 DOI: 10.5483/bmbrep.2018.51.1.237]