Health benefits of liquid smoke from various biomass sources: a systematic review
1Department of Oral Medicine, Faculty of Dental Medicine, Universitas Airlangga, Surabaya 60132, Indonesia
2Department of Chemical and Materials Engineering, The University of Auckland, Auckland 1010, New Zealand
3Circular Innovations (CIRCUIT) Research Centre, The University of Auckland, Auckland 1010, New Zealand
4NGĀ ARA WHETŪ Centre for Climate, Biodiversity and Society, The University of Auckland, Auckland 1010, New Zealand
5Department of Biology, Faculty of Science and Technology, Universitas Airlangga, Surabaya 60132, Indonesia
6Faculty of Dental Medicine, Universitas Airlangga, Surabaya 60132, Indonesia
7Research Centre for Chemistry, National Research and Innovation Agency, Kawasan PUSPIPTEK – Serpong, Tangerang Selatan, Banten, Indonesia
8Department of Pharmaceutical Technology, Sekolah Tinggi Ilmu Farmasi (STIFAR), Riau 28292, Indonesia
*Correspondence to: Professor Diah Savitri Ernawati, DDS., PhD, Department of Oral Medicine, Faculty of Dental Medicine, Universitas Airlangga, Jalan Prof. Dr. Moestopo No 47 Surabaya 60132, Indonesia, Tel.: +6231-5030255, E-mail: diah-s-e@fkg.unair.ac.id; Meircurius Dwi Condro Surboyo, DDS, MDS, Department of Oral Medicine, Faculty of Dental Medicine, Universitas Airlangga, Jalan Prof. Dr. Moestopo No 47 Surabaya 60132, Indonesia, Tel.: +6231-5030255, E-mail: meircurius-2015@fkg.unair.ac.id
Received: September 7 2024; Revised: October 21 2024; Accepted: October 29 2024; Published Online: November 14 2024
Cite this paper:
Surboyo MDC, Baroutian S, Puspitasari W et al. Health benefits of liquid smoke from various biomass sources: a systematic review. BIO Integration 2024; 5: 1–19.
DOI: 10.15212/bioi-2024-0083. Available at: https://bio-integration.org/
Download citation
© 2024 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
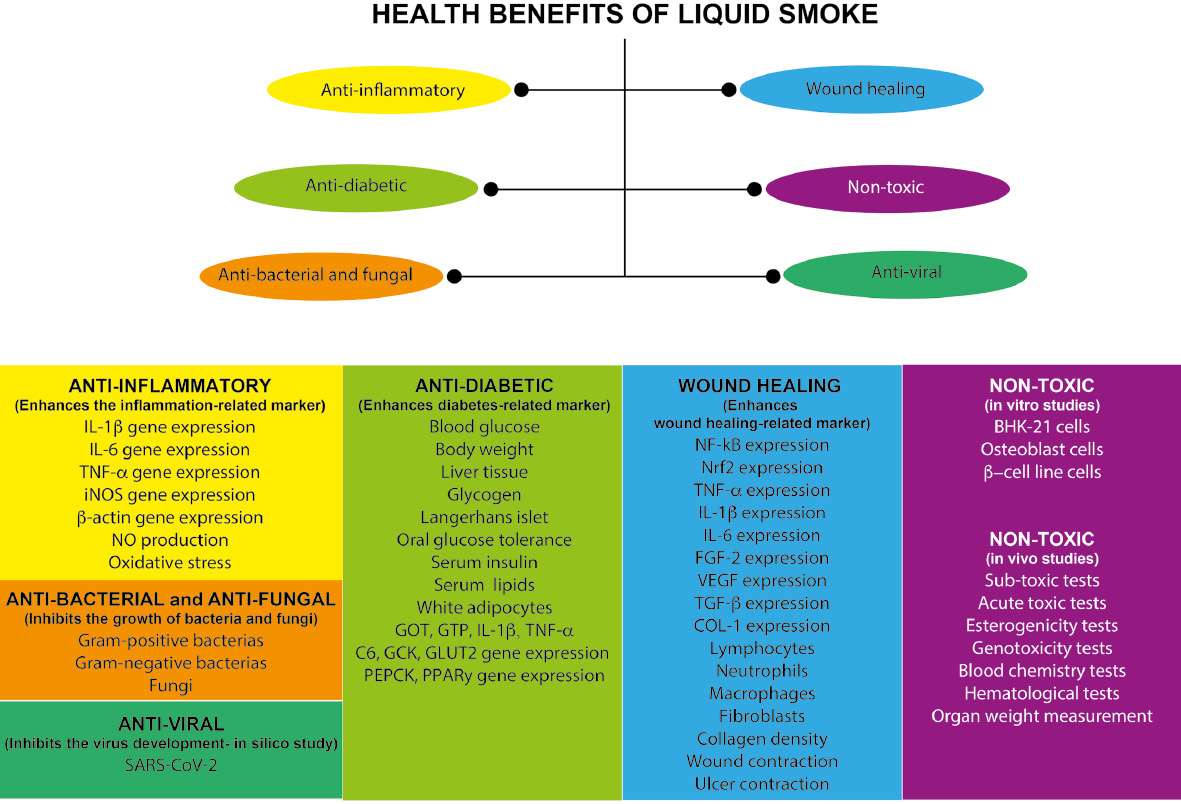
Liquid smoke, a product of the pyrolysis process, includes components such as phenol, furfural, and ketones, and has acidic characteristics. Liquid smoke from various biomass sources has been used as a natural preservative worldwide and reported to be safe in humans. As a bio-economic product, liquid smoke has human health benefits. This review analyzes and describes the health benefits of liquid smoke from various biomass sources, according to in silico, in vitro, and in vivo studies. A systematic review following PRISMA guidelines was conducted to identify published reports of liquid smoke from various biomass sources. The anti-inflammatory, anti-bacterial, anti-diabetic, wound healing, and anti-periodontitis activity of liquid smoke was analyzed. Prior research has investigated liquid smoke produced through pyrolysis of various biomass types, such as rice husks (Oryza sativa), coconut shells (Cocos nucifera L.), palm kernels (Elaeis guineensis Jacq.), cocoa pods (Theobroma cacao L.), tian op, and hickory (Carya tomentosa (Lam.) Nutt.), as well as commercial liquid smoke. Toxicity testing, and in vitro and in vivo studies, are required for the assessment of health benefits. Therapeutic benefits of liquid smoke including anti-oxidant, anti-inflammatory, anti-nociceptive, anti-bacterial, anti-fungal, and anti-viral activity have been described. Further health benefits include anti-diabetic, anti-periodontitis, wound healing, and ulcer healing activity. These findings increase the use value of liquid smoke as a natural product with human health benefits.
Keywords
Bio-economy, health benefit, human health, liquid smoke, pyrolysis.
Introduction
Liquid smoke is created by condensing the smoke produced during the pyrolysis of lignocellulosic biomass in a controlled environment. The process involves four stages of thermal decomposition: the evaporation of water, and the decomposition of hemicellulose, cellulose, and lignin. Consequently, liquid smoke is recovered in a liquid form containing water and oxygenated compounds. Liquid smoke is a bio-economic product that is derived from pyrolysis and has acidic characteristics. Liquid smoke was initially used as a natural food preservative. Early research on liquid smoke as a food preservative was published in 1975 [1]. As a food preservative, liquid smoke has anti-bacterial properties toward both gram-positive and gram-negative bacteria, as well as anti-fungal activity [2]. Liquid smoke has been demonstrated to act against lactic acid bacteria, namely Pediococcus cerevisiae and Lactobacillus plantarum [3, 4]; Salmonella sp. [5]; Clostridium botulinum [6]; Aeromonas hydrophila and Staphylococcus aureus [7]; Listeria monocytogenes [8–13]; and Listeria innocua [14]. In addition, it has anti-fungal activity against Penicillium roqueforti, Penicillium camemberti, and Aspergillus oryzae [15].
Liquid smoke can maintain the quality of food, including physical properties such as color [16, 17], meat texture [18], pH [19], tenderization [20] by preventing lipid oxidation processes [21–23], and preservation of shells life [24–28], particularly in various types of meat. Fish treated with liquid smoke flavoring shows low brightness and pH, but high firmness, elasticity, color intensity, and expressible water content [25, 29]. In other marine products, the application of liquid smoke does not affect the amino acid content [30], including that of lysine [31]. In addition, liquid smoke can improve organoleptic properties and antioxidant properties [32], because of the presence of phenolic compounds [33], primarily phenol and its derivatives [34–36]. In addition, liquid smoke contains aldehydes, ketones, diketones, esters, alcohols, acids, furan and pyran derivatives, pyrocatechol derivatives, and alkyl and aryl ethers [37].
Controversy exists regarding the use of liquid smoke as a food preservative, because of the presence of by-products of the pyrolysis process, particularly PAHs such as BaP; however, research has indicated that the levels of these by-products are very low and therefore safe for consumption [38–41].
The first report on the effects of liquid smoke on health, performed in 1994, conducted cytogenic analysis indicating that liquid smoke administration does not increase the frequency of chromosome aberrations [42]. The first toxicity tests of liquid smoke with respect to health were performed in experimental animals and indicated its safety [43]. Furthermore, liquid smoke administered as a food supplement in experimental diabetic animal models has been shown to ameliorate diabetes status and prevent inflammation, according to various indicators [44].
Given the complex composition of liquid smoke, comprising primarily phenolic compounds with antioxidant and anti-inflammatory properties, several studies have investigated therapeutic effects on human health. These observed benefits have shifted perspectives on the use and economic potential of liquid smoke. The expanding applications and economic value of liquid smoke could play a crucial role in its future development, particularly in creating new health-related products. Analyzing global usage patterns is necessary to validate its high utility, economic significance, and potential in drug development. This review was conducted because of the lack of systematic reviews in this field, and because most prior reviews have focused on other aspects liquid smoke, such as production.
Materials and methods
Study design
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed for this systematic review. This review was aimed at analyzing whether liquid smoke from various biomass sources might have any health benefits.
Search methods and strategy
A comprehensive search of the PubMed (https://pubmed.ncbi.nlm.nih.gov), Scopus (https://www.scopus.com/search/form.uri?zone=TopNavBar&origin=searchadvanced&display=basic#basic), Science Direct (https://www.sciencedirect.com), Web of Science (https://www.webofscience.com/wos/woscc/basic-search), and Embase databases until December 2023 was conducted. The keyword “liquid smoke” was used to search available articles. In addition, the reference lists of eligible articles were searched manually to identify additional relevant publications.
Study selection criteria
The inclusion criteria for studies followed the Population, Intervention and Outcome (PIO) framework:
- Type of population: all in vitro, in vivo, and in silico studies reporting the potential of liquid smoke from various biomass sources or commercial liquid smoke. The studies were required to state the source of liquid smoke
- Type of intervention: health benefits, such as anti-oxidant, anti-inflammatory, anti-nociceptive, anti-bacterial, anti-fungal, and anti-viral effects
- Type of outcome: the ability of liquid smoke to confer health benefits, such as anti-inflammatory, anti-diabetic, anti-periodontitis, wound healing, ulcer healing, and hepatic protection properties.
Data collection and extraction
Initially, four reviewers independently screened the titles and abstracts of articles from the databases matching the selection criteria of the study, to identify potentially eligible articles. Four reviewers eliminated duplicate articles and discussed the articles that were selected. Disagreements among reviewers were itemized, and the fifth reviewer was consulted to reach a consensus. The key information in each selected article was recorded and included in the study design, and relevant findings were tabulated.
The following information was extracted from the studies: first author’s name, year of publication, biomass source for production of liquid smoke, and health benefits. In cases of disagreement, a consensus was reached through discussion with the other authors.
Result
Study selection and screening result
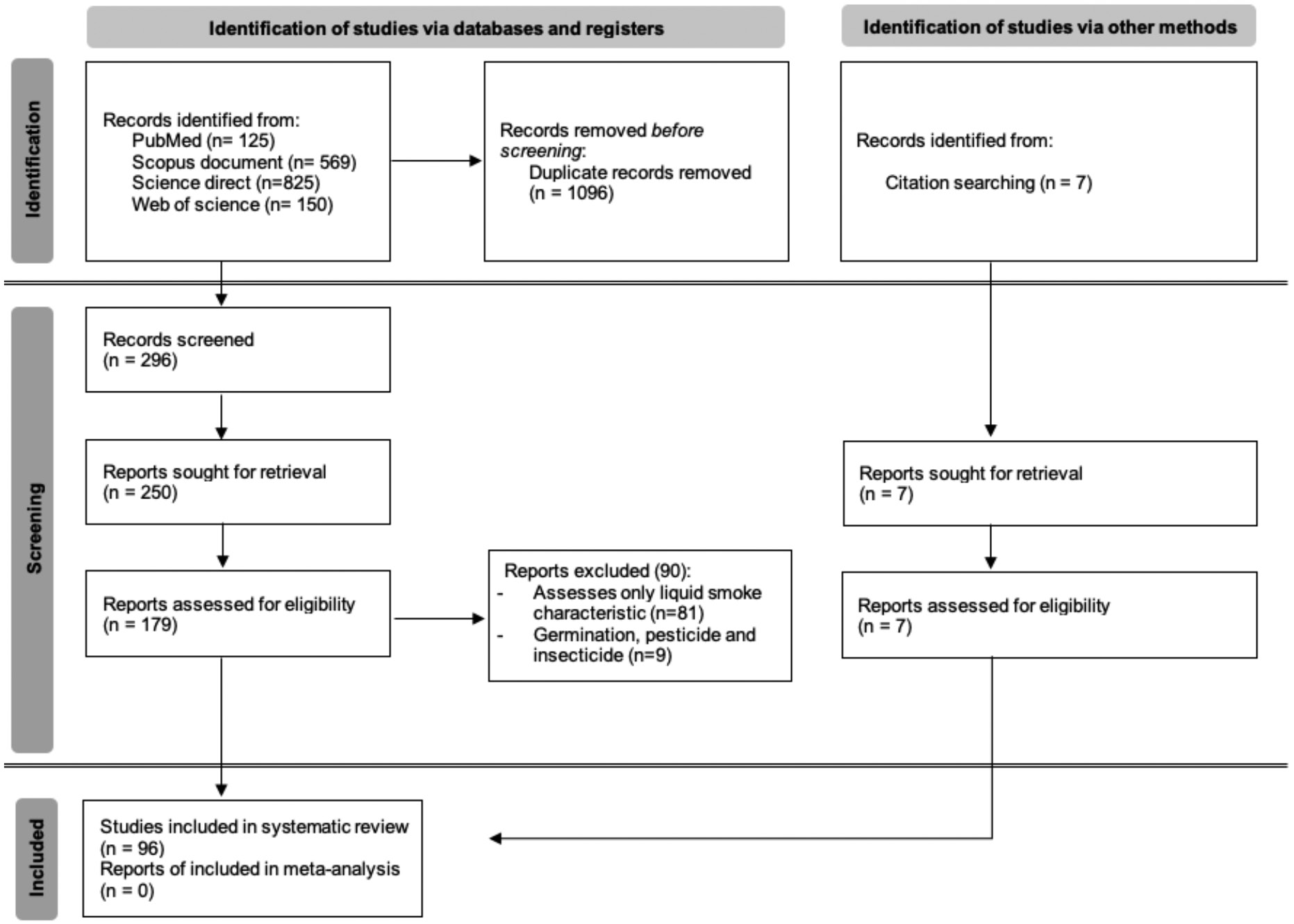
Searching of the databases identified 1669 articles, 1069 of which were identified as duplicates; thus, 269 articles remained. These 269 articles were screened on the basis of their titles and abstracts, thus leaving 250 articles. Only 179 articles were available in full text, among which 89 met the inclusion criteria, and 90 articles were excluded. During the article review process, a citation search identified 7 additional relevant articles. A total of 96 articles were finally included in the systematic review (Figure 1).
Figure 1 PRISMA flow diagram.
Liquid smoke sources
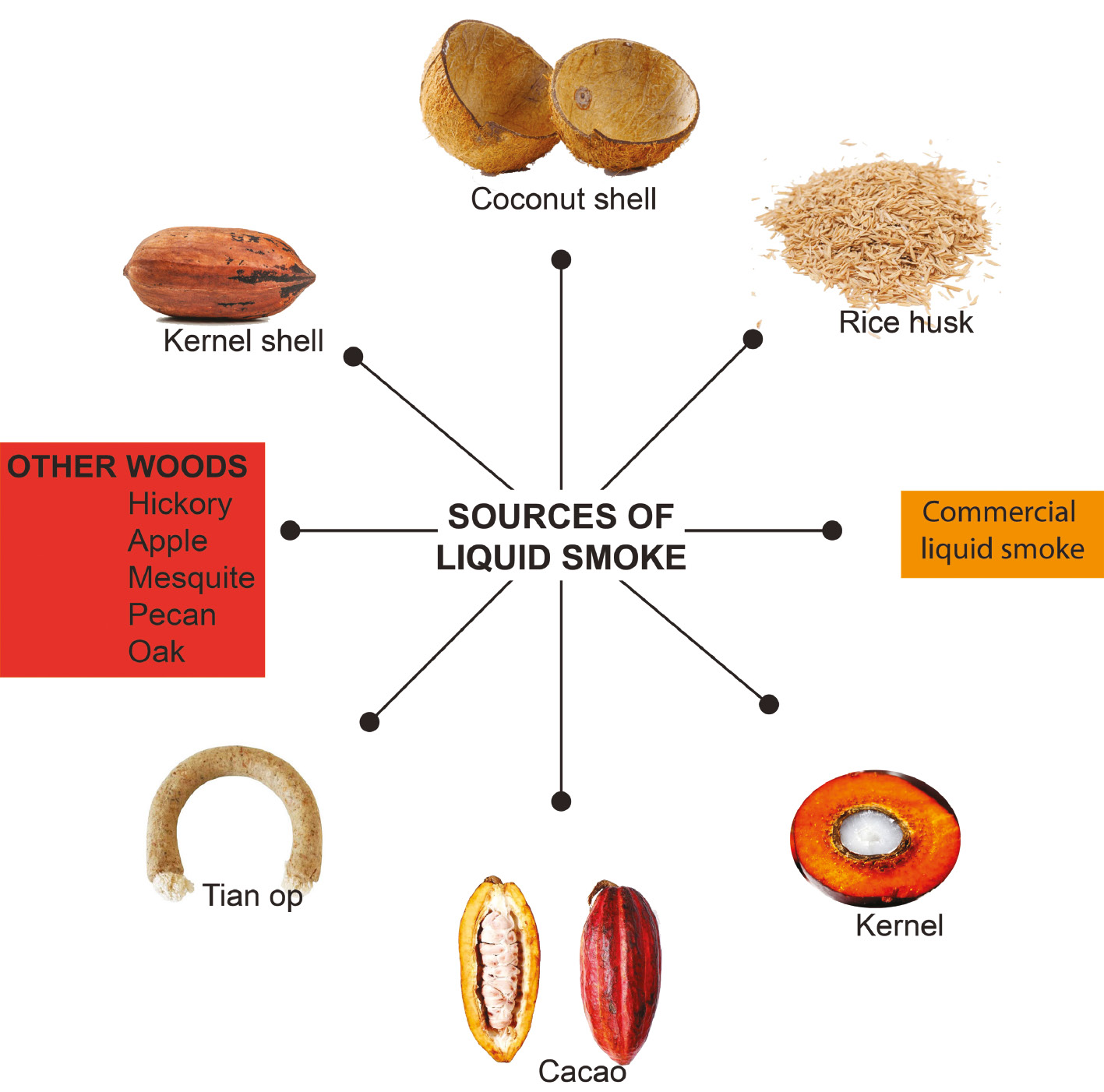
Various biomass types are used for liquid smoke production. The possible health benefits of liquid smoke produced from rice husks (Oryza sativa) [44–58], coconut shells (Cocos nucifera L.) [59–70], palm kernels (Elaeis guineensis Jacq.) [71], cocoa pods (Theobroma cacao L.) [72], tian op [73], hickory (Carya tomentosa (Lam.) Nutt.) [74], apple (Punica granatum L.), mesquite (Prosopis pubescens Benth.), pecan (Carya illinoinensis), and oak (Quercus robur L.) [75], as well as commercial liquid smoke [42, 76], have been reported (Figure 2). Commercial liquid smoke was defined as that produced by companies and derived from single or multiple biomass sources.
Figure 2 Biomass sources for production of liquid smoke providing health benefits for humans.
The production of liquid smoke was performed by pyrolysis at 200 °C [72], 300 °C [72], 400 °C [44–56, 59–69, 72], or 340–420 °C [71]. Some studies did not describe the temperature of pyrolysis [70, 73, 75].
Toxicity of liquid smoke
Materials suitable for human use must be non-toxic. Several studies have demonstrated that liquid smoke derived from rice husks (Oryza sativa) [44, 46, 51, 54, 56], cocoa pods (Theobroma cacao L.) [72], tian op [73], apple, hickory, mesquite, pecan, and oak [75], as well as commercial liquid smoke [42], exhibit no toxicity in vitro or in vivo (Supp Table 1).
Toxicity testing of liquid smoke derived from rice husks conducted in vitro has revealed no toxicity toward INS-1 rat insulinoma β-cells [44, 56], RBL-2H3, RAW264.7 [56], BHK-21 [46], and osteoblast cells [51, 54]. In in vivo studies, liquid smoke from rice husks (Oryza sativa) has also shown no signs of acute toxicity [45]. Oral administration of liquid smoke from tian op has not been found to affect body weight, hematological parameters, and organ weight [73]. Similarly, liquid smoke from apple, hickory, mesquite, pecan, and oak has demonstrated no toxicity or genotoxicity, while enhancing Nfr2 expression and decreasing AhR activity [75]. Additionally, a study of commercial liquid smoke has found no increase in the frequency of chromosome aberrations [42].
Anti-inflammatory properties
The anti-inflammatory properties of liquid smoke from rice husks (Oryza sativa) have been observed both in vitro and in vivo. Liquid smoke affects oxidative stress [44, 56] markers such as NO production [44, 56, 57]; iNOS genes and proteins [44, 56]; tissue gene expression of 5-LOX, COX-2, ICAM, and β-actin [56]; and cytokine genes and proteins, such as TNF-α, IL-1β, and IL-6 [44, 56]. By modulating gene expression, liquid smoke from rice husks affects the production of proteins, resulting in altered levels of TNF-α, IL-1β, IL-6, PGE2, and LTB4 [56]. Other proteins such as myeloperoxidase and β-hexosaminidase are also affected [56]. In in vivo studies, liquid smoke from rice husks (Oryza sativa) has shown anti-inflammatory properties by decreasing inflammation in the ear [56] (Supp Table 2).
Anti-diabetic properties
The anti-diabetic properties of liquid smoke from rice husks (Oryza sativa) have been examined both in vitro and in vivo. The addition of liquid smoke to mouse fat has been found to ameliorate damage to liver tissue and islets of Langerhans in a model of diabetic model induced by a high-fat diet [44, 53]. Enhanced gene expression and enzyme production in the liver has been reported, including that of GOT, GTP, C6, PEPK, GCK [44, 53], GLUT2, PPAR-γ, TNF-α, IL-1β, and IL-6 [53]. Consequently, insulin release [44] and serum insulin [44, 53] increase, whereas blood glucose decreases [44, 53], and glycogen restoration is improved [44] (Supp Table 2).
Anti-periodontitis properties
Topical application of liquid smoke from rice husks (Oryza sativa) has been shown to have anti-periodontitis effects by decreasing inflammatory markers and stimulating growth factors such as NF-kB [47], Nrf2 [52], IL-1β [52], TGF-β [47], FGF2 [47], and COL-1 [47] (Supp Table 2). The anti-periodontitis effect is also demonstrated by liquid smoke’s ability to inhibit Porphyromonas gingivalis, a key etiological agent of periodontitis [50, 54].
Ulcer healing properties
Liquid smoke from rice husks (Oryza sativa) has been found to influence ulcer healing. Topical application of liquid smoke increases inflammatory cells, such as macrophages, lymphocytes, and fibroblasts [48]. Effects have also been observed on cytokines and growth factors, such as IL-6 [48], TGF-β [48], FGF-2, COL-1, VEGF, and PDGF [55] (Supp Table 3).
Liquid smoke has also shown health benefits in humans. In an in vitro study, liquid smoke from coconut shells (Cocos nucifera L.) has been shown to be non-toxic [66], and to have anti-bacterial activity against Streptococcus aureus [70], anti-inflammatory activity [66], and anti-nociceptive properties [66, 68]. Liquid smoke from coconut shells (Cocos nucifera L.) stimulates burn wound healing properties by increasing the number of fibroblasts and wound contraction [59]. Oral ulcers, wounds occurring in the oral mucosa, have shown significant healing after topical administration of liquid smoke from coconut shells (Cocos nucifera L.), by decreasing NF-kB [61, 62] and increasing Nrf2 [64]. Effects have also been observed on the production of cytokines such as TNF-α [62, 64], IL-6, and IL-1β [64], and growth factors, such as FGF-2 and VEGF [63]. Liquid smoke also impacts inflammatory and proliferative cells, including neutrophils [65], lymphocytes [65], macrophages [62, 64], fibroblasts [63, 65], and collagen production [69] (Supp Table 3).
Anti-bacterial properties
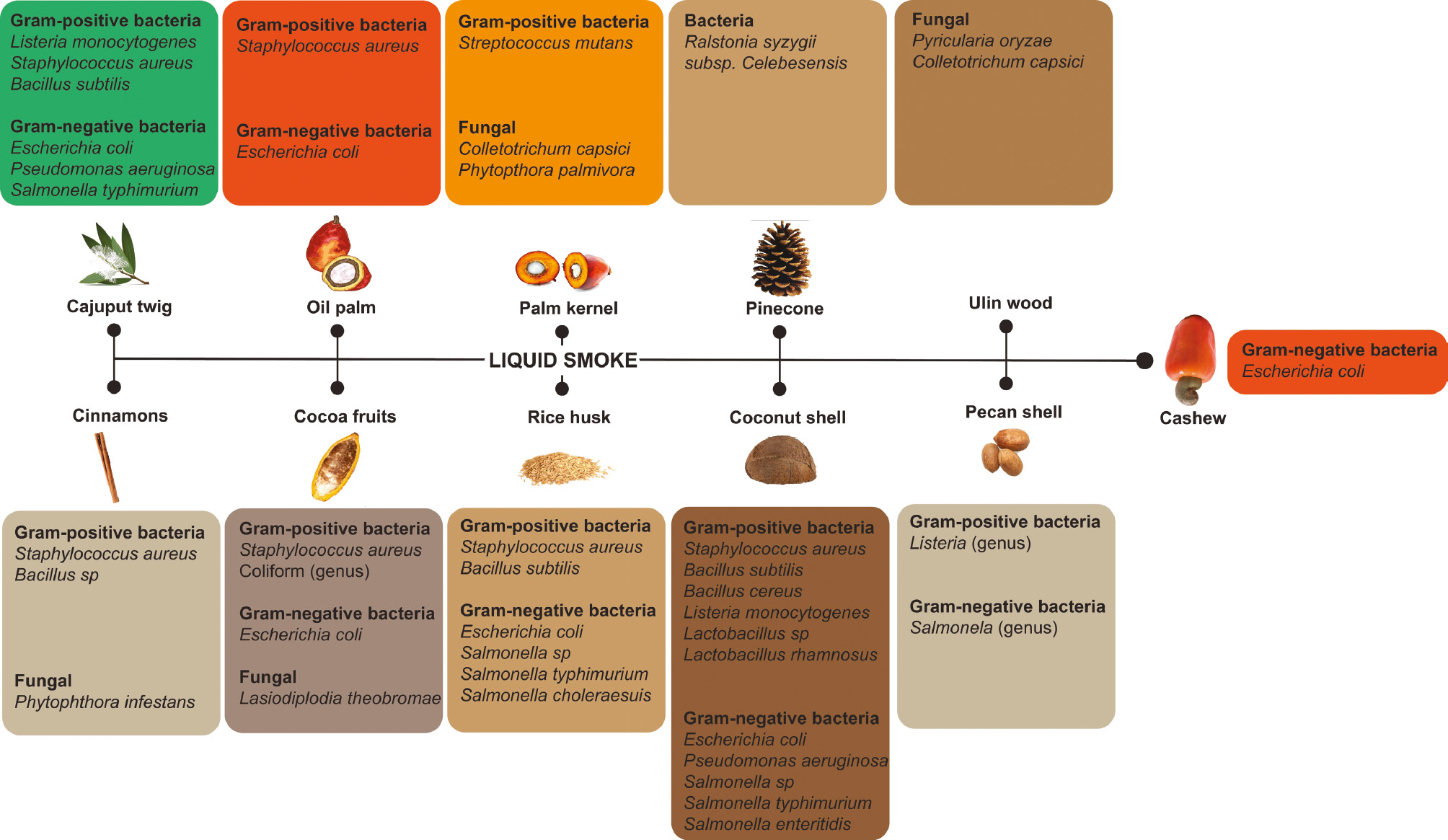
Liquid smoke also has anti-bacterial effects. Liquid smoke with such properties has been derived from various biomass sources including cajuput twigs (Melaleuca leucadendra), oil palm (Elaeis guineensis Jacq.), palm kernels (Elaeis guineensis Jacq.), pinecones (Pinus coulteri), ulin wood (Eusideroxylon zwageri), cinnamon (Cinnamomum verum), cocoa fruits (Theobroma cacao L.), rice husks (Oryza sativa), coconut shells (Cocos nucifera L.), and pecan shells (Carya illinoinensis) (Figure 3).
Figure 3 Antibacterial and anti-fungal properties of various liquid smoke types.
Coconut shell (Cocos nucifera L.) is the most studied liquid smoke origin in the literature. The antibacterial properties of liquid smoke from coconut shells (Cocos nucifera L.) have been reported in several studies on gram-positive bacteria, such as Staphylococcus aureus [77–80], Bacillus subtilis [77], Bacillus cereus [79], Listeria monocytogenes [77, 79], Lactobacillus sp. [81], and Lactobacillus rhamnosus [82], as well as gram-negative bacteria, such as Escherichia coli [77, 79, 80, 83, 84], Pseudomonas aeruginosa [77, 78, 85, 86], Salmonella sp. [81], Salmonella typhimurium [77, 81], and Salmonella enteritidis [79].
Rice husks (Oryza sativa), one of the most common sources of liquid smoke, have antibacterial properties toward several gram-positive bacteria, such as Staphylococcus aureus [87] and Bacillus subtilis [87], and gram-negative bacteria, such as Escherichia coli [87, 88], Salmonella sp. [88], Salmonella typhimurium [57, 89], and Salmonella choleraesmus [87].
Liquid smoke obtained from cajuput twigs (Melaleuca leucadendra) has antibacterial properties toward several gram-positive bacteria, such as Listeria monocytogenes, Staphylococcus aureus, and Bacillus subtilis, as well as gram-negative bacteria, such as Escherichia coli, Pseudomonas aeruginosa, and Salmonella typhimurium [77].
Oil palm (Elaeis guineensis Jacq.), including shells and branches, is another source of liquid smoke. Oil palm shells (Elaeis guineensis Jacq.) have antibacterial potential toward gram-positive and gram-negative bacteria, such as Escherichia coli [90] and Staphylococcus aureus [90, 91]. Furthermore, liquid smoke from palm kernels (Elaeis guineensis Jacq.) has antibacterial properties toward the gram-positive bacterium Streptococcus mutants [71].
Cocoa skin (Theobroma cacao L.) has been demonstrated by several studies to have anti-bacterial potential against gram-positive bacteria such as Staphylococcus aureus [92, 93] and Coliform [93], and gram-negative bacteria such as Escherichia coli [92]. Cinnamon (Cinnamomum verum), a raw material for liquid smoke production, has been found to have anti-bacterial properties toward the gram-positive bacteria Staphylococcus aureus and Bacillus sp. [94]. Liquid from cashews (Anacardium occidentale) and pecan shells (Carya illinoinensis) has anti-bacterial activity toward Escherichia coli [95], Listeria sp., and Salmonella sp. [96].
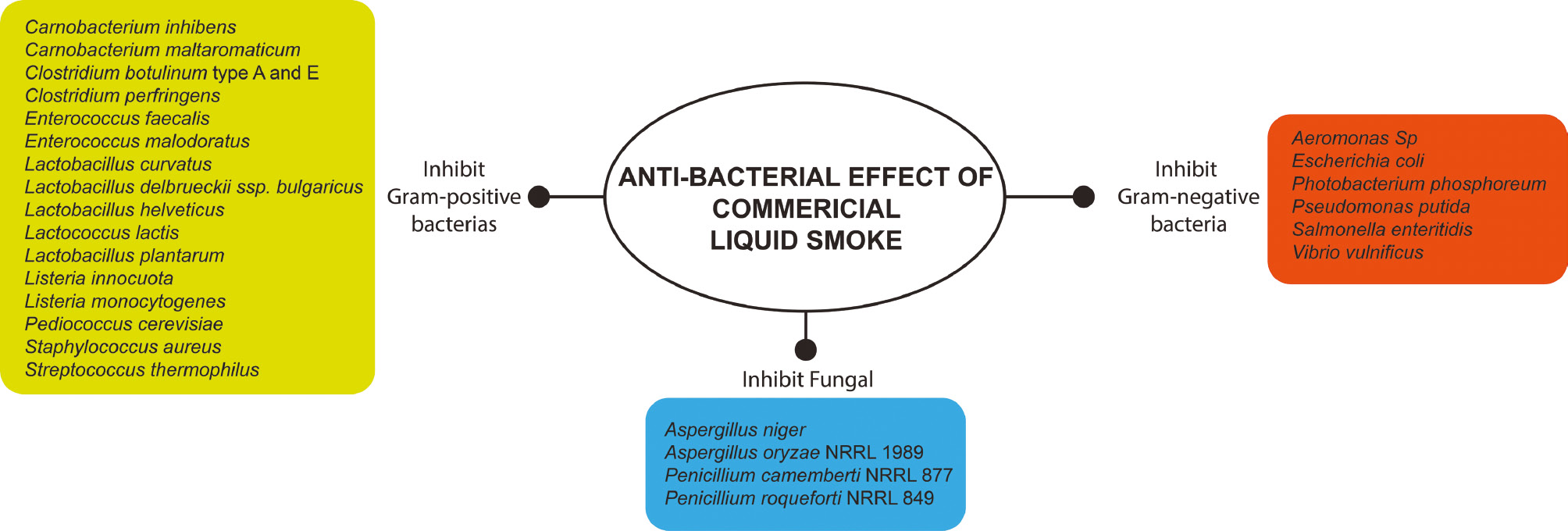
Commercial liquid smoke also has anti-bacterial, gram-positive, gram-negative, and anti-fungal activity (Figure 4). Effects on gram-positive bacteria, such as Bacillus cereus [97], Carnobacterium inhibens [98], Carnobacterium maltaromaticum [98, 99], Clostridium botulinum types A and E [6], Clostridium perfringens [100, 101], Enterococcus faecalis [98], Enterococcus malodoratus [99], Lactobacillus curvatus [98], Lactobacillus deibrueckii ssp. bulgaricus [102], Lactobacillus helveticus [102], Lactococcus lactis [98], Lactobacillus plantarum [2–4, 99], Listeria innocua [2, 12, 14, 98], Listeria monocytogenes [8, 10–12, 24, 26, 103–109], Listeria monocytogenes Scott A [9, 13], Pediococcus cerevisiae [3], Staphylococcus aureus [7, 67, 97, 100, 101, 110–113], and Streptococcus thermophilus [99, 102], have been reported.
Figure 4 Antibacterial and anti-fungal properties of commercial liquid smoke.
Furthermore, antimicrobial properties toward gram-negative bacteria, such as Aeromonas sp. [7, 114], Escherichia coli [2, 97, 112, 113], Photobacterium phosphoreum [98], Pseudomonas putida [2, 98], Salmonella sp. [2, 97], Salmonella enteritidis [112], and Vibrio vulnificus [98], have also been reported.
Anti-fungal properties
Antifungal properties of liquid smoke from coconut shells (Cocos nucifera L.), cocoa shells (Theobroma cacao L.), cinnamon (Cinnamomum verum), ulin wood (Eusideroxylon zwageri), palm kernels (Elaeis guineensis Jacq.), and pinecones (Pinus coulteri) have been reported. Some anti-fungal activity has been observed toward Calathea utilis [84], Fusarium oxysporum [115], Candida albicans [80], Lasiodiplodia theobromae [116], Phytophthora infestans [117], Phytophthora palmivora [118], Colletotrichum capsica [119, 120], Pyricularia oryzae [121], and Ralstonia syzygii subsp. celebesensis [122] (Table 1).
Table 1 Liquid Smoke from Various Wood Sources with Anti-Fungal Benefits for Human Health
| Raw material | Anti-fungal properties | References |
|---|---|---|
| Coconut shells (Cocos nucifera L.) | Calathea utilis | [84] |
| Candida albicans | [80] | |
| Fusarium oxysporum | [115] | |
| Cocoa shells (Theobroma cacao L.) | Lasiodiplodia theobromae | [116] |
| Cinnamon (Cinnamomum verum) | Phytophthora infestans | [117] |
| Ulin wood (Eusideroxylon zwageri) | Colletotrichum capsici | [119] |
| Pyricularia oryzae | [121] | |
| Palm kernels (Elaeis guineensis Jacq.) | Colletotrichum capsici | [120] |
| Phytopthora palmivora | [118] | |
| Pinecones (Pinus coulteri) | Ralstonia syzygii subsp. celebesensis | [122] |
The antifungal properties of commercially available liquid smoke toward fungi such as Aspergillus niger [2], Aspergillus oryzae NRRL 1989, Penicillium camemberti NRRL 877, Penicillium roqueforti NRRL 849 [15], Saccharomyces cerevisiae [2], and Phytophthora palmivora [123] have also been described.
Anti-viral properties
Anti-viral properties of liquid smoke from rice husks (Oryza sativa) toward SARS-CoV-2 have been reported. The liquid smoke components 6-octadecenoic acid and oleic acid have been shown to be potential SARS-Cov-2 inhibitors in an in silico study [49].
Hepatic protection properties
The administration of liquid smoke from rice husks has shown potential to ameliorate hepatic damage from bacteria including Salmonella [57, 58]. The addition of liquid smoke from rice husks to the diet has been found to decrease hepatocyte necrosis in mice, by decreasing catalase activity, SOD activity, TNF-α, MPO [58], and IFN and NO production [57]. Decreased hepatocyte necrosis has been demonstrated, on the basis of recovery of GOT and GPT in the liver [58]. Liquid smoke from rice husks has protective effects against not only hepatic function but also lung and kidney damage [58].
Other properties
The incorporation of liquid smoke as a food additive has indicated interactions between the constituent primary emitter pyrolysis product (EMP) and the taste receptor TA2R1, thus elucidating the mechanism through which liquid smoke enhances flavor perception when used as a food flavoring agent [76]. Other research has indicated that the addition of liquid smoke does not cause adverse effects such as inflammation or ulceration of the digestive tract, although liquid smoke is acidic [124]. However, exposure to liquid smoke derived from hickory and mesquite wood has been found to induce oxidative stress and compromise the protective function of the skin [74].
Discussion and future development
Liquid smoke has been proposed as an ingredient with health effects on humans, primarily because it has long been used as a food preservative. As a food preservative, liquid smoke improves the characteristics of foods such as meat [1, 18, 20, 21, 125–136], bacon [1, 22, 137–139], quail [140], mackerel [141–144], fish [16, 25, 27, 29, 31, 145–164], fish balls [113, 165], meatballs [166–171], sausages [23, 172–176], nuggets [28, 177], shellfish or mussels [30, 178–181], shrimp [182], prawns [183], mushrooms [184], and potatoes [17, 185].
Adding liquid smoke can maintain storage time and life [25, 27, 28, 31, 131, 144, 156, 161, 162, 164, 166, 169, 180], freshness [143], color [125, 129, 142, 147, 167], acidity [29, 126, 132, 136, 155, 158, 171, 174, 184], and sensory or organoleptic (flavor, taste, or texture) qualities [16–18, 20, 23, 25, 29, 31, 128, 129, 132, 135–138, 141–143, 146, 150, 153, 155, 157, 158, 160, 164–167, 170, 172–175, 177, 180, 183, 185]. The aroma of food containing liquid smoke is also distinctively smoky [176, 181].
In addition to various chemical properties of meat, such as total TVB-N [128, 143, 149, 160, 164, 184, 186], TBA [186], lysine [31, 141], NDMA, NPYR, and NTHZ can also be influenced [139]. Other important characteristics such as water content [125–127, 158, 163, 170, 171, 184, 187], moisture [136, 141, 163, 165, 182], protein [30, 125, 134, 141, 145, 151, 159, 165, 170, 171], and cholesterol [182] are remain stable. Total lipid [125, 140, 145, 148, 152, 159, 163, 165, 170, 182, 188] can also be maintained through mechanisms of increased lipid oxidation [131, 159, 179] or decreased lipid oxidation [21–23]. Liquid smoke addition does not affect the blood urea nitrogen and creatinine content in poultry [133]. With technologic development, liquid smoke has been transformed into edible film [144, 189], gelatin film [190], and even forms of nanoparticles [156] or nanocapsules [159] to improve its availability. Interestingly, in marine products, the application of liquid smoke can decrease the content of heavy metals [178]. The various advantages of liquid smoke may maintain nutritional content and food characteristics, thus improving human health. Other important characteristics include anti-bacterial effects against foodborne bacteria [130, 144, 146, 154, 157, 164, 166, 167, 169, 172, 174, 180, 188], such as Coliform, Staphylococcus aureus [136], Escherichia coli, Salmonella [132], and Listeria monocytogenes [31]. The anti-bacterial effects are due to the high content of phenol, acetic acid, and water in liquid smoke [1, 158, 165, 168, 174, 184].
Empirically and historically, liquid smoke has been a widely used food preservative, as supported by multiple studies; however, safety aspects remain debated, because by-products of combustion, namely PAH and BaP, are considered harmful to human health [191]. Some studies have reported that the content of this PAH in food after liquid smoke addition is low [40, 192, 193], at approximately 6.3–43.7 μg/kg [35], while other studies found no detectable levels of PAH [194]. Both PAH and BaP have been detected in some studies [38–40], at approximately 0.18–0.80 μg/kg [36], whereas in other studies, it was not found [135]. Pyrolysis temperature is associated with the PAH and BaP content; however, in some studies using higher pyrolysis temperatures of 400–500 °C, the PAH and BaP component has been found [41, 191, 195].
If an ingredient is used as a drug or therapy, fundamental questions include whether the active ingredient is contained, and what the ingredient’s the mechanism of action in the body might be. Liquid smoke contains multiple components, dominated by phenolic compounds and carboxyl components. Liquid smoke is acidic. The acidity (pH) of liquid smoke is determined by the temperature of the pyrolysis itself and the biomass type used. Lower pyrolysis temperatures result in lower pH values. Temperatures of 150–250 °C produce a pH of 2–3 [196, 197]; temperatures of 300–400 °C produce a pH pf 3–4 [198, 199]; and temperatures of 400–500 °C produce pH values of 2, 3.82–4.69 [200–202], or 4.8 [158].
Not only the acidity but also the components of liquid smoke are influenced by temperature. At temperatures of 250–600 °C, phenolic compounds are dominant (7.32–56.8%) [195, 196, 200, 203, 204]; some identified components include carbonyl, carboxylate, furan, and acidic compounds [158, 198, 200, 201, 203, 205–213]. At higher temperatures (350–550 °C), various phenolic compounds, such as 2-methoxy-4-methyl phenol, 4-ethyl-2 methoxy phenols [191, 214, 215], 2 methoxy phenol, and methoxy-4-methyl phenol, are detected [200, 214, 216–218]. Lower temperatures (150–250 °C) produce higher levels of acid (up to 51%) compared to phenolic compounds [195–197, 219]. Moreover, temperatures of 300–600 °C also produce high levels of acidic compounds (ranging from 40% to 92.30%) [195, 204, 208, 213, 220–225], 1,2-ethanediol (up to 20%) [226], and carbonyl compounds (ranging from 32% to 73%) [208, 222–224, 227].
Although the content of PAH and BaP, and the acidity, are controversial aspects regarding the safety of liquid smoke as a food ingredient, no toxicity effects have been reported. Various in vitro and in vivo studies have demonstrated that liquid smoke is safe. The first study, reported in 1994, indicated that the liquid smoke does not have any mutagenic effects and does not affect chromosome aberrations [5, 42]. In an in vitro study, liquid smoke from rice husks (Oryza sativa) has shown no toxicity to fibroblast cells [46] and osteoblast cells [51]. Other parameters, such as oxidative stress, genotoxicity, AhR, and esterogenicity, have not been detected [75]. Furthermore, toxicity tests in animals have been performed. Liquid smoke from coconut shells (Cocos nucifera L.) has been orally administered to animals, and no death resulted [43]. Moreover, liquid smoke from tian op has been tested in an animal model, and no body weight, hematological, and organ changes have been observed [73]. Liquid smoke from rice husks (Oryza sativa) has shown similar results to those of tian op. Oral administration of liquid smoke from rice husks (Oryza sativa) and cocoa (Theobroma cacao L.) has been shown to be safe in mice, and no signs of acute toxicity have been observed [45, 72].
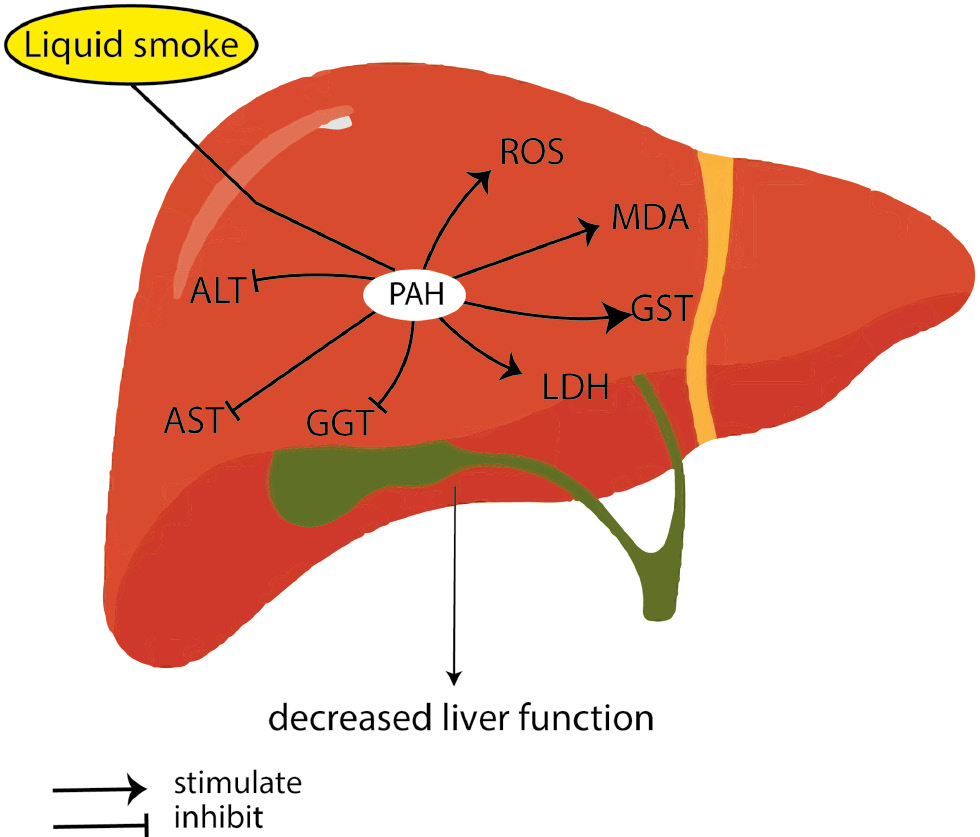
The presence of PAH in liquid smoke is a concern. PAH inhalation, ingestion, and dermal contact can be toxic in the human body [228]. PAH accumulates in the body and damages the cardiovascular system, respiratory system [229], nerve cells, and liver cells [230], and may lead to cancer [231, 232], such as breast cancer [233], laryngeal cancer [234], esophageal cancer [235], and lung cancer [236]. Via the ingestion route, PAH is absorbed in the gastrointestinal tract [237], and causes hepatic toxicity and increased lipid metabolism [230]. PAH also affects oxidative stress in the human body, including MDA, GST, and LDH [238]. In carcinogenesis, PAH increases serum p53 and p21 proteins [238]. In the liver, PAH is associated with increased ALT, AST and GGT, and affects liver function [239]. At the cellular level, PAH affects cellular viability, induces free radical-like ROS production, and decreases anti-oxidant ability, including glutathione GSH levels, PSSG, and the activity of GPx, GST, and GR [240]. Together, these processes affect the physiological function of the liver (Figure 5).
Figure 5 Possible effects of PAH on liver function by increasing oxidative stress (ROS, MDA, GST, and LDH) and inhibiting the liver enzymes such as ALT, AST, and GGT.
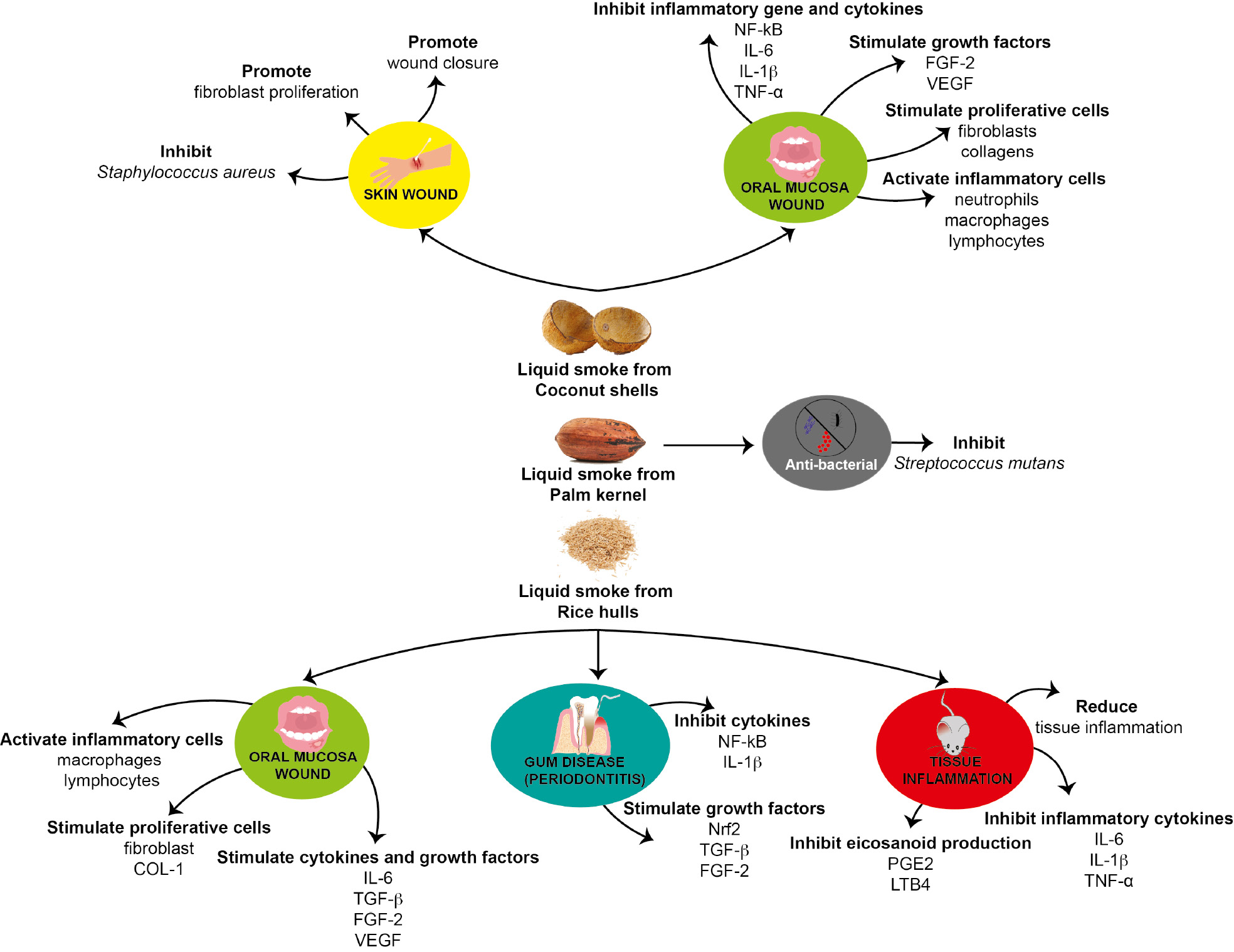
Health benefits of liquid smoke from coconut shells (Cocos nucifera L.) and rice husks (Oryza sativa) have been demonstrated in humans. Topical treatment with liquid smoke from rice husks (Oryza sativa) has beneficial effects in gum disease (periodontitis) [47, 52] and oral mucosa wounds (ulcer) [48, 55]. Liquid smoke from rice husks (Oryza sativa) has anti-periodontitis effects by inhibiting inflammation and stimulating healing. The mechanism is associated with inhibition of NF-kB [47] and IL-1β [52], and stimulation of Nrf2 [52] to produce growth factors including TGF-β, FGF2, and COL-1 [47]. Liquid smoke from rice husks (Oryza sativa) promotes the activity of inflammatory cells, such as macrophages and lymphocytes [48], and stimulates the production of cytokines and growth factors, including IL-6 [48], TGF-β [48], FGF-2 [55], VEGF [55], COL-1 [55], and PDGF [55]. It also enhances fibroblast proliferation, contributing to wound healing [48] (Figure 6).
Figure 6 Possible mechanism underlying the health benefits of three liquid smoke types, from coconut shells (Cocos nucifera L.), rice husks (Oryza sativa), and palm kernels (Elaeis guineensis Jacq.). Liquid smoke from coconut shells (Cocos nucifera L.) stimulates skin wound healing and oral mucosal healing; liquid smoke from rice husks (Oryza sativa) stimulates gum disease (periodontitis) healing and oral mucosal healing. Liquid smoke from palm kernels (Elaeis guineensis Jacq.) has anti-bacterial properties against Streptococcus mutants.
Like liquid smoke from rice husks (Oryza sativa), liquid smoke from coconut shells (Cocos nucifera L.) has a similar health benefits in wound healing support [59, 70], and mucosal wound healing (ulcer healing) [60–65, 69]. The topical application of liquid smoke from coconut shells (Cocos nucifera L.) increases wound healing by increasing fibroblast proliferation [59] and wound contraction [59, 70], and also has anti-bacterial activity toward Staphylococcus aureus [70] (Figure 7).
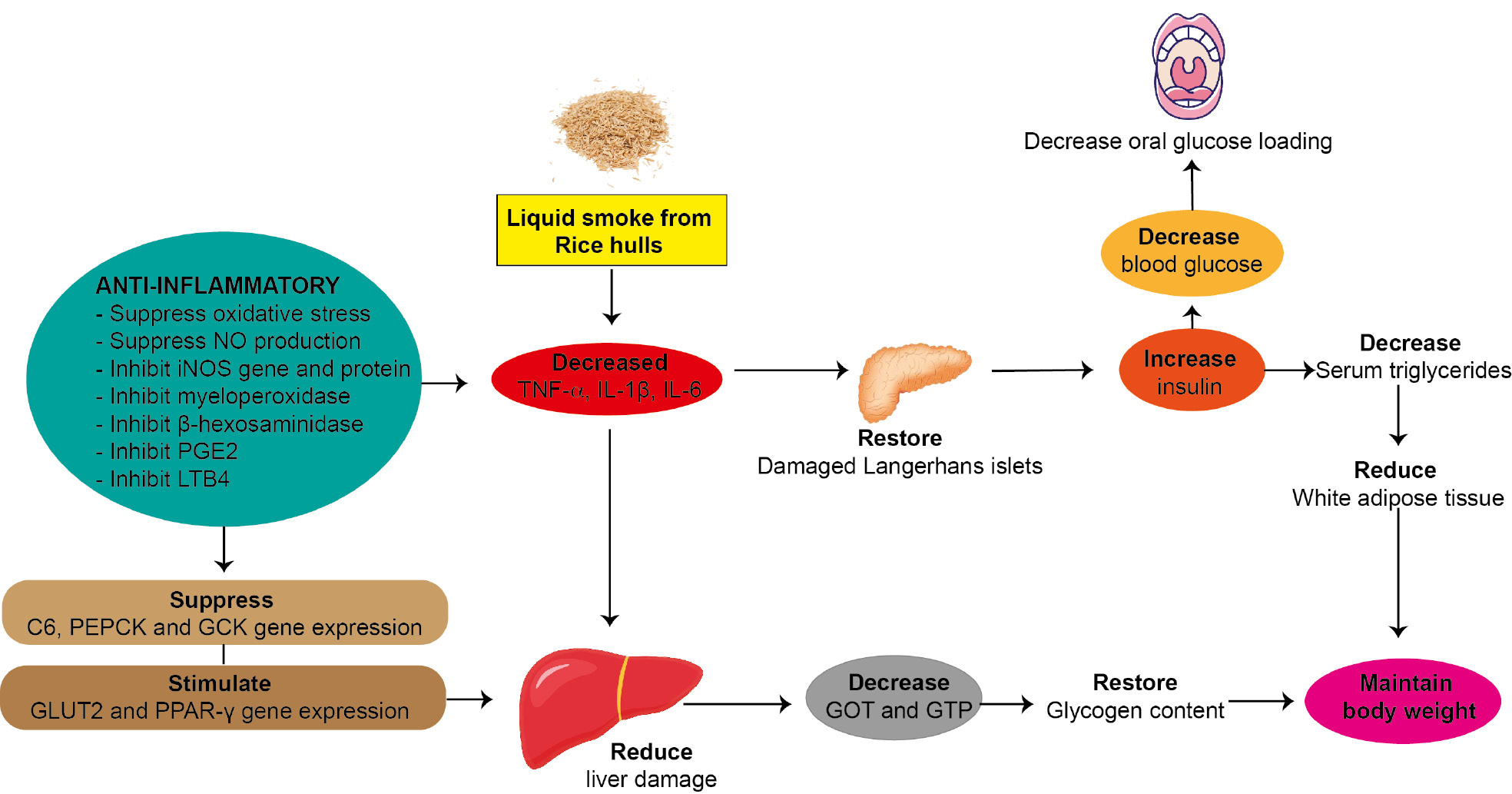
Figure 7 Possible mechanism of anti-diabetic properties of liquid smoke from rice husks (Oryza sativa), according to an in vivo model study. The anti-diabetic properties were demonstrated through food supplementation in an animal diet for 4 weeks.
For mucosal healing (ulcer healing), topical application of liquid smoke from coconut shells (Cocos nucifera L.) increases healing by inhibiting pro-inflammatory cytokines and genes, such as NF-kB [61, 62], IL-6, IL-1β [64], and TNF-α [62, 64], and stimulating growth factors supporting healing, such as Nrf2 [64], FGF-2, and VEGF [63]. The healing response also includes a decreased neutrophil response [65], as well as increased lymphocytes [65], macrophages [62, 64], fibroblasts [63, 65], and collagen [69], thus accelerating ulcer closure [60]. Liquid smoke from palm kernels (Elaeis guineensis Jacq.) has antibacterial effects and has been used as a mouthwash to inhibit tooth decay caused by Streptococcus mutans [71] (Figure 6).
Research on the health benefits of liquid smoke from rice husks (Oryza sativa) has indicated anti-diabetic properties (Figure 7). Liquid smoke from rice husks (Oryza sativa), in accordance with the requirements for drug candidates, has shown low toxicity both in vitro and in vivo. In in vitro studies, liquid smoke from rice husks (Oryza sativa) has been found to maintain the viability of INS-1 rat insulinoma β-cells [44, 56], RBL-2H3, RAW264.7 [56], BHK-21 [46], and osteoblasts [51, 54]. In an in vivo study, liquid smoke from rice husks (Oryza sativa) has been found to have low acute toxicity [45].
The anti-diabetic properties of liquid smoke from rice husks (Oryza sativa) have been demonstrated in a food supplementation animal study. The addition of liquid smoke to animal diet improves diabetes status. Mechanistically, liquid smoke from rice husks (Oryza sativa) ameliorates liver damage [44, 53], and restores the damage and size of Langerhans cells in the pancreas (the source of insulin) [44, 53], thereby restoring hepatic and pancreatic function. Improvements in markers have been observed, including decreased levels of GOT and GTP; suppressed C6, PEPCK, and GCK gene and protein expression [44, 53]; and increased GLUT2 and PPAR-γ [53]. The changes these markers suppress the inflammatory state in diabetes through decreasing pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6 [53]. Through this mechanism, insulin production and release were increased [44, 53], helping to maintain blood glucose levels [44, 53]. Additionally, glycogen stores were restored [44], and this effect contributed to a reduction in serum lipid levels and maintenance of body weight [53].
Liquid smoke from rice husks (Oryza sativa) also has anti-inflammatory properties by inhibiting oxidative stress, NO production, iNOS gene expression, and the production of pro-inflammatory cytokines [44, 56]. Furthermore, the inhibition of myeloperoxidase β-hexosaminidase, PGE2, and LTB4 stimulates 5-LOX, COX-2, and ICAM expression, thereby supporting the anti-diabetic activity [56].
Most liquid smoke has broad spectrum antibacterial effects and therefore may be developed as an anti-microbial agent. Some gram-positive bacteria, such as Bacillus cereus [79, 97] and Listeria monocytogenes [8, 10–12, 24, 26, 77, 79, 103–109], cause human illness, including gastrointestinal and diarrheal syndrome. These bacteria are food-borne pathogens [241]. Bacteria such as Staphylococcus aureus [7, 67, 77–80, 87, 90–94, 97, 100, 101, 110–113] cause skin disease and respiratory disease [242]. Streptococcus thermophilus [99, 102], Streptococcus mutants [71], and Lactobacillus plantarum [2–4, 99] are gram-positive bacteria causing diseases in the oral cavity, particularly tooth decay or caries [243–245].
Gram-negative bacteria such as Escherichia coli [2, 77, 79, 80, 83, 84, 87, 88, 90, 92, 95, 97, 112, 113], Salmonella sp. [2, 81, 88, 96, 97], Salmonella typhimurium [77, 81, 89], Salmonella enteritidis [79], and Salmonella choleraesmus [87, 112] are also foodborne pathogens that can cause human illness, such as gastrointestinal and diarrheal syndrome. All the antibacterial properties of liquid smoke are currently focused on combating foodborne pathogens. Future discovery of other anti-bacterial properties of liquid smoke is needed.
The anti-fungal effects of liquid smoke have been widely studied in various fungi causing plant diseases, such as Calathea utilis [84], Fusarium oxysporum [115], Lasiodiplodia theobromae [116], Phytophthora infestans [117], Phytophthora palmivora [118], Colletotrichum capsica [119, 120], and Pyricularia oryzae [121]. Only one fungal species associated with human disease has been found to be inhibited by liquid smoke: Candida albicans [80].
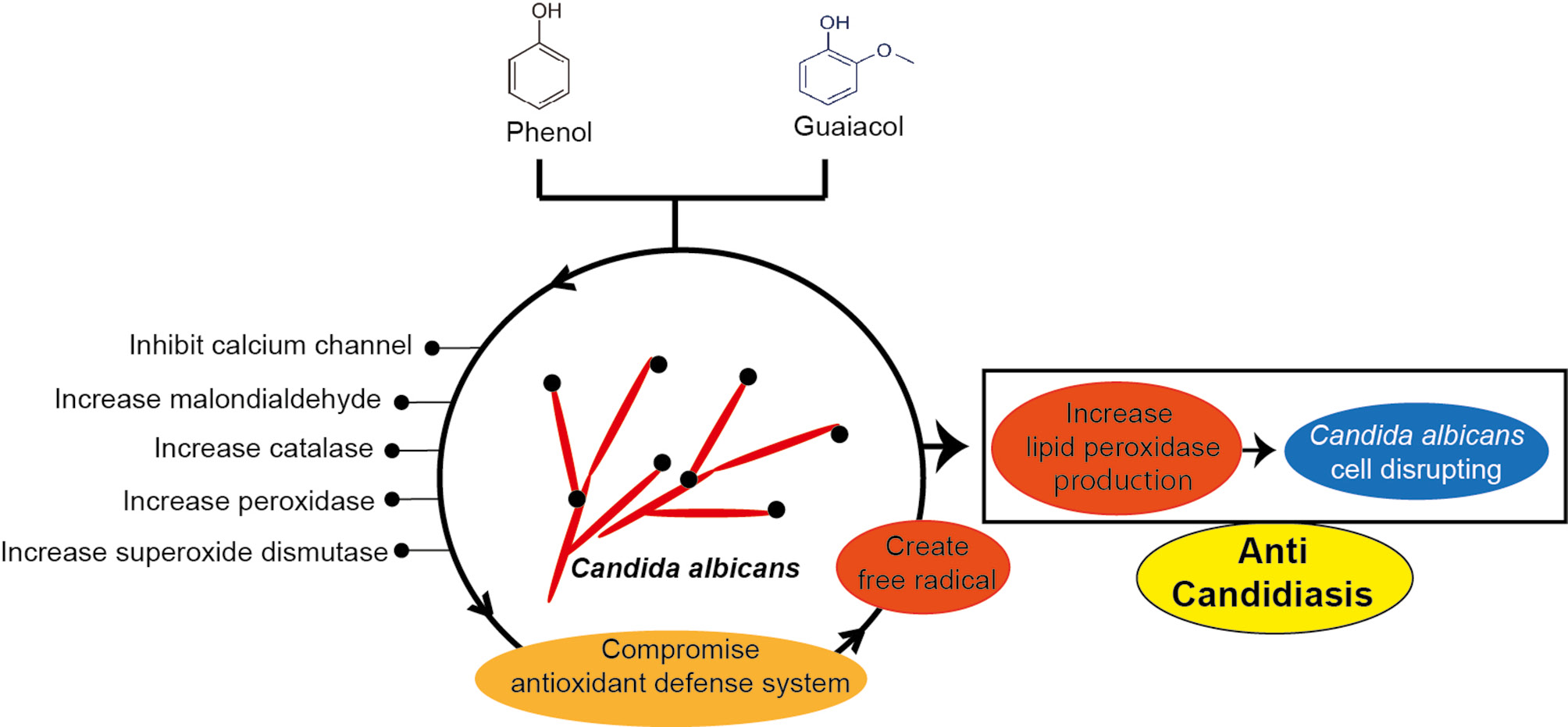
Among the fungi affected by liquid smoke, only Candida albicans causes both mucosal or skin and systemic infections in humans. Liquid smoke from coconut shells (Cocos nucifera L.), cajuput twigs (Melaleuca leucadendra) [80], eucalyptus, and Mimosa tenuiflora [246] inhibits the growth of Candida albicans. The main components of liquid smoke responsible for its anti-fungal properties are phenolic compounds, such as phenol and guaiacol [247]. Phenol has benzene rings that interact with membranes and disrupt mitochondrial balance [248]. Moreover, the antifungal effects of guaiacol might be attributable to damage to Candida albicans membranes through disruption of Ca2+ transport channels. In addition, guaiacol increases the production of MDA, CAT, POD, and SOD, thus decreasing the oxidative response [249, 250]. These mechanisms together induce oxidative stress and compromise the antioxidant defense system in Candida albicans through a toxic free radical cascade mediated by lipid peroxidation [251]. This mechanism may provide a basis for the development of liquid smoke for candidiasis treatment (Figure 8).
Figure 8 Possible anti-Candida albicans properties of liquid smoke.
The limitations of this review arise from a lack of information regarding the final temperature of pyrolysis in the included studies. The final temperature of pyrolysis significantly influences the composition of liquid smoke, including its phenolic compounds, acidity, and residual products such as PAH and benzopyrene. Another limitation is the lack of compound analysis provided or conducted in most of the reviewed articles. Information on compound analysis is crucial to facilitate understanding of the active components of liquid smoke responsible for therapeutic effects. Despite these limitations, this review offers comprehensive insights into the potential health effects of liquid smoke, drawing from various studies using in vitro, in vivo, and in silico methods. Future research should focus on human trials to validate the potential therapeutic applications of liquid smoke as a drug regimen.
Conclusion
The therapeutic benefits of liquid smoke, including anti-oxidant, anti-inflammatory, anti-nociceptive, anti-bacterial, anti-fungal, and anti-viral effects, have been analyzed. Furthermore, health benefits have been demonstrated, including anti-inflammatory, anti-diabetic, anti-periodontitis, wound healing, and ulcer healing effects, as indicated by various markers associated with diseases that liquid smoke may influence. These findings may increase the use value of liquid smoke as a natural product with benefits for human health. Importantly, the liquid smoke reviewed herein is a condensed form of smoke containing water and some oxygenated compounds, thus mitigating inhalation toxicity concerns. Nevertheless, although current studies suggest minimal toxicity in vitro and in vivo, further research on the long-term health risks and safety of liquid smoke in various applications is recommended.
Funding
This research was funded by Universitas Airlangga in the schema Hibah Mandat Artikel Review, grant number 208/UN3.15/PT/2022
Author contribution
Conceptualization, MDCS and DSE; methodology, MDCS; software, MDCS; validation, SR and DM; formal analysis, MDCS and DSE; investigation, MDCS, UZ, WP, and PHC; resources, MDCS; data curation, MDCS and PHC; writing—original draft preparation, MDCS and PHC; writing—review and editing, SR, DM, BI, and DSE; visualization, MDCS and SR; supervision, DSE; project administration, DSE; funding acquisition, MDCS and DSE. All authors have read and agreed to the published version of the manuscript.
Data availability statement
The data are available on personal request to diah-s-e@fkg.unair.ac.id (DSE) and Meircurius-2015@fkg.unair.ac.id (MDCS).
Conflicts of interest
Diah Savitri Ernawati reports financial support provided by Airlangga University. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported herein.
Abbreviation
5-LOX, 5-lipoxygenase; AhR, aryl hydrocarbon receptor; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BaP, benzo[a]pyrene; BHK-21, baby hamster kidney 21; C6, complement component 6; CAT, catalase; FGF-2, fibroblast growth factor 2; CGT, gamma-glutamyl transpeptidase; COL-1, collagen 1; COX-2, cyclooxygenase 2; GCK, glucokinase; GLUT2, glutamate transporter 2; GOT, aspartate aminotransferase; GPx, glutathione peroxidase; GR, glutathione reductase; GSH, glutathione; GST, glutathione S-transferase; GTP, phosphoglycerate kinase; ICAM, intercellular adhesion molecules; IL-1β, interleukin 1β; IL-6, interleukin 6; iNOS, inducible nitric oxide; MDA, malondialdehyde; NDMA, N-nitroso dimethylamine; MPO, myeloperoxidase; NF-kB, nuclear factor kappa B; NO, nitric oxide; NPYR, N-nitroso pyrrolidine; Nrf2, nuclear factor erythroid 2–related factor 2; NTHZ, N-nitroso thiazolidine; LDH, lactate dehydrogenase; LTB4, leukotriene B4; PAH, polycyclic aromatic hydrocarbons; PDGF, platelet-derived growth factor; PEPK, phosphoenolpyruvate carboxykinase; PGE2, prostaglandin E2; POD, peroxidase; PPAR-γ, peroxisome proliferator-activated receptor gamma; PSSG, protein-S-glutathionylation; RAW264.7; RBL-2H3; ROS, reactive oxygen species; SARS-CoV-2, severe acute respiratory syndrome-corona virus-2; SOD, superoxide dismutase; TBA, thiobarbituric acid; TGF-β, transforming growth factor β; TNF-α, tumor necrosis factor α; TVB-N, volatile basic nitrogen; VEGF, vascular endothelial growth factor
Graphical abstract
The health benefits of liquid smoke derived from various biomass sources, including anti-inflammatory, antibacterial, anti-fungal, anti-viral, anti-diabetic, wound-healing and non-toxic.
Supplementary materials
Supplementary Material can be downloaded from https://bio-integration.org/wp-content/uploads/2024/11/bioi20240083_Supplemental.zip.
References
- Knowles ME, Gilbert J, McWeeny DJ. Phenols in smoked cured meats. Phenolic composition of commercial liquid smoke preparations and Derived Bacon. J Sci Food Agric 1975;26(2):189-96. [PMID: 1169661 DOI: 10.1002/jsfa.2740260209]
- Milly PJ, Toledo RT, Ramakrishnan S. Determination of minimum inhibitory concentrations of liquid smoke fractions. J Food Sci 2005;70(1):M12-7. [DOI: 10.1111/j.1365-2621.2005.tb09040.x]
- Donnelly LS, Ziegler GR, Acton JC. Effect of liquid smoke on the growth of lactic acid starter cultures used to manufacture fermented sausage. J Food Sci 1982;47(6):2074-5. [DOI: 10.1111/j.1365-2621.1982.tb12953.x]
- Catte M, Gancel F, Dzierszinski F, Tailliez R. Effects of water activity, NaCl and smoke concentrations on the growth of Lactobacillus plantarum ATCC 12315. Int J Food Microbiol 1999;52(1-2):105-8. [PMID: 10573397 DOI: 10.1016/s0168-1605(99)00118-x]
- Putnam KP, Bombick DW, Avalos JT, Doolittle DJ. Comparison of the cytotoxic and mutagenic potential of liquid smoke food flavourings, cigarette smoke condensate and wood smoke condensate. Food Chem Toxicol 1999;37(11):1113-8. [PMID: 10566883 DOI: 10.1016/s0278-6915(99)00104-0]
- Eklund MW, Pelroy GA, Paranjpye R, Peterson ME, Teeny FM. Inhibition of clostridium botulinum types A and E toxin production by liquid smoke and NaCI in hot-process smoke-flavored fish. J Food Prot 1982;45(10):935-41. [PMID: 30866266 DOI: 10.4315/0362-028X-45.10.935]
- Sofos JN, Maga JA, Boyle DL. Effect of ether extracts from condensed wood smokes on the growth of Aeromonas hydrophila and Staphylococcus aureus. J Food Sci 1988;53(6):1840-3. [DOI: 10.1111/j.1365-2621.1988.tb07856.x]
- Messina MC, Ahmad HA, Marchello JA, Gerba CP, Paquette MW. The effect of liquid smoke on Listeria monocytogenes. J Food Prot 1988;51(8):629-31. [PMID: 30991599 DOI: 10.4315/0362-028X-51.8.629]
- Faith NG, Yousef AE, Luchansky JB. Inhibition of Listeria monocytogenes by liquid smoke and isoeugenol, a phenolic component found in smoke. J Food Saf 1992;12(4):303-14. [DOI: 10.1111/j.1745-4565.1992.tb00086.x]
- Poysky FT, Paranjpye RN, Peterson ME, Pelroy GA, Guttman AE, et al. Inactivation of Listeria monocytogenes on hot-smoked salmon by the interaction of heat and smoke or liquid smoke†. J Food Prot 1997;60(6):649-54. [PMID: 31195567 DOI: 10.4315/0362-028X-60.6.649]
- Thurette J, Membre JM, Ching LH, Talkkiez R, Catteau M. Behavior of Listeria spp. in smoked fish products affected by liquid smoke, NaCI concentration, and temperature. J Food Prot 1998;61(11):1475-9. [DOI: 10.4315/0362-028X-61.11.1475]
- Vitt SM, Himelbloom BH, Crapo CA. Inhibition of Listeria innocua and L. Monocytogenes in a laboratory medium and cold-smoked salmon containing liquid smoke. J Food Saf 2001;21(2):111-25. [DOI: 10.1111/j.1745-4565.2001.tb00311.x]
- Lebois M, Connil N, Onno B, Prevost H, Dousset X. Effects of divercin V41 combined to NaCl content, phenol (liquid smoke) concentration and pH on Listeria monocytogenes ScottA growth in BHI broth by an experimental design approach. J Appl Microbiol 2004;96(5):931-7. [PMID: 15078508 DOI: 10.1111/j.1365-2672.2004.02221.x]
- Milly PJ, Toledo RT, Chen J. Evaluation of liquid smoke treated ready-to-eat (RTE) meat products for control of Listeria innocua M1. J Food Sci 2008;73(4):M179-83. [PMID: 18460134 DOI: 10.1111/j.1750-3841.2008.00714.x]
- Wendroff WL, Riha WE, Muehlenkamp E. Growth of molds on cheese treated with heat or liquid smoke. J Food Prot 1993;56(11):963-6. [PMID: 31113088 DOI: 10.4315/0362-028X-56.11.963]
- Siskos I, Zotos A, Taylor KA. The effect of drying, pressure and processing time on the quality of liquid-smoked trout (Salmo gairdnerii) fillets. J Sci Food Agric 2005;85(12):2054-60. [DOI: 10.1002/jsfa.2220]
- Abu-Ali JM, Barringer SA. Color and texture development of potato cylinders with liquid smoke during baking, frying and microwaving. J Food Process Preserv 2007;31(3):334-44. [DOI: 10.1111/j.1745-4549.2007.00129.x]
- Martinez O, Salmerón J, Guillén MD, Casas C. Texture profile analysis of meat products treated with commercial liquid smoke flavourings. Food Control 2004;15(6):457-61. [DOI: 10.1016/S0956-7135(03)00130-0]
- Kolsarici N, Güven T. Sivi Tütsü Kullaniminin Frankfurter Sosislerin Depolama Stabilitesine Etkisi. Turk J Vet Anim Sci 1998;22(4):379-88.
- Cordray JC, Huffman DL, Jones WR. Restructured pork from hot processed sow meat: effect of mechanical tenderization and liquid smoke. J Food Prot 1986;49(8):639-42. [PMID: 30959695 DOI: 10.4315/0362-028X-49.8.639]
- Schwanke S, Ikins WG, Kastner C, Brewer MS. Effect of liquid smoke on lipid oxidation in a beef model system and restructured roasts. J Food Lipids 1996;3(2):99-113. [DOI: 10.1111/j.1745-4522.1996.tb00058.x]
- Coronado SA, Trout GR, Dunshea FR, Shah NP. Effect of dietary vitamin E, fishmeal and wood and liquid smoke on the oxidative stability of bacon during 16 weeks’ frozen storage. Meat Sci 2002;62(1):51-60. [PMID: 22061191 DOI: 10.1016/s0309-1740(01)00226-1]
- Schwert R, Verlindo R, Cichoski AJ, Oliveira D, Valduga E. Comparative evaluation of liquid and traditional smoke on oxidative stability, color and sensory properties of Brazilian calabrese sausage. CyTA – J Food 2011;9(2):131-4. [DOI: 10.1080/19476337.2010.491581]
- Gedela S, Gamble RK, Macwana S, Escoubas JR, Muriana PM. Effect of inhibitory extracts derived from liquid smoke combined with postprocess pasteurization for control of Listeria monocytogenes on ready-to-eat meats†. J Food Prot 2007;70(12):2749-56. [PMID: 18095426 DOI: 10.4315/0362-028x-70.12.2749]
- Martinez O, Salmerón J, Guillén MD, Casas C. Effect of freezing on the physicochemical, textural and sensorial characteristics of salmon (Salmo salar) smoked with a liquid smoke flavouring. LWT – Food Sci Technol 2010;43(6):910-8. [DOI: 10.1016/j.lwt.2010.01.026]
- Martin EM, O’Bryan CA, Lary RY, Griffis CL, Vaughn KLS, et al. Spray application of liquid smoke to reduce or eliminate Listeria monocytogenes surface inoculated on frankfurters. Meat Sci 2010;85(4):640-4. [PMID: 20416807 DOI: 10.1016/j.meatsci.2010.03.017]
- Alcicek Z, Atar HH. The effects of salting on chemical quality of vacuum packed liquid smoked and traditional smoked rainbow trout (Oncorhyncus mykiss) fillets during chilled storage. J Ani Vet Adv 2010;9(22):2778-83. [DOI: 10.3923/javaa.2010.2778.2783]
- Alcicek Z. Determination shelf life and PAHs content of smoked anchovy (Engraulis encrasicolus, Linneaus, 1758) nugget with different level liquid smoke flavors during chilled storage. J Ani Vet Adv 2011;10(20):2691-5. [DOI: 10.3923/javaa.2011.2691.2695]
- Martinez O, Salmerón J, Guillén MD, Casas C. Sensorial and physicochemical characteristics of salmon (Salmo salar) treated by different smoking processes during storage. Food Sci Technol Int 2007;13(6):477-84. [DOI: 10.1177/1082013207087816]
- Şengör GF, Gün H, Kalafatoǧlu H. Determination of the amino acid and chemical composition of canned smoked mussels (Mytilus galloprovincialis, L.). Turk J Vet Anim Sci 2008;32(1):1-5.
- Dimitriadou D, Zotos A, Petridis D, Taylor AKD. Improvement in the production of smoked trout fillets (Salmo Gairdnerii) steamed with liquid smoke. Food Sci Technol Int 2008;14(1):67-77. [DOI: 10.1177/1082013208090077]
- Guillén MD, Ibargoitia ML. New components with potential antioxidant and organoleptic properties, detected for the first time in liquid smoke flavoring preparations. J Agric Food Chem 1998;46(4):1276-85. [DOI: 10.1021/jf970952x]
- Soldera S, Sebastianutto N, Bortolomeazzi R. Composition of phenolic compounds and antioxidant activity of commercial aqueous smoke flavorings. J Agric Food Chem 2008;56(8):2727-34. [PMID: 18348527 DOI: 10.1021/jf072117d]
- Guillén MD, Manzanos MJ. Extractable components of the aerial parts of Salvia lavandulifolia and composition of the liquid smoke flavoring obtained from them. J Agric Food Chem 1999;47(8):3016-27. [PMID: 10552602 DOI: 10.1021/jf981260r]
- Gomaa EA, Gray JI, Rabie S, Lopez-Bote C, Booren AM. Polycyclic aromatic hydrocarbons in smoked food products and commercial liquid smoke flavourings. Food Addit Contam 1993;10(5):503-21. [PMID: 8224319 DOI: 10.1080/02652039309374174]
- Anastasio A, Mercogliano R, Vollano L, Pepe T, Cortesi ML. Levels of benzo[a]pyrene (BaP) in “mozzarella di bufala campana” cheese smoked according to different procedures. J Agric Food Chem 2004;52(14):4452-5. [PMID: 15237951 DOI: 10.1021/jf049566n]
- Guillén MD, Manzanos MJ. Study of the volatile composition of an aqueous oak smoke preparation. Food Chem 2002;79(3):283-92. [DOI: 10.1016/S0308-8146(02)00141-3]
- Yabiku HY, Martins MS, Takahashi MY. Levels of benzo[a]pyrene and other polycyclic aromatic hydrocarbons in liquid smoke flavour and some smoked foods. Food Addit Contam 1993;10(4):399-405. [PMID: 8405579 DOI: 10.1080/02652039309374163]
- Guillen MD, Sopelana P, Partearroyo MA. Occurrence of polycyclic aromatic hydrocarbons in smoke flavourings. Polycycl Aromat Compd 2000;21(1-4):215-29. [DOI: 10.1080/10406630008028535]
- Hattula T, Elfving K, Mroueh UM, Luoma T. Use of liquid smoke flavouring as an alternative to traditional flue gas smoking of rainbow trout fillets (Oncorhynchus mykiss). LWT – Food Sci Technol 2001;34(8):521-5. [DOI: 10.1006/fstl.2001.0794]
- Varlet V, Serot T, Monteau F, Le Bizec B, Prost C. Determination of PAH profiles by GC–MS/MS in salmon processed by four cold-smoking techniques. Food Addit Contam 2007;24(7):744-57. [PMID: 17613060 DOI: 10.1080/02652030601139946]
- Kažimírová A, Jablonická A. Evaluation of potential mutagenic effect of the liquid smoke preparation UTP in vivo: cytogenetic analysis of mouse bone marrow. Mutat Res 1994;323(1-2):89-92. [PMID: 7508573 DOI: 10.1016/0165-7992(94)90050-7]
- Budijanto S, Hasbullah R, Prabawati S, Zuraida I. Identifikasi dan Uji Keamanan Asap Cair Tempurung Kelapa untuk Produk Pangan. J Pascapanen 2008;5(1):32-40.
- Yang JY, Kang MY, Nam SH, Friedman M. Antidiabetic effects of rice hull smoke extract in alloxan-induced diabetic mice. J Agric Food Chem 2012;60(1):87-94. [PMID: 22129064 DOI: 10.1021/jf2035077]
- Arundina I, Tantiana T, Diyatri I, Surboyo MDC, Adityasari R. Acute toxicity of liquid smoke of rice hull (Oryza sativa) on mice (Mus musculus). J Int Dent Med Res 2020;13(1):91-6.
- Arundina I, Diyatri I, Surboyo MDC. The component analysis of liquid smoke from rice hulls and its toxicity test on baby hamster kidney cells. J Pharm Phytother Res 2021;9(1):78-87. [DOI: 10.56499/jppres20.928_9.1.78]
- Budhy TI, Arundina I, Surboyo MDC, Halimah AN. The effects of rice husk liquid smoke in Porphyromonas gingivalis-induced periodontitis. Eur J Dent 2021;15(4):653-9. [PMID: 34041725 DOI: 10.1055/s-0041-1727554]
- Arundina I, Diyatri I, Kusumaningsih T, Surboyo MDC, Monica E, et al. The role of rice hull liquid smoke in the traumatic ulcer healing. Eur J Dent 2021;15:33-8. [PMID: 32777835 DOI: 10.1055/s-0040-1714445]
- Arundina I, Frimayanti N, Surboyo MDC, Budhy TI, Iskandar B, et al. In silico study of liquid smoke rice husk against COVID-19. Eur J Dent 2023;17:492-6. [PMID: 36075262 DOI: 10.1055/s-0042-1750776]
- Arundina I, Diyatri I, Juliastuti WS, Budhy TI, Surboyo MDC, et al. Nanoparticles of liquid smoke rice husk inhibit Porphyromonas gingivalis. Eur J Dent 2023;17:337-41. [PMID: 35820441 DOI: 10.1055/s-0042-1749154]
- Arundina I, Diyatri I, Juliastuti WS, Budhy TI, Surboyo MDC, et al. Osteoblast viability of liquid smoke rice hull and nanoparticles form as periodontitis treatment. Eur J Dent 2023;17:450-5. [PMID: 35803277 DOI: 10.1055/s-0042-1745772]
- Arundina I, Budhy T, Juliastuti W, Surboyo MC, Halimah A, et al. The expression of interleukin-1β and nuclear factor erythroid-2 in the periodontitis after treatment of liquid smoke rice hull. J Adv Pharm Technol Res 2022;13(2):95-9. [PMID: 35464663 DOI: 10.4103/2231-4040.321508]
- Yang JY, Moon E, Nam SH, Friedman M. Antidiabetic effects of rice hull smoke extract on glucose-regulating mechanism in type 2 diabetic mice. J Agric Food Chem 2012;60(30):7442-9. [PMID: 22803686 DOI: 10.1021/jf3017749]
- Arundina IRA, Diyatri I, Surboyo MDC, Halimah AN, Chusnurrafi FI. The antibacterial effect of liquid smoke rice hull on Porphyromonas gingivalis and its proliferative effects on osteoblast as periodontitis remedies: an invitro study. Int J Pharm Res 2020;12(3):3466-71. [DOI: 10.31838/ijpr/2020.12.03.490]
- Arundina I, Diyatri I, Surboyo MDC, Monica E, Afanda NM. Growth factor stimulation for the healing of traumatic ulcers with liquid rice hull smoke. J Taibah Univ Med Sci 2021;16(3):431-9. [PMID: 34140871 DOI: 10.1016/j.jtumed.2021.01.003]
- Kim SP, Yang JY, Kang MY, Park JC, Nam SH, et al. Composition of liquid rice hull smoke and anti-inflammatory effects in mice. J Agric Food Chem 2011;59(9):4570-81. [PMID: 21438497 DOI: 10.1021/jf2003392]
- Kim SP, Kang MY, Park JC, Nam SH, Friedman M. Rice hull smoke extract inactivates Salmonella Typhimurium in laboratory media and protects infected mice against mortality. J Food Sci 2012;77(1):80-5. [PMID: 22132793 DOI: 10.1111/j.1750-3841.2011.02478.x]
- Kim SP, Nam SH, Friedman M. Rice hull smoke extract protects mice against a salmonella lipopolysaccharide-induced endotoxemia. J Agric Food Chem 2014;62(31):7753-9. [PMID: 25068861 DOI: 10.1021/jf501533s]
- Tarawan VM, Mantilidewi KI, Dhini IM, Radhiyanti PT, Sutedja E. Coconut shell liquid smoke promotes burn wound healing. J Evid Based Complementary Altern Med 2017;22(3):436-40. [PMID: 27821610 DOI: 10.1177/2156587216674313]
- Surboyo MDC, Ernawati DS, Arundina I, Rahayu RP. Oral ulcer healing after treatment with distilled liquid smoke of coconut shell on diabetic rats. J Krishna Inst Med Sci Univ 2019;8(2):70-9.
- Surboyo MDC, Arundina I, Rahayu RP, Mansur D, Bramantoro T. Potential of distilled liquid smoke derived from coconut (Cocos nucifera L) shell for traumatic ulcer healing in diabetic rats. Eur J Dent 2019;13(2):271-9. [PMID: 31487751 DOI: 10.1055/s-0039-1693527]
- Surboyo MDC, Mahdani FY, Ernawati DS, Sarasati A, Rezkita F. The macrophage responses during diabetic oral ulcer healing by liquid coconut shell smoke: an immunohistochemical analysis. Eur J Dent 2020;14(3):410-4. [PMID: 32447753 DOI: 10.1055/s-0040-1712776]
- Ayuningtyas NF, Surboyo MDC, Ernawati DS, Parmadiati AE. The role of liquid smoke coconut shell in the proliferation phase of an oral traumatic ulcer. J Pharm Pharmacogn Res 2020;8(6):549-57.
- Surboyo MDC, Ernawati DS, Radithia D, Soebadi B, Mahdani FY, et al. Distilled liquid smoke coconut shell attenuates the cytokine profile of macrophages in oral ulcer in experimental model of diabetes mellitus. J Appl Pharm Sci 2021;11(08):62-9. [DOI: 10.7324/JAPS.2021.110809]
- Ernawati DS, Surboyo MDC, Ayuningtyas NF, Nagoro AAB. Role of inflammatory cell responses in stimulating fibroblasts in diabetic oral ulcer after treatment with liquid smoke of coconut endocarp: a histological assessment. Eur J Dent 2021;15(1):71-6. [DOI: 10.1055/s-0040-1715913]
- Surboyo MDC, Mahdani FY, Ayuningtyas NF, Santosh ABR, Ernawati DS, et al. The cytotoxicity, anti-inflammation, anti-nociceptive and oral ulcer healing properties of coconut shell liquid smoke. J Herbmed Pharmacol 2021;10(4):459-67. [DOI: 10.34172/jhp.2021.53]
- Saputra AAS, Prihatiningsih T, Santoso O. Antibacterial properties of hydrogel membranes infused with liquid smoke on growth inhibition of Staphylococcus aureus. Malaysian J Med Health Sci 2021;17(3):2636-9346.
- Surboyo MDC, Tantiana T, Arundina I. Analgesic effect of coconut shell (Cocos nucifera L) liquid smoke on mice. Dental J (Majalah Kedokteran Gigi) 2012;45(3):156-60. [DOI: 10.20473/j.djmkg.v45.i3.p156-160]
- Surboyo MDC, Arundina I, Rahayu RP. Increase of collagen in diabetes-related traumatic ulcers after the application of liquid smoke coconut shell. Dental J (Majalah Kedokteran Gigi) 2017;50(2):71-5. [DOI: 10.20473/j.djmkg.v50.i2.p71-75]
- Saputra S, Wibisono G, Ramadhani E, Dewi T. Hydrogel patch from liquid smoke and vitamin K Collard Greens extract for wound healing applications. IOP Conf Ser Earth Environ Sci 2021;762(1):012025. [DOI: 10.1088/1755-1315/762/1/012025]
- Faisal M. A preliminary study of the utilization of liquid smoke from Palm Kernel shells for organic mouthwash. Int J GEOMATE 2017;13(37):116-20. [DOI: 10.21660//2017.37.2734]
- Budaraga IK, Putra DP. Test liquid smoke toxicity for cocoa skin [Theobroma Cacao L.] with the BSLT method at different pyrolysis temperatures. IOP Conf Ser Earth Environ Sci 2021;741(1):012011. [DOI: 10.1088/1755-1315/741/1/012011]
- Watcharananun W, Chivapat S, Rangsripipat A, Attawish A, Chavalittumrong P, et al. Subchronic toxicity of liquid smoke from ‘Tian Op’ in Wistar rats. Kasetsart J Nat Sci 2013;47(1):42-52.
- Lin LW, Denison MS, Rice RH. Woodsmoke extracts cross-link proteins and induce cornified envelope formation without stimulating keratinocyte terminal differentiation. Toxicol Sci 2021;183(1):128-38. [PMID: 34086961 DOI: 10.1093/toxsci/kfab071]
- Selin E, Mandava G, Vilcu AL, Oskarsson A, Lundqvist J. An in vitro-based hazard assessment of liquid smoke food flavourings. Arch Toxicol. 2022;96(2):601-11. [PMID: 34799742 DOI: 10.1007/s00204-021-03190-1]
- Hu K, Chang R, Zhu Q, Wan J, Tang P, et al. Exploring the mechanism of liquid smoke and human taste perception based on the synergy of the electronic tongue, molecular docking, and multiple linear regression. Food Biophys 2020;15(4):482-94. [DOI: 10.1007/s11483-020-09632-0]
- Mansur D, Sugiwati S, Rizal WA, Suryani R, Maryana R. Pyrolysis of cajuput (Melaleuca leucadendron) twigs and rice (Oryza sativa) husks to produce liquid smoke-containing fine chemicals for antibacterial agent application. Biomass Conv Bioref 2023;13:10561-10574. [DOI: 10.1007/s13399-021-01896-x]
- Zuraida I, Sukarno S, Budijanto S. Antibacterial activity of coconut shell liquid smoke (CS-LS) and its application on fish ball preservation. Int Food Res J 2011;18:405-10.
- Rahmasari Y, Yemiş GP. Characterization of ginger starch-based edible films incorporated with coconut shell liquid smoke by ultrasound treatment and application for ground beef. Meat Sci 2022;188:108799. [PMID: 35303656 DOI: 10.1016/j.meatsci.2022.108799]
- Kailaku SI, Syakir M, Mulyawanti I, Syah ANA. Antimicrobial activity of coconut shell liquid smoke. IOP Conf Ser Mater Sci Eng 2017;206(1):012050. [DOI: 10.1088/1757-899X/206/1/012050]
- Widodo E, Pranibilan AAR, Ardilla YNN, Natsir MH, Djunaidi IH. Effect on encapsulated liquid smoke in combination with formic acid on intestinal development and microbial counts in broiler. IOP Conf Ser Earth Environ Sci 2021;788(1):012185. [DOI: 10.1088/1755-1315/788/1/012185]
- Nurliyani, Harmayani E. Characteristics of Goat Milk Cheese added with liquid smoke and porang glucomannan ripened with Lactobacillus rhamnosus. Int J Dairy Sci 2018;13(1):7-14. [DOI: 10.3923/ijds.2018.7.14]
- Dien HA, Montolalu RI, Berhimpon S. Liquid smoke inhibits growth of pathogenic and histamine forming bacteria on skipjack fillets. IOP Conf Ser Earth Environ Sci 2019;278(1):012018. [DOI: 10.1088/1755-1315/278/1/012018]
- Pasaribu T, Sinurat AP, Wina E, Cahyaningsih T. Evaluation of the phytochemical content, antimicrobial and antioxidant activity of Cocos nucifera liquid smoke, Garcinia mangostana pericarp, Syzygium aromaticum leaf, and Phyllanthus niruri L. extracts. Vet World 2021;14(11):3048-55. [DOI: 10.14202/vetworld.2021.3048-3055]
- Rahmawati M, Sudarno, Subekti S. The effect of coconut shell liquid smoke in commercial feed on total bacteria of Pseudomonas Aeruginosa in the Tilapia’s Kidney (Orechromis niloticus). IOP Conf Ser Earth Environ Sci 2019;236(1):012077. [DOI: 10.1088/1755-1315/236/1/012077]
- Rahmadini S, Sudarno, Subekti S. The effect of coconut shell liquid smoke in commercial feed towards total Pseudomonasaeruginosa bacteria on gastrointestinal tract tilapia (Oreochromis Niloticus). IOP Conf Ser Earth Environ Sci 2019;236(1):012078. [DOI: 10.1088/1755-1315/236/1/012078]
- Muriady, Meilina H, Faisal M. Antibacterial activity of liquid smoke powder from rice husk. Int J GEOMATE. 2022;23(95):89-96. [DOI: 10.21660/2022.95.7522]
- Desvita H, Faisal M, Mahidin, Suhendrayatna. Preservation of meatballs with edible coating of chitosan dissolved in rice hull-based liquid smoke. Heliyon 2020;6(10):e05228. [PMID: 33102852 DOI: 10.1016/j.heliyon.2020.e05228]
- Kim SP, Lee SJ, Nam SH, Friedman M. Mechanism of antibacterial activities of a rice hull smoke extract (RHSE) against multidrug-resistant salmonella typhimurium in vitro and in mice. J Food Sci 2018;83(2):440-5. [PMID: 29266224 DOI: 10.1111/1750-3841.14020]
- Achmadi SS, Mubarik NR, Nursyamsi R, Septiaji P. Characterization of redistilled liquid smoke of oil-palm shells and its application as fish preservatives. J Appl Sci 2013;13(3):401-8. [DOI: 10.3923/jas.2013.401.408]
- Achmadi SS, Kusumaningrum HD, Anggara I, Matematika F, Ilmu D, et al. Redistilled liquid smoke of oil-palm shells as a preservative for beef meatballs. J Teknol dan Industri Pangan 2015;26(1):1-8. [DOI: 10.6066/jtip.2015.26.1.1]
- Budaraga IK, Putra DP. Liquid smoke antimicrobial test of cocoa fruit peel against eschericia coli and staphylococcus aureus bacteria. IOP Conf Ser Earth Environ Sci 2019;365(1):012049. [DOI: 10.1088/1755-1315/365/1/012049]
- Budaraga IK, Yulita N, Yessirita N. Antibacterial study of cocoa skin liquid smoke in raw milk. IOP Conf Ser Earth Environ Sci 2021;803(1):012034. [DOI: 10.1088/1755-1315/803/1/012034]
- Putri RE, Kasim A, Emriadi, Asben A. Antibacterial effectiveness of cinnamon wood (Cinnamomum burmannii BL) liquid smoke obtained from different pyrolysis time. Asian J Plant Sci 2021;20(4):665-72. [DOI: 10.3923/ajps.2021.665.672]
- Pasaribu T, Kostaman T, Saenab A. The influence of the mixture of some plant powder with Anacardium occidentale shell liquid smoke on total bacteria and Escherichia coli in the ileum of broiler. IOP Conf Ser Earth Environ Sci 2021;888(1):012029. [DOI 10.1088/1755-1315/888/1/012029]
- Lingbeck JM, Cordero P, O’Bryan CA, Johnson MG, Ricke SC, et al. Temperature effects on the antimicrobial efficacy of condensed smoke and lauric arginate against Listeria and Salmonella. J Food Prot 2014;77(6):934-40. [PMID: 24853515 DOI: 10.4315/0362-028X.JFP-13-459]
- Tuesta-Chavez T, Monteza J, Silva Jaimes MI, Ruiz-Pacco GA, Changanaqui K, et al. Characterization and evaluation of antioxidant and antimicrobial capacity of prepared liquid smoke-loaded chitosan nanoparticles. J Food Eng 2021;319(4):110912. [DOI: 10.1016/j.jfoodeng.2021.110912]
- Saha A, Birkeland S, Løvdal T. The effect of K-lactate salt and liquid smoke on bacterial growth in a model system. J Aquatic Food Product Technol 2017;26(2):192-204. [DOI: 10.1080/10498850.2015.1110221]
- Takeda S, Uchiyama J, Sugita K, Enomoto H, Ahhmed AM, et al. Functionality of liquid smoke as an antimicrobial in cooked meat products: liquid smoke suppresses spoilage-related lactic acid bacteria. Food Sci Technol Res 2021;27(5):759-68. [DOI: 10.3136/fstr.27.759]
- Taormina PJ, Bartholomew GW. Validation of bacon processing conditions to verify control of Clostridium perfringens and Staphylococcus aureus. J Food Prot 2005;68(9):1831-9. [PMID: 16161681 DOI: 10.4315/0362-028x-68.9.1831]
- Cardinal M, Cornet J, Serot T, Baron R. Effects of the smoking process on odour characteristics of smoked herring (Clupea harengus) and relationships with phenolic compound content. Food Chem 2006;96(1):137-46. [DOI: 10.1016/j.foodchem.2005.02.040]
- Aydinol P, Ozcan T. The effect of natural and liquid smokes on the benzo[a] pyrene content and quality parameters of Circassian cheese. Int J Dairy Technol 2013;66(4):498-504. [DOI: 10.1111/1471-0307.12060]
- Gedela S, Escoubas JR, Muriana PM. Effect of inhibitory liquid smoke fractions on Listeria monocytogenes during long-term storage of frankfurters. J Food Prot 2007;70(2):386-91. [PMID: 17340873 DOI: 10.4315/0362-028x-70.2.386]
- Guilbaud M, Chafsey I, Pilet MF, Leroi F, Prévost H, et al. Response of Listeria monocytogenes to liquid smoke. J Appl Microbiol 2008;104(6):1744-53. [PMID: 18266702 DOI: 10.1111/j.1365-2672.2008.03731.x]
- Kin S, Schilling MW, Kim T, Smith BS, Silva JL, et al. Effects of potassium lactate and acetate on Listeria monocytogenes inhibition, physicochemical and sensory properties of smoked catfish fillets. J Aquatic Food Product Technol 2012;21(4):338-50. [DOI: 10.1080/10498850.2011.601436]
- Ekonomou SI, Bulut S, Karatzas KAG, Boziaris IS. Inactivation of Listeria monocytogenes in raw and hot smoked trout fillets by high hydrostatic pressure processing combined with liquid smoke and freezing. Innov Food Sci Emerg Technol 2020;64:102427. [DOI: 10.1016/j.ifset.2020.102427]
- Montazeri N, Himelbloom BH, Oliveira ACM, Leigh MB, Crapo CA. Refined liquid smoke: a potential antilisterial additive to cold-smoked sockeye salmon (Oncorhynchus nerka). J Food Prot 2013;76(5):812-9. [PMID: 23643122 DOI: 10.4315/0362-028X.JFP-12-368]
- Morey A, Bratcher CL, Singh M, McKee SR. Effect of liquid smoke as an ingredient in frankfurters on Listeria monocytogenes and quality attributes. Poult Sci 2012;91(9):2341-50. [PMID: 22912472 DOI: 10.3382/ps.2012-02251]
- Kilinç B, Çakli Ş. Growth of Listeria monocytogenes as affected by thermal treatments of rainbow trout fillets prepared with liquid smoke. Turk J Fish Aquat Sci 2012;12(2):285-90. [DOI: 10.4194/1303-2712-v12_2_13]
- Paranjpye RN, Peterson ME, Poysky FT, Pelroy GA, Eklund MW. Control of bacterial pathogens by liquid smoke and sodium lactate during processing of cold-smoked and dried salmon strips. J Aquatic Food Product Technol 2005;13(4):29-39. [DOI: 10.1300/J030v13n04_03]
- Pilevar Z, Hosseini H, Hajimehdipoo H, Shahraz F, Alizadeh L, et al. The anti-Staphylococcus aureus effect of combined Echinophora platyloba essential oil and liquid smoke in beef. Food Technol Biotechnol 2017;55(1):117-24. [DOI: 10.17113/ftb.55.01.17.4633]
- Van Loo EJ, Babu D, Crandall PG, Ricke SC. Screening of commercial and pecan shell-extracted liquid smoke agents as natural antimicrobials against foodborne pathogens. J Food Prot 2012;75(6):1148-52. [PMID: 22691487 DOI: 10.4315/0362-028X.JFP-11-543]
- Özpolat E. The effect of vacuum packaging on fish balls prepared from Capoeta trutta with different concentrations of liquid smoke. Food Sci Technol 2022;42:e28722. [DOI: 10.1590/fst.28722]
- Gram L. Inhibition of mesophilic spoilage Aeromonas spp. on fish by salt, potassium sorbate, liquid smoke, and chilling. J Food Prot 1991;54(6):436-42. [PMID: 31051620 DOI: 10.4315/0362-028X-54.6.436]
- Ristiani W, Yuniati R, Lestari R, Wardhana W. Application of coconut shell liquid smoke to control fusarium wilt disease on Hevea brasiliensis Muell. Arg. Agrivita J Agric Sci 2022;44(1):11-20. [DOI: 10.17503/agrivita.v44i1.2355]
- Budaraga IK, Putra DP, Yanti Y. Microbial activities and minimum liquid smoke killing concentration made of cacao pod toward Lasiodiplodia theobromae growth. IOP Conf Ser Earth Environ Sci 2022;1059(1):012068. [DOI: 10.1088/1755-1315/1059/1/012068]
- Budaraga IK, Tukiran, Syamsuwirman. Influence of liquid smoke cinnamon against attacks leaf rot disease (Phytophthora Infestans) on potato (Solanum Tuberosum L.). IOP Conf Ser Earth Environ Sci 2019;347(1):012036. [DOI: 10.1088/1755-1315/347/1/012036]
- Faisal M. A preliminary study of the utilization of Cu(II) modified liquid smoke to inhibit the activity of white-rot fungi (Schizophyllum commune Fr) in a pinewood in-vitro. Int J GEOMATE 2019;17(61):56-61. [DOI: 10.21660/2019.61.4679]
- Imaningsih W, Mariana M, Junaedi AB, Rasyidah R. Antifungal activities of the combination of ulin wood liquid smoke and hiyung cayenne pepper root endophytic fungi against Colletothricum capsici. AGRIVITA J Agricultural Sci 2021;43(1):69-78. [DOI: 10.17503/agrivita.v1i1.2458]
- Faisal M, Gani A, Husni H, Baihaqi A, Daimon H. Pyrolysis of oil Palm Kernel Shell into liquid smoke and its application to control anthracnose disease on Chili (Capsicum annum L.). J Eng Appl Sci 2016;11(12):2583-7. [DOI: 10.3923/jeasci.2016.2583.2587]
- Imaningsih W, Adventaria D, Mariana, Junaidi AB. Inhibitory effect of ulin wood liquid smoke and gogo rice endophytic fungi against pathogen Pyricularia oryzae. Biotropia (Bogor) 2022;29(1):23-32. [DOI: 10.11598/btb.2022.29.1.1568]
- Aisyah I, Sinaga MS, Nawangsih AA, Giyanto, Pari G. Utilization of liquid smoke to suppress blood diseases on bananas and its effects on the plant growth. AGRIVITA J Agric Sci 2018;40(3):453-60. [DOI: 10.17503/agrivita.v40i3.1390]
- Bande LOS, Muhidin, Gusnawaty HS, Mariadi, Nuriadi, et al. Effectiveness of botanical pesticides composite to decrease of Phytophthora palmivora caused black pod rot on cocoa. J Agronomy 2018;17(3):154-60. [DOI: 10.3923/ja.2018.154.160]
- Shimizu M, Nakama A, Yamano T, Noda T, Fujita T, et al. Role of gastric glutathione in smoke flavouring-induced gastric injury in rats. Food Chem Toxicol 1992;30(12):1005-9. [PMID: 1473793 DOI: 10.1016/0278-6915(92)90110-7]
- Malelak GEM, Benu I, Manu AE, Jelantik IGN. Nutritional value and color of se’i processed from cull cow meat from different body condition score and smoked at different smoke method. IOP Conf Ser Earth Environ Sci 2021;653(1):012041. [DOI 10.1088/1755-1315/653/1/012041]
- Abustam E, Said MI, Nahariah, Yusuf M. The influence of Moringa leaf flour ratio with smoke flour and maturation time on performance Bali beef Pectoralis profundus muscle characteristics. IOP Conf Ser Earth Environ Sci 2020;492(1):012039. [DOI: 10.1088/1755-1315/492/1/012039]
- Budaraga IK, Putra DP. Study of the physical properties of liquid smoke from cocoa rind on moisture content and different pyrolysis temperature. IOP Conf Ser Earth Environ Sci 2020;542(1):012045. [DOI: 10.1088/1755-1315/542/1/012045]
- Desvita H, Faisal M, Mahidin M, Suhendrayatna S. Edible coating for beef preservation from chitosan combined with liquid smoke. Int J Technol 2020;11(4):817. [DOI: 10.14716/ijtech.v11i4.4039]
- Yusnaini, Suryanto E. The effect of heating process using electric and gas ovens on sensory properties of cooked smoked-meat. IOP Conf Ser Earth Environ Sci 2019;247(1):012023. [DOI: 10.1088/1755-1315/247/1/012023]
- Abustam E, Said MI, Yusuf M. The effect of antioxidant activity of liquid smoke in feed supplement block on meat functional of muscle Longissimus dorsi. IOP Conf Ser Earth Environ Sci 2018;119(1):012046. [DOI: 10.1088/1755-1315/119/1/012046]
- Dimakopoulou-Papazoglou D, Katsanidis E. Effect of maltodextrin, sodium chloride, and liquid smoke on the mass transfer kinetics and storage stability of osmotically dehydrated beef meat. Food Bioproc Tech 2017;10(11):2034-45. [DOI: 10.1007/s11947-017-1973-5]
- Malelak GEM, Sipahelut GM, Jelantik IGN, Ratu MRD, Lalel HJD. Characteristics of se’i (Rotenesse Smoked Meat) treated with coconut shell liquid smoked and Citrus aurantifolia extract. Media Peternakan 2015;38(2):89-94. [DOI: 10.5398/medpet.2015.38.2.89]
- Yosi F, Sandi S. Meat quality, blood profile, and fecal ammonia concentration of broiler supplemented with liquid smoke. Media Peternakan 2014;37(3):169-74. [DOI: 10.5398/medpet.2014.37.3.169]
- Yusnaini, Soeparno, Suryanto E, Armunanto R. Physical, chemical and sensory properties of Kenari (Canariun indicum L.) shell liquid smoke-immersed-beef on different level of dilution. J Indonesian Trop Anim Agric 2012;37(1):27-33. [DOI: 10.14710/jitaa.37.1.27-33]
- Alçiçek Z, Zencir Ö, Çelik Çakiroğullari G, Atar HH. The effect of liquid smoking of anchovy (Engraulis encrasicolus, L. 1758) fillets on sensory, meat yield, polycyclic aromatic hydrocarbon (PAH) content, and chemical changes. J Aquat Food Prod Technol 2010;19(3-4):264-73. [DOI: 10.1080/10498850.2010.512995]
- Gonulalan Z, Kose A, Yetim H. Effects of liquid smoke on quality characteristics of Turkish standard smoked beef tongue. Meat Sci 2004;66(1):165-70. [PMID: 22063944 DOI: 10.1016/S0309-1740(03)00080-9]
- Saldaña E, Saldarriaga L, Cabrera J, Behrens JH, Selani MM, et al. Descriptive and hedonic sensory perception of Brazilian consumers for smoked bacon. Meat Sci 2019;147:60-9. [PMID: 30196202 DOI: 10.1016/j.meatsci.2018.08.023]
- Saldaña E, Castillo LS, Sánchez JC, Siche R, de Almeida MA, et al. Descriptive analysis of bacon smoked with Brazilian woods from reforestation: methodological aspects, statistical analysis, and study of sensory characteristics. Meat Sci 2018;140:44-50. [PMID: 29501932 DOI: 10.1016/j.meatsci.2018.02.014]
- Ikins WG, Gray JI, Mandagere AK, Booren AM, Pearson AM, et al. N-Nitrosamine formation in fried bacon processed with liquid smoke preparations. J Agric Food Chem 1986;34(6):980-5. [DOI: 10.1021/jf00072a012]
- Mujnisa A, Wijaya F, Etty. Liquid smoke supplementation in coconut water multi-nutrient block to nutrient consumption of quail. IOP Conf Ser Earth Environ Sci 2020;492(1):012018. [DOI: 10.1088/1755-1315/492/1/012018]
- Pertiwi MD, Swastawati F, Amalia U. Quality of brine boiled Indian mackerel (Rastrelliger sp.) with different cooking methods and additions of liquid smoke. IOP Conf Ser Earth Environ Sci 2020;404(1):012079. [DOI: 10.1088/1755-1315/404/1/012079]
- Faisal M, Gani A, Mulana F. Preliminary assessment of the utilization of durian peel liquid smoke as a natural preservative for mackerel. F1000Res 2019;8:240. [PMID: 32055394 DOI: 10.12688/f1000research.18095.6]
- Faisal M, Gani A, Husni. Utilization of liquid smoke from oil palm kernel shell to preserve mackerel. Rasayan J Chem 2018;11(3):1120-5. [DOI: 10.31788/RJC.2018.1132090]
- Setiadi, Karina S. Effect of edible coating based on whey protein-liquid smoke on preservation of Spanish mackerel fish (Scomberomorus commersoni). In: AIP Conference Proceedings. American Institute of Physics Inc.; 2020. pp. 040001. [DOI: 10.1063/5.0014133]
- de Souza MLR, Fernandes VRM, Gasparino E, Coutinho ME, Vianna VO, et al. Pantanal yacare (Caiman yacare) tail fillets subjected to traditional hot smoking and liquid smoke. J Sci Food Agric 2022;102(14):6423-31. [PMID: 35562846 DOI: 10.1002/jsfa.12009]
- Ruiz-Alonso SA, Girón-Hernández LJ, López-Vargas JH, Muñoz-Ramírez AP, Simal-Gandara J. Optimizing salting and smoking conditions for the production and preservation of smoked-flavoured tilapia fillets. LWT 2021;138:110733. [DOI: 10.1016/j.lwt.2020.110733]
- Çakir F, Ayvaz Z. Investigation of the effect of different immersion times of anchovy fillets in liquid smoke flavoring on color by image analysis. J Aquat Food Prod Technol 2020;29(9):865-70. [DOI: 10.1080/10498850.2020.1813857]
- Wijayanti I, Swastawati F, Ambariyanto A, Cahyono B, Chilmawati D. Application of filtration and re-distillation of liquid smoke as flavouring agent on texture, proximate and sensory characteristics of milkfish (Chanos chanos) fishballs. Afr J Food Agric Nutr Dev 2020;20(2):15569-81. [DOI: 10.18697/ajfand.90.17940]
- Swastawati F, Al-Baari AN, Susanto E, Purnamayati L. The effect of antioxidant and antibacterial liquid smoke nanocapsules on catfish fillet (Pangasius sp.) during storage at room temperature and cold temperature. Carpathian J Food Sci Technol 2019;11(4):165-75. [DOI: 10.34302/2019.11.4.16]
- Sari E, Khatab U, Burmawi, Rahman ED, Afriza F, et al. Production of liquid smoke from the process of carbonization of durian skin biomass, coconut shell and palm shell for preservation of tilapia fish. IOP Conf Ser Mater Sci Eng 2019;543(1):012075. [DOI: 10.1088/1757-899X/543/1/012075]
- Hadanu R, Lomo CP. Organoleptic test analysis and effect of liquid smoke concentration on smoked fish. IOP Conf Ser Earth Environ Sci 2019;382(1):012017. [DOI: 10.1088/1755-1315/382/1/012017]
- Apituley DAN, Loppies CRM. Fatty acid profile of smoked tuna processed with liquid smokes. Sci Stud Res: Chem Chem Eng Biotechnol Food Ind 2019;20(3):329-38.
- Martínez O, Salmerón J, Epelde L, Vicente MS, de Vega C. Quality enhancement of smoked sea bass (Dicentrarchus labrax) fillets by adding resveratrol and coating with chitosan and alginate edible films. Food Control 2018;85:168-76. [DOI: 10.1016/j.foodcont.2017.10.003]
- Ozpolat E, Patir B. Combined effect of different casing and liquid smoked concentration on the shelf-life of sausages produced from fish (Capoeta umbla). Indian J Anim Res 2016;51(5):956-61. [DOI: 10.18805/ijar.v0iOF.6821]
- Kong KJW, Alçiçek Z, Balaban MO. Effects of dry brining, liquid smoking and high-pressure treatment on the physical properties of aquacultured King salmon (Oncorhynchus tshawytscha) during refrigerated storage. J Sci Food Agric 2015;95(4):708-14. [PMID: 24862325 DOI: 10.1002/jsfa.6754]
- Saloko S, Darmadji P, Setiaji B, Pranoto Y. Antioxidative and antimicrobial activities of liquid smoke nanocapsules using chitosan and maltodextrin and its application on tuna fish preservation. Food Biosci 2014;7:71-9. [DOI: 10.1016/j.fbio.2014.05.008]
- Alçiçek Z. The effects of thyme (Thymus vulgaris L.) oil concentration on liquid-smoked vacuum-packed rainbow trout (Oncorhynchus mykiss Walbaum, 1792) fillets during chilled storage. Food Chem 2011;128(3):683-8. [DOI: 10.1016/j.foodchem.2011.03.087]
- Berhimpon S, Montolalu RI, Dien HA, Mentang F, Meko AUI. Concentration and application methods of liquid smoke for exotic smoked Skipjack (Katsuwonus pelamis L.). Int Food Res J 2018;25(5):1864-9.
- Swastawati F, Purnamayati L, Purnawati RD. Application of liquid smoke from corncob and coconut shell to the fillet of catfish (Pangasius sp.). AACL Bioflux 2019;12(6):2339-46.
- Chatzikyriakidou K, Katsanidis E. Effect of liquid smoke dipping and packaging method on the keeping quality of raw and cooked chub mackerel (Scomber japonicus) fillets. J Aquat Food Prod Technol 2012;21(5):445-54. [DOI: 10.1080/10498850.2011.608918]
- Suprapto H, Kumalaningsih S, Wignyanto W, Santoso I, Sucipto S. Optimization of stingray (Trygon sephen) smoking to extend shelf life and consumer preferences using response surface methodology. IOP Conf Ser Earth Environ Sci 2021;733(1):012114. [DOI: 10.1088/1755-1315/733/1/012114]
- Rakhmayeni DA, Yuniarti T, Sukarno S. Application of liquid smoke from coconut shell in tandipang (Dussumeiria acutta) smoked fish to extend shelf life. J Ilm Perikan dan Kelaut 2020;12(2):315-23. [DOI: 10.20473/jipk.v12i2.20790]
- Martinez O, Salmerón J, Guillén MD, Casas C. Textural and physicochemical changes in salmon (Salmo salar) treated with commercial liquid smoke flavourings. Food Chem 2007;100(2):498-503. [DOI: 10.1016/j.foodchem.2005.09.071]
- Siskos I, Zotos A, Melidou S, Tsikritzi R. The effect of liquid smoking of fillets of trout (Salmo gairdnerii) on sensory, microbiological and chemical changes during chilled storage. Food Chem 2007;101(2):458-64. [DOI: 10.1016/j.foodchem.2006.02.002]
- Leviyani RA, Kurniasih RA, Swastawati F. Application of liquid smoke for Chikuwa tilapia. IOP Conf Ser Earth Environ Sci 2019;246(1):012084. [DOI: 10.1088/1755-1315/246/1/012084]
- Faisal M, Desvita H, Abubakar Y, Azwar. A preliminary study on the use of rice husk-based smoke powder for meatball preservatives. J Food Qual 2022;2022:1-8. [DOI: 10.1155/2022/7915258]
- Indiarto R, Nurhadi B, Tensiska, Subroto E, Istiqamah YK. Effect of liquid smoke on microbiological and physico-chemical properties of beef meatballs during storage. Food Res 2019;4(2):522-31. [DOI: 10.26656/fr.2017.4(2).341]
- Abustam E, Said MI, Yusuf M, Ali HM. Effect of aging time on changes in smoke flour compounds on meatballs and fresh meat of Bali beef. IOP Conf Ser Earth Environ Sci 2019;247(1):012026. [DOI: 10.1088/1755-1315/247/1/012026]
- Widayat W, Arifiani SN, Yaqin N, Al Baarri AN. Study of utilization liquid smoke and carrageenan as a natural antibacterial in manufacturing beef meatballs. IOP Conf Ser Earth Environ Sci 2018;102(1):012060. [DOI: 10.1088/1755-1315/102/1/012060]
- Swastawati F, Ambaryanto, Cahyono B, Wijayanti I, Chilmawati D. Characterizations of milkfish (Chanos chanos) meatballs as effect of nanoencapsulation liquid smoke addition. IOP Conf Ser Earth Environ Sci 2018;116(1):012027. [DOI: 10.1088/1755-1315/116/1/012027]
- Arnim, Ferawati, Marlida Y. The effect of liquid smoke utilization as preservative for meatballs quality. Pak J Nutr 2012;11(11):1078-80. [DOI: 10.3923/pjn.2012.1078.1080]
- de Araújo IB, Raúl LJ, Maciel MIS, Shinohara NKS, Campagnoli de Oliveira Filho PR. Effect of traditional and liquid smoke on the quality of sea catfish sausages (Sciades herzbergii, Bloch, 1794). J Aquat Food Prod Technol 2020;29(6):553-66. [DOI: 10.1080/10498850.2020.1774021]
- Bhuyan D, Das A, Laskar SK, Bora DP, Tamuli S, Hazarika M. Effect of different smoking methods on the quality of pork sausages. Vet World 2018;11(12):1712-9. [PMID: 30774263 DOI: 10.14202/vetworld.2018.1712-1719]
- Mahastishotorbani P, Majidi P, Ahari H. Investigation of liquid smoke’s composition from peach tree wood using GC-Massand its microbial and physiochemical properties on hotdogs. Orient J Chem 2017;33(1):439-49. [DOI: 10.13005/ojc/330151]
- Dos Santos Alves LAA, Lorenzo JM, Gonçalves CAA, Dos Santos BA, Heck RT, et al. Impact of lysine and liquid smoke as flavor enhancers on the quality of low-fat Bologna-type sausages with 50% replacement of NaCl by KCl. Meat Sci 2017;123:50-6. [PMID: 27614180 DOI: 10.1016/j.meatsci.2016.09.001]
- Yin X, Chen Q, Liu Q, Wang Y, Kong B. Influences of smoking in traditional and industrial conditions on flavour profile of Harbin red sausages by comprehensive two-dimensional gas chromatography mass spectrometry. Foods 2021;10(6):1180. [PMID: 34073832 DOI: 10.3390/foods10061180]
- Swastawati F, Ambariyanto A, Cahyono B, Wijayanti I, Chilmawati D, et al. Physicochemical changes and sensory quality of liquid smoked milkfish nuggets. Afr J Food Agric Nutr Dev 2021;21(07):18261-78. [DOI: 10.18697/ajfand.102.19440]
- Swastawati F, Riyadi PH, Mulyono M, Nugraheni A, Muniroh M, Hidayati AN. Effectiveness of liquid smoke as a source of acetic acid in lowering heavy metals levels in blood cockle (Anadara granosa). IOP Conf Ser Earth Environ Sci 2022;1036(1):012010. [DOI: 10.1088/1755-1315/1036/1/012010]
- Nieva-Echevarría B, Goicoechea E, Guillén MD. Effect of liquid smoking on lipid hydrolysis and oxidation reactions during in vitro gastrointestinal digestion of European sea bass. Food Res Int 2017;97:51-61. [PMID: 28578064 DOI: 10.1016/j.foodres.2017.03.032]
- Alcicek Z. Effects of different liquid smoke flavor levels on the shelf life of venus clam (Chamelea Gallina, L 1758) meat. J Food Process Preserv 2014;38(3):964-70. [DOI: 10.1111/jfpp.12052]
- Petridis D, Zotos A, Kampouris T, Roumelioti Z. Optimization of a steaming with liquid smoke smoking process of Mediterranean mussel (Mytilus galloprovincialis). Food Sci Technol Int 2013;19(1):59-68. [PMID: 23239761 DOI: 10.1177/1082013212442183]
- Daniswara NP, Swastawati F, Purnamayati L. Fatty acid profile and cholesterol level of smoked whiteleg shrimp (Litopenaeus vannamei) with different liquid smoke concentrations. IOP Conf Ser Earth Environ Sci 2020;404(1):012053. [DOI: 10.1088/1755-1315/404/1/012053]
- Portella CG, Sant’Ana LS, Valenti WC. Sensory aspects of liquid smoking of giant river prawn: comparison with traditional smoking. Int J Food Sci Technol 2011;46(4):834-9. [DOI: 10.1111/j.1365-2621.2011.02564.x]
- Kurniawan CW, Atmaka W, Manuhara GJ, Sanjaya AP. Quality characteristic of liquid smoked straw mushroom (Volvariella volvacea) ball during storage. IOP Conf Ser Earth Environ Sci 2018;102(1):012094. [DOI: 10.1088/1755-1315/102/1/012094]
- Yost M, Abu-Ali JM, Barringer SA. Kinetics of potato color and texture development during baking, frying, and microwaving with the addition of liquid smoke. J Food Sci 2006;71(9):E364-9. [DOI: 10.1111/j.1750-3841.2006.00171.x]
- Nithin CT, Joshy CG, Chatterjee NS, Panda SK, Yathavamoorthi R, et al. Liquid smoking – A safe and convenient alternative for traditional fish smoked products. Food Control 2020;113:107186. [DOI: 10.1016/j.foodcont.2020.107186]
- Qomariyah N, Retnani Y, Jayanegara A, Wina E, Permana IG. Influence of biochar and liquid smoke additives from cacao-pod husks on in vitro ruminal fermentation characteristics. Adv Anim Vet Sci 2020;9(4):533-43. [DOI: 10.17582/journal.aavs/2021/9.4.533.543]
- Tiven NC, Hartati L, Simanjorang TM. Liquid smoke as fat protector and its effect on rumen fermentation characteristics and microbial activity. Trop Anim Sci J 2021;44(2):152-9. [DOI: 10.5398/tasj.2021.44.2.152]
- Piccirilli GN, Soazo M, Pérez LM, Delorenzi NJ, Verdini RA. Effect of storage conditions on the physicochemical characteristics of edible films based on whey protein concentrate and liquid smoke. Food Hydrocoll 2019;87:221-8. [DOI: 10.1016/j.foodhyd.2018.08.015]
- Wang W, Li C, Zhang H, Ni Y. Using liquid smoke to improve mechanical and water resistance properties of gelatin films. J Food Sci 2016;81(5):E1151-7. [PMID: 27061211 DOI: 10.1111/1750-3841.13282]
- Mulyawanti I, Kailaku SI, Syah ANA, Risfaheri. Chemical identification of coconut shell liquid smoke. IOP Conf Ser Earth Environ Sci. 2019;309(1):012020. [DOI: 10.1088/1755-1315/309/1/012020]
- Esposito M, Citro A, Marigliano L, Urbani V, Seccia G, et al. Influence of different smoking techniques on contamination by polycyclic aromatic hydrocarbons in traditional smoked Mozzarella di Bufala Campana. Int J Dairy Technol. 2015;68(1):97-104. [DOI: 10.1111/1471-0307.12179]
- Nithin CT, Chatterjee NS, Joshy CG, Yathavamoorthi R, Ananthanarayanan TR, et al. Source-dependent compositional changes in coconut flavoured liquid smoke and its application in traditional Indian smoked fishery products. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2020;37(10):1610-20. [PMID: 32790498 DOI: 10.1080/19440049.2020.1798030]
- Visciano P, Perugini M, Conte F, Amorena M. Polycyclic aromatic hydrocarbons in farmed rainbow trout (Oncorhynchus mykiss) processed by traditional flue gas smoking and by liquid smoke flavourings. Food Chem Toxicol 2008;46(5):1409-13. [PMID: 18262709 DOI: 10.1016/j.fct.2008.01.001]
- Maulina S, Sinaga FAF. The opportunities of oil palm fronds become a commercial liquid smoke. Mater Sci Forum 2019;948:159-66. [DOI: 10.4028/www.scientific.net/MSF.948.159]
- Maulina S, Silia F. Liquid smoke characteristics from the pyrolysis of oil palm fronds. IOP Conf Ser Mater Sci Eng 2018;309(1):012073. [DOI: 10.1088/1757-899X/309/1/012073]
- Rizal WA, Nisa’ K, Maryana R, Prasetyo DJ, Pratiwi D, et al. Chemical composition of liquid smoke from coconut shell waste produced by SME in Rongkop Gunungkidul. IOP Conf Ser Earth Environ Sci 2020;462(1):012057. [DOI: 10.1088/1755-1315/462/1/012057]
- Desvita H. Characteristic of liquid smoke produced from slow pyrolysis of cacao pod shells (Theobroma cacao L). Int J GEOMATE 2021;20(80):17-22. [DOI: 10.21660/2021.80.6154]
- Putri RE, Kasim A, Emriadi, Asben A. Pyrolysis and characterization of liquid smoke from cacao pod husks. IOP Conf Ser Earth Environ Sci 2019;327(1):012011. [DOI: 10.1088/1755-1315/327/1/012011]
- Ni’mah L, Setiawan MF, Prabowo SP. Utilization of waste palm kernel shells and empty palm oil bunches as raw material production of liquid smoke. IOP Conf Ser Earth Environ Sci 2019;366(1):012032. [DOI: 10.1088/1755-1315/366/1/012032]
- Komarayati S, Gusmailina, Hendra D, Winarni I. Characteristics of liquid smoke from several kinds of waste. IOP Conf Ser Mater Sci Eng 2020;935(1):012041. [DOI: 10.1088/1757-899X/935/1/012041]
- Swastawati F, Romadhon. Utilization of liquid smoke nanoencapsulation in fresh fish fillets as a preservation material. IOP Conf Ser Earth Environ Sci 2020;530(1):012001. [DOI: 10.1088/1755-1315/530/1/012001]
- Hidayati B, Sipahutar R, Bizzy I, Faizal M. Increased productivity of liquid smoke through fast thawing with refrigeration systems at low air temperatures. J Appl Eng Sci 2022;20(1):79-84. [DOI: 10.5937/jaes0-30849]
- Faisal M, Gani A, Mulana F, Desvita H, Kamaruzzaman S. Effects of pyrolysis temperature on the composition of liquid smoke derived from oil palm empty fruit bunches. Rasayan J Chem 2020;13(1):514-20. [DOI: 10.31788/RJC.2020.1315507]
- Faisal M, YelviaSunarti AR, Desvita H. Characteristics of liquid smoke from the pyrolysis of durian peel waste at moderate temperatures. Rasayan J Chem 2018;11(2):871-6. [DOI: 10.31788/RJC.2018.1123035]
- Ifa L, Yani S, Mandasini, Sabara Z, Nurjannah N, et al. Production of phenol from liquid smoke resulted by the pyrolysis of cashew nut shells. IOP Conf Ser Earth Environ Sci 2018;175(1):012033. [DOI: 10.1088/1755-1315/175/1/012033]
- Suryanto E, Taroreh MRI, Momuat LI. Purification and characterization of phenolic antioxidant from corncob liquid smoke. Asian J Chem 2020;32(12):2985-90. [DOI: 10.14233/ajchem.2020.22486]
- Maulina S, Amalia R, Kamny ER. Effect of pyrolisis temperature and time on liquid smoke characteristics. In: Soleh Setiyawan A, Dwi Ariesyady H, Nastiti A, Roosmini D, Sonny Abfertiawan M, editors. E3S Web of Conferences. 2020;148:02007. [DOI: 10.1051/e3sconf/202014802007]
- Handayani T, Xyzquolyna D, Pranoto Y, Suratman A. Reduction of Pb(II) ion in soybean seeds (Glycine max) using corncob liquid smoke. IOP Conf Ser Earth Environ Sci 2019;292(1):012003. [DOI: 10.1088/1755-1315/292/1/012003]
- Xin X, Bissett A, Wang J, Gan A, Dell K, et al. Production of liquid smoke using fluidised-bed fast pyrolysis and its application to green lipped mussel meat. Food Control 2021;124:107874. [DOI: 10.1088/1755-1315/292/1/012003]
- Sriharti, Indriati A, Saparita R. Utilization of liquid smoke corn cobs for germination tomato (Solanum lycopersicum) seeds. IOP Conf Ser Earth Environ Sci 2020;462(1):012049. [DOI: 10.1088/1755-1315/462/1/012049]
- Andy, Malaka R, Purwanti S, Ali HM, Aulyani TL. Liquid smoke characteristic from coconut shell and rice husk. IOP Conf Ser Earth Environ Sci. 2021;788(1):012078. [DOI: 10.1088/1755-1315/788/1/012078]
- Rahmawati D, Mansur D, Sulaswatty A, Diastuti H. Conversion of cardamom by-product into liquid smoke and biochar by pyrolysis. Malays J Chem 2022;24(2):293-301.
- Sulhatun, Hasibuan R, Harahap H, Iriani, Fithra H. Improving production of liquid smoke from candlenut shell by pyrolisis process. In: Proceedings of MICoMS 2017. Emerald Reach Proceedings Series. Leeds: Emerald Group Holdings Ltd.; 2018. pp. 143-9. [DOI: 10.1108/978-1-78756-793-1-00056]
- Alphonse ST, Mbougueng PD, Sachindra NM, Douanla NFN, Léopold TN. Characterization of volatile compounds of liquid smoke flavourings from some tropical hardwoods. Sci Afr 2020;8:e00443. [DOI: 10.1016/j.sciaf.2020.e00443]
- Sulhatun, Hasibuan R, Harahap H, Iriani. Effect of pyrolysis temperature and time of liquid smoke product from candlenut shell by pyrolysis process. Int J Recent Technol Eng 2019;8(3S):343-7. [DOI: 10.35940/ijrte.C1073.1083S19]
- Sulhatun S, Muhammad M, Suryati S, Meriatna M, Hakim L, et al. Determining and evaluating the pyrolysis of candlenut shell as an alternative energy sources. In: Proceedings of the 4th European International Conference on Industrial Engineering and Operations Management. Rome, Italy: IEOM Society International; 2021. pp. 263-8. [DOI: 10.46254/EU04.20210776]
- Yusnaini, Suryanto E, Hasan S, Wulansari A, Dewi EK. The characteristics of volatile compounds of Kenari (Canarium indicum L.) shell liquid smoke. IOP Conf Ser Earth Environ Sci 2021;709(1):012032. [DOI: 10.1088/1755-1315/709/1/012032]
- Maulina S, Sinaga FA. Improving the quality of liquid smoke from oil palm fronds through adsorption and distillation processes. IOP Conf Ser Mater Sci Eng 2020;801(1):012062. [DOI: 10.1088/1757-899X/801/1/012062]
- Maulina S, Amalia R. Quality improvement of liquid smoke from oil palm frond pyrolysis through the adsorption-distillation process using activated carbon as adsorbent. IOP Conf Ser Mater Sci Eng. 2020;801(1):012064. [DOI: 10.1088/1757-899X/801/1/012064]
- Wijaya M, Wiharto M, Auliah A. Characterization compound chemical from cocoa waste as acetic acid and phenol. J Phys Conf Ser. 2021;1918(3):032014. [DOI: 10.1088/1742-6596/1918/3/032014]
- Ratnani RD, Hadiyanto H, Widiyanto W, Adhi MA. Characterization of liquid smoke from dried water hyacinth using GCMS (gas chromatography-mass spectrophotometry) to utilize weeds as food preservative. J Pendidik IPA Indones. 2022;11(2):208-18. [DOI: 10.15294/jpii.v11i2.34501]
- Sulhatun, Hasibuani R, Harahap H, Iriani. Influence temperatur of pyrolisis process on production of liquid smoke from candlenut shell by examining its potensial coumpound. Int J Recent Technol Eng 2019;8(3S):285-90. [DOI: 10.35940/ijrte.C1064.1083S19]
- Faisal M, Gani A, Hidayati ARN, Mardhatillah P, Husni H. In-vitro antifungal effect of the cayenne leaf extract modified with liquid smoke from cocoa pod husk to control F. oxysporum on chili (Capsicum annuum L.). Rasayan J Chem 2021;14(2):705-11. [DOI: 10.31788/RJC.2021.1426239]
- Majid ZANM, Rahmawati L, Riyani C. Identification of bio-oil chemical compounds from pyrolysis process of oil palm empty fruit bunches. IOP Conf Ser Earth Environ Sci 2022;1063(1):012001. [DOI: 10.1088/1755-1315/1063/1/012001]
- Oramahi HA, Kustiati, Wardoyo ERP. Optimization of liquid smoke from Shorea pachyphylla using response surface methodology and its characterization. Sci Technol Indones 2022;7(2):257-62. [DOI: 10.26554/sti.2022.7.2.257-262]
- Junaidi AB, Nursyifa A, Abdullah. Redistillation and characterization of liquid smoke from ulin wood (Eusideroxylon zwageri Teijsm. & Binn.) and its ability as a chitosan solvent. IOP Conf Ser Mater Sci Eng 2020;980(1):012024. [DOI: 10.1088/1757-899X/980/1/012024]
- Mallah MA, Changxing L, Mallah MA, Noreen S, Liu Y, et al. Polycyclic aromatic hydrocarbon and its effects on human health: an overeview. Chemosphere 2022;296:133948. [PMID: 35151703 DOI: 10.1016/j.chemosphere.2022.133948]
- Shiue I. Are urinary polyaromatic hydrocarbons associated with adult hypertension, heart attack, and cancer? USA NHANES, 2011–2012. Environ Sci Pollut Res Int 2015;22(21):16962-8. [PMID: 26111752 DOI: 10.1007/s11356-015-4922-8]
- Hu Z, Li Y, Yang Y, Yu W, Xie W, et al. Serum lipids mediate the relationship of multiple polyaromatic hydrocarbons on non-alcoholic fatty liver disease: a population-based study. Sci Total Environ 2021;780:146563. [PMID: 34030288 DOI: 10.1016/j.scitotenv.2021.146563]
- Khillare PS, Hasan A, Sarkar S. Accumulation and risks of polycyclic aromatic hydrocarbons and trace metals in tropical urban soils. Environ Monit Assess 2014;186(5):2907-23. [PMID: 24374784 DOI: 10.1007/s10661-013-3589-1]
- Szulcek R, Grune J, Kuebler WM. The updated cancer paradigm of PAH: recognizing complexity. Am J Physiol Lung Cell Mol Physiol 2020;318(6):L1111-4. [PMID: 32320264 DOI: 10.1152/ajplung.00159.2020]
- Lee DG, Burstyn I, Lai AS, Grundy A, Friesen MC, et al. Women’s occupational exposure to polycyclic aromatic hydrocarbons and risk of breast cancer. Occup Environ Med 2019;76(1):22-9. [PMID: 30541747 DOI: 10.1136/oemed-2018-105261]
- Wagner M, Bolm-Audorff U, Hegewald J, Fishta A, Schlattmann P, et al. Occupational polycyclic aromatic hydrocarbon exposure and risk of larynx cancer: a systematic review and meta-analysis. Occup Environ Med 2015;72(3):226-33. [PMID: 25398415 DOI: 10.1136/oemed-2014-102317]
- Okaru AO, Rullmann A, Farah A, Gonzalez de Mejia E, Stern MC, et al. Comparative oesophageal cancer risk assessment of hot beverage consumption (coffee, mate and tea): the margin of exposure of PAH vs very hot temperatures. BMC Cancer 2018;18(1):236. [PMID: 29490609 DOI: 10.1186/s12885-018-4060-z]
- Singh A, Kamal R, Ahamed I, Wagh M, Bihari V, et al. PAH exposure-associated lung cancer: an updated meta-analysis. Occup Med (Lond). 2018;68(4):255-61. [PMID: 29579260 DOI: 10.1093/occmed/kqy049]
- Umeh AC, Duan L, Naidu R, Esposito M, Semple KT. In vitro gastrointestinal mobilization and oral bioaccessibility of PAHs in contrasting soils and associated cancer risks: focus on PAH nonextractable residues. Environ Int. 2019;133:105186. [PMID: 31639608 DOI: 10.1016/j.envint.2019.105186]
- Adly HM, Saleh SA. The association of increased oxidative stress and tumor biomarkers related to polyaromatic hydrocarbons exposure for different occupational workers in Makkah, Saudi Arabia. Cureus 2022;14(12):e32981. [PMID: 36578859 DOI: 10.7759/cureus.32981]
- Xu C, Liu Q, Liang J, Weng Z, Xu J, et al. Urinary biomarkers of polycyclic aromatic hydrocarbons and their associations with liver function in adolescents. Environ Pollut 2021;278:116842. [PMID: 33711626 DOI: 10.1016/j.envpol.2021.116842]
- Branco V, Matos B, Mourato C, Diniz M, Carvalho C, et al. Synthesis of glutathione as a central aspect of PAH toxicity in liver cells: a comparison between phenanthrene, Benzo[b]Fluoranthene and their mixtures. Ecotoxicol Environ Saf 2021;208:111637. [PMID: 33396157 DOI: 10.1016/j.ecoenv.2020.111637]
- Ishida R, Ueda K, Kitano T, Yamamoto T, Mizutani Y, et al. Fatal community-acquired Bacillus cereus pneumonia in an immunocompetent adult man: a case report. BMC Infect Dis 2019;19(1):197. [PMID: 30813918 DOI: 10.1186/s12879-019-3836-3]
- Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015;28(3):603-61. [PMID: 26016486 DOI: 10.1128/CMR.00134-14]
- Marco S, Rullo R, Albino A, Masullo M, De Vendittis E, et al. The thioredoxin system in the dental caries pathogen Streptococcus mutans and the food-industry bacterium Streptococcus thermophilus. Biochimie 2013;95(11):2145-56. [PMID: 23954859 DOI: 10.1016/j.biochi.2013.08.008]
- Pribadi N, Rachmawati AN, Sari SK, Putri CR. Effect of LTA Lactobacillus plantarum (LP) exposure duration in inflammatory response (as study model of dental pulp inflammation wistar rat). Malays J Med Health Sci 2021;17(Supp 13):12-7.
- Pribadi N, Rahayu RP, Ismiyatin K, Putri CR, Surboyo MDC. The effect of lipoteichoic acid from Lactobacillus plantarum on dental pulp inflammation. Eur J Dent 2021;15(4):682-6. [PMID: 34416767 DOI: 10.1055/s-0041-1728238]
- de Souza Araújo E, Pimenta AS, Feijó FMC, Castro RVO, Fasciotti M, et al. Antibacterial and antifungal activities of pyroligneous acid from wood of Eucalyptus urograndis and Mimosa tenuiflora. J Appl Microbiol 2018;124(1):85-96. [PMID: 29095556 DOI: 10.1111/jam.13626]
- Surboyo MDC, Ernawati DS, Arundina I, Mansur D, Iskandar B, et al. The potential of liquid smoke as an oral ulcer remedies : a proposed mechanism based on systematic review. J Pharm Pharmacogn Res 2021;9(6):905-20.
- Gallucci MN, Carezzano ME, Oliva MM, Demo MS, Pizzolitto RP, et al. In vitro activity of natural phenolic compounds against fluconazole-resistant Candida species: a quantitative structure-activity relationship analysis. J Appl Microbiol 2014;116(4):79-804. [PMID: 24387763 DOI: 10.1111/jam.12432]
- Gao T, Zhang Y, Shi J, Mohamed SR, Xu J, et al. The antioxidant guaiacol exerts fungicidal activity against fungal growth and deoxynivalenol production in Fusarium graminearum. Front Microbiol 2021;12:762844. [PMID: 34867894 DOI: 10.3389/fmicb.2021.762844]
- Abegg MA, Alabarse PVG, Casanova A, Hoscheid J, Salomon TB, et al. Response to oxidative stress in eight pathogenic yeast species of the genus candida. Mycopathologia 2010;170(1):11-20. [PMID: 20229037 DOI: 10.1007/s11046-010-9294-5]
- Khan A, Ahmad A, Ahmad Khan L, Padoa CJ, van Vuuren S, et al. Effect of two monoterpene phenols on antioxidant defense system in Candida albicans. Microb Pathog 2015;80:50-6. [PMID: 25681060 DOI: 10.1016/j.micpath.2015.02.004]