Multiparticulate Drug Delivery of Losartan Potassium via Extrusion-Spheronization: Formulation and Dissolution Comparisons
1Arvind Gavali College of Pharmacy, Jaitapur, Satara 415004, Maharashtra, India
*Correspondence to: Dr. Vishal D. Yadav, Associate Professor and Head, Department of Pharmaceutics, Arvind Gavali College of Pharmacy, Jaitapur, Satara 415004, Maharashtra, India, Tel: 07447551122. E-mail: v.yadavagcop@gmail.com
Received: August 28 2024; Revised: October 8 2024; Accepted: October 29 2024; Published Online: November 11 2024
Cite this paper:
Yadav VD, Salunkhe DS, Lokhande VY. Multiparticulate Drug Delivery of Losartan Potassium via Extrusion-Spheronization: Formulation and Dissolution Comparisons. BIO Integration 2024; 5: 1–11.
DOI: 10.15212/bioi-2024-0079. Available at: https://bio-integration.org/
Download citation
© 2024 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
Background: Losartan potassium, an antihypertensive medication, has high solubility and a short half-life that result in potential adverse effects and rapid drug clearance. Multiparticulate drug delivery systems enhance the drug’s bioavailability, decrease patient-to-patient variability, and optimize drug distribution. Herein, losartan potassium pellets for sustained drug release were developed and characterized.
Methods: The formulation process involved varying the concentrations of Eudragit RSPO (200 mg, 400 mg, or 600 mg) and Eudragit L100 (200 mg, 400 mg, or 600 mg) across nine pellet batches, and adjusting the triethyl citrate concentrations accordingly. The pellets’ bulk density, tapped density, flow properties (Carr’s index, Hausner’s ratio, and angle of repose), drug content, particle size distribution, and in vitro drug release were evaluated. Interactions between losartan potassium and the excipients were analyzed with FTIR and DSC.
Results: FTIR spectra indicated physical interactions without major chemical alterations, whereas DSC thermograms revealed changes in thermal behavior due to excipient interactions. In vitro drug release studies indicated that formulations with higher concentrations of Eudragit RSPO and triethyl citrate achieved controlled, prolonged drug release. The optimized batch (F7) demonstrated balanced characteristics including favorable bulk and tapped density, good flow properties, and a sustained release profile. Varying the polymer and plasticizer concentrations significantly influenced pellet performance, and F7 was found to be the most promising formulation for sustained-release applications.
Conclusion: This study underscores the importance of polymer selection and formulation optimization in developing effective sustained-release drug delivery systems, and has potential implications for enhancing therapeutic outcomes in clinical practice.
Keywords
Bioavailability, dissolution, oral drug delivery, pellets, sustained release..
Introduction
Hypertension, a predominant cardiovascular disease risk factor, is rising in incidence globally, thus increasing the need for effective therapeutic interventions [1]. This condition is frequently managed with various classes of medications, including angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta blockers, diuretics, and calcium channel blockers [2]. Among these, losartan potassium (LP), an angiotensin II receptor antagonist, is widely prescribed for hypertension and certain nephropathies. Losartan, along with its active metabolite E-3174, targets the AT-I receptor subtype with high specificity and potency. Additionally, losartan does not induce withdrawal symptoms after therapy discontinuation, thus providing a valuable option for long-term hypertension management [3, 4]. Although LP’s efficacy in controlling blood pressure is well established, challenges remain in optimizing its delivery to maximize therapeutic outcomes and enhance patient adherence.
LP has a low oral bioavailability of approximately 33%, because of first-pass metabolism by the CYP-450 enzyme system; therefore, only a fraction of the drug enters systemic circulation, and elevated doses are necessary to achieve efficacy [5–7]. LP is also susceptible to metabolism in the proximal region of the gastrointestinal tract, where CYP enzymes are abundant. [8] Despite a BCS class III classification, a water solubility of 0.0216 mg/ml, and a logP of 5.37, LP’s pharmacokinetics further complicate its clinical use. Approximately 14% of LP is converted into an active metabolite; although the terminal half-life is 6–9 hours, the parent drug has a short half-life of approximately 2 hours [9]. This rapid clearance, coupled with potential adverse effects such as diarrhea, muscle cramps, dizziness, insomnia, and elevated serum potassium, underscores the need for alternative dosage forms enabling either decreased dosing frequency or enhanced drug efficacy [10, 11]. Various techniques have been explored for LP formulation, including direct compression [12], matrices [13], and solid dispersions [14]. However, these methods often face challenges in achieving consistent drug release profiles, stability, and patient-friendly dosage forms.
Sustained-release formulations indeed aim to maintain stable drug plasma levels over an extended period, which minimizes fluctuations and can enhance therapeutic efficacy, especially by bypassing or reducing the effects of first-pass metabolism. However, few prior studies have used such approaches to address LP’s limitations. The bilosomes described by Qushawy et al. [15] effectively extend drug release, achieving losartan release percentages of 64.31 ± 1.03% to 88.48 ± 0.93% over 12 hours. HPMC/carbopol-based floating gastroretentive capsules have achieved extended release of LP in the upper gastrointestinal tract; this formulation significantly affects the pharmacokinetic parameters of LP and its active metabolite, E3174 [9]. Therefore, exploring extended-release dosage forms to increase losartan’s oral bioavailability and effectiveness in cardiac disorders is a critical and promising research area.
Eudragit RSPO and L100 are key polymers used in multiparticulate delivery systems for controlled and targeted drug release. Eudragit RSPO forms a semi-permeable matrix that provides sustained drug release and therefore is ideal for extended-release formulations. Eudragit L100, a pH-sensitive polymer, dissolves at pH >6.0, thus enabling enteric coatings that protect drugs from gastric degradation and ensure targeted release in the intestines. Combined use of these polymers enables precise modulation of drug release profiles, and enhances the effectiveness and stability of pharmaceutical formulations.
Pelletization, a technique used in multiparticulate drug delivery systems, offers an effective approach to improve drug release control, enhance bioavailability, and increase patient compliance [16]. Technological advancements have opened new horizons in the manufacturing and scalability of drug delivery systems [17]. The need for pelletization arises from the limitations of conventional dosage forms, such as tablets and capsules, which might not achieve uniform drug release, or might require frequent dosing and lead to patient non-compliance [18]. Pellets are multiparticulate systems consisting of small, free-flowing, spherical particles that can fill capsules or can be compressed into tablets [19]. This system offers several advantages, including controlled and sustained drug release, diminished risk of dose dumping, and an ability to combine multiple drugs or different release profiles in a single dosage form [20–24].
Moreover, multiparticulate systems such as pellets aid in uniform distribution of drugs in the gastrointestinal tract, thereby decreasing localized irritation and providing more predictable pharmacokinetic profiles. These benefits not only enhance patient compliance but also allow for flexible dosing regimens tailored to individual patients’ needs [20, 25–28]. Therefore, addressing the challenges associated with losartan through advanced drug delivery systems, such as pelletization, offers a promising approach to increasing drug bioavailability, achieving controlled drug release, enhancing patient compliance, and ultimately improving therapeutic outcomes.
Among various techniques for pellet production, extrusion-spheronization is frequently applied because of its effectiveness and versatility [29]. This key technique offers substantial advantages in drug formulation, particularly in achieving controlled and sustained release profiles [30]. Extrusion-spheronization provides consistent pellet sizes and shapes, thereby enhancing drug release uniformity while minimizing dose dumping. Its adaptability to various drugs and excipients improves drug distribution and stability within pellets, and contributes to optimized therapeutic outcomes and increased patient compliance [19, 29, 31]. Extrusion-spheronization is therefore an excellent choice for addressing the challenges associated with drugs such as LP.
Given the intriguing characteristics of pellets and the limited oral bioavailability of losartan, this study was aimed at developing and evaluating LP pellets, focusing on the critical formulation parameters influencing pellet characteristics, drug release profiles, and overall therapeutic efficacy. By optimizing these parameters, we sought to create a dosage form to not only meet the therapeutic needs of patients with hypertension, but also enhance their treatment experience by increasing compliance and decreasing adverse effects.
Materials and methods
Materials
LP was provided by Lupin Ltd. in Boisar. Polyvinyl pyrrolidone (PVP) K-30, Eudragit RSPO, and Eudragit L-100 were purchased from Research-Lab Fine Chem Industries in Mumbai. Microcrystalline cellulose (MCC) was obtained from the S.D. Lab Chemical Centre in Mumbai. The remaining chemicals were all of analytical grade.
Preparation of pellets
Pellets of LP were prepared with the extrusion-spheronization technique. The materials were accurately weighed, and Eudragit L100 and Eudragit RSPO were combined in the appropriate proportions. Triethyl citrate was added to the formulations as a plasticizer, as detailed in Table 1, and the mixture was triturated in a mortar for 5 minutes. The drug and PVP were then mixed thoroughly by trituration for 15 minutes to produce a homogeneous mixture. This mixture was extruded with an extruder fitted with a screen with a 1.2 mm diameter, at a speed of 40 rpm. The extrudates were immediately transferred to the rotating plate of a spheronizer. Spheronization was performed in two stages: initially for 5 minutes at 500 rpm and subsequently for 15 minutes at 1000 rpm. During the first 5 minutes, MCC was added to the rotating extrudates to prevent pellet adhesion [32–34]. Table 1 lists the compositions of several batches of LP pellets.
Table 1 Composition of Losartan Potassium Pellets
| Drugs/Polymers (mg) | Formulation (Quantities in mg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | |
| Losartan potassium | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Eudragit RSPO | 200 | 200 | 200 | 400 | 400 | 400 | 600 | 600 | 600 |
| Eudragit L100 | 200 | 400 | 600 | 200 | 400 | 600 | 200 | 400 | 600 |
| Triethyl citrate | 20 | 40 | 60 | 80 | 100 | 120 | 140 | 160 | 180 |
| Polyvinyl pyrrolidone | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| MCC | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
Characterization of pellets
Bulk density
The pellets were first weighed accurately with a balance to obtain their initial weight (M). They were then poured into a graduated cylinder, and the initial volume (Vb) occupied by the pellets was recorded. The bulk density (ρb) was calculated by division of the weight of the pellets by the measured volume. The bulk density of coated pellets is useful for assessing their packing characteristics and handling properties [35].
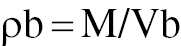
where ρb = bulk density, M = weight of the pellets, and Vb = bulk volume.
Tapped density
A graduated cylinder was filled with a predetermined mass of pellets. The cylinder was then placed in an electric tapper device, which was operated for a predetermined number of taps until the volume of the pellets reached a stable, predetermined minimum level. The final volume after tapping was recorded. The tapped density was computed by division of the weight of the pellets by their tapped volume. The tapped density, a measure of changes in the density of a material under tapping or vibration, aids in assessment of pellet packing behavior [36].
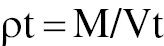
where ρt = tapped density, M = weight of the pellets, and Vt = tapped volume.
Carr’s index
To determine Carr’s index, we used the bulk density and tapped density values of the pellets. Carr’s index, also known as the compressibility index, was calculated with the following equation [37].
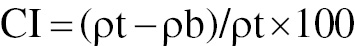
Angle of repose
To determine the angle of repose, we analyzed the flow characteristics of the pellets by forming a conical mound on a flat surface. A smooth, level table was used for this purpose. The material was gradually poured onto the surface to create a conical heap, which was allowed to settle naturally. After the mound had stabilized, the height and radius of the heap were measured. The angle of repose was then determined with the following equation:
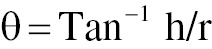
where h = height of the heap, and r = radius of the heap.
This angle, along with the recorded height and radius of the heap, provided insights into the material’s flowability and stability [38].
Particle size
The particle size of the pellets was measured with an eyepiece micrometer. First, the ocular micrometer was calibrated to ensure accurate readings. Calibration was performed by placing a stage micrometer with a known scale on the stage of the microscope. The number of divisions on the ocular micrometer corresponding to the known divisions on the stage micrometer was recorded, thus enabling conversion between ocular micrometer divisions and micrometers. After calibration, a prepared slide with the test pellets was placed on the microscope stage. The number of ocular micrometer divisions occupied by each pellet was counted. To obtain the pellet diameter in micrometers, we multiplied the number of divisions d by the calibration factor derived from the calibration step. This process ensured accurate measurement of the pellet particle size [34, 39].
Drug content
Pellets containing 10 mg powdered drug were dissolved in 10 mL methanol. The solution was thoroughly agitated for 20 minutes to ensure complete dissolution. After agitation, the mixture was filtered through a 0.45 μm membrane filter to remove any particulate matter. The filtrate was then analyzed with a spectrophotometer at 233 nm. The drug content was determined by comparison of the spectrophotometric measurements to a regression equation y = 18.44x + 0.350 (R2 = 0.997) derived from a calibration curve [40–42].
Solid state characterization
FTIR spectroscopy
To study the interactions between drugs and polymers, we conducted FTIR spectroscopy on LP drug formulations with excipients of Eudragit L100, Eudragit RSPO, and PVP. Spectra were obtained from optimized pellet formulations containing these components. The FTIR spectra were analyzed over a range from 4500 to 750 cm−1 to identify any new or altered peaks indicating potential interactions between LP and the excipients after formulation [43–45].
Differential scanning calorimetry of optimized pellet formulation
To assess the thermal behavior and thermotropic properties of the optimized pellet formulation, we conducted differential scanning calorimetry (DSC). This study focused on examining how LP was physically incorporated into the enhanced pellet formulation. DSC thermograms of the LP pellets were recorded over a temperature range from 30°C to 450°C, with a scanning speed of 10°C/min. This analysis provided insights into the drug’s thermal properties, and confirmed the stability and interaction of the drug within the pellet formulation [46, 47].
In vitro dissolution studies
Using a basket-style dissolution apparatus, we evaluated in vitro drug release from pellets containing 30 mg LP. The LP pellets were enclosed in capsule shells and placed in the dissolution test apparatus. Dissolution was conducted at a constant temperature of 37 ± 0.5°C, and the dissolution medium (900 mL) was stirred at 100 rpm. Initially, 0.1 N HCl (pH 1.2) was used as the dissolving medium for the first 2 hours. The medium was then replaced with phosphate buffer (pH 6.8), and the dissolution process continued for as many as 10 hours. At predetermined intervals, 5 mL aliquots of dissolution medium were collected and replaced with an equal volume of fresh dissolution medium. The samples were filtered, diluted, and analyzed with a UV-visible spectrophotometer at 233 nm. The phosphate buffer (pH 6.8) and 0.1 N HCl (pH 1.2) served as references in UV analysis [41, 48].
Results and discussion
The pellets were successfully prepared with the extrusion-spheronization technique, thereby ensuring uniform size distribution and a spherical shape, which are crucial for consistent drug release.
Bulk and tapped densities
Bulk density values ranged from 0.7964 g/cm3 (F5) to 0.8533 g/cm3 (F1), whereas tapped density ranged from 0.8697 g/cm3 (F5) to 0.9123 g/cm3 (F3). For batches F1–F3, the bulk density values were relatively high and were highest for F1 (0.8533 g/cm3). The tapped density followed a similar trend, with F3 having the highest tapped density (0.9123 g/cm3). The relatively small differences between bulk and tapped densities reflected the formulations’ good packing properties and minimal air entrapment.
For batches F4–F6, the bulk density values were slightly lower than those in F1–F3 and were lowest for F5 (0.7964 g/cm3). The tapped density showed similar trends, with a small but noticeably greater difference between bulk and tapped densities compared to those in F1–F3. This difference indicates slightly looser packing of the granules due to the increased polymer content.
The bulk and tapped densities in formulations F7–F9 were consistent and fell within a narrow range. The difference between bulk and tapped densities, although slightly lower than those in F1–F3, remained minimal, thus indicating that the granules retained good packing properties despite the higher polymer content. The differences between bulk tapped densities were statistically significant (p < 0.05), thus confirming that polymer amounts influence pellet packing and density.
Flow properties: Carr’s index, Hausner’s ratio, and angle of repose
Flow properties are summarized in Table 2. Carr’s index, indicating powder flowability, ranged from 5.605% in F1 to 10.54% in F3. Hausner’s ratio, indicating flowability and compressibility, varied from 1.060 in F1 to 1.117 in F3. The angle of repose varied from 14° (F5) to 19° (F8).
Table 2 Characterization of Losartan Potassium Pellets (mean ± SD; n = 3)
| Batches | Particle Size (mm) | Bulk Density (g/cm3) | Tapped Density (g/cm3) | Carr’s Index (%) | Hausner’s Ratio | Angle of Repose (°) | Drug Content (%) |
|---|---|---|---|---|---|---|---|
| F1 | 0.4–1.7 | 0.8533 ± 0.016 | 0.8723 ± 0.003 | 5.605 ± 2.189 | 1.060 ± 0.024 | 15 ± 0.29 | 96.93 ± 0.52 |
| F2 | 0.2–1.9 | 0.8229 ± 0.003 | 0.9077 ± 0.004 | 9.336 ± 0.807 | 1.103 ± 0.009 | 19 ± 0.27 | 97.03 ± 0.31 |
| F3 | 0.7–2.0 | 0.8126 ± 0.003 | 0.9123 ± 0.007 | 10.54 ± 0.528 | 1.117 ± 0.006 | 15 ± 0.33 | 98.23 ± 0.41 |
| F4 | 1.2–3.2 | 0.8383 ± 0.004 | 0.9106 ± 0.001 | 8.435 ± 0.268 | 1.092 ± 0.003 | 16 ± 0.19 | 92.43 ± 0.25 |
| F5 | 0.8–1.8 | 0.7964 ± 0.002 | 0.8697 ± 0.009 | 8.427 ± 0.200 | 1.094 ± 0.005 | 14 ± 0.48 | 96.56 ± 0.89 |
| F6 | 0.5–2.2 | 0.8178 ± 0.003 | 0.8939 ± 0.003 | 8.518 ± 0.425 | 1.093 ± 0.005 | 14 ± 0.17 | 97.36 ± 1.02 |
| F7 | 0.5–1.5 | 0.8254 ± 0.002 | 0.9064 ± 0.004 | 9.496 ± 0.766 | 1.098 ± 0.005 | 18 ± 0.27 | 98.97 ± 0.92 |
| F8 | 0.7–2.4 | 0.8120 ± 0.008 | 0.8791 ± 0.001 | 7.641 ± 0.766 | 1.087 ± 0.005 | 19 ± 018 | 97.83 ± 0.79 |
| F9 | 0.1–3.2 | 0.8094 ± 0.002 | 0.8901 ± 0.003 | 9.173 ± 0.313 | 1.099 ± 0.002 | 14 ± 0.86 | 99.12 ± 1.03 |
Formulations F1–F3, containing low concentrations of Eudragit RSPO, exhibited the best flow properties, and F1 had the lowest Carr index (5.605%) and Hausner ratio (1.060). The angle of repose was also low (15° for both F1 and F3), thus indicating excellent flowability, probably because lower polymer content resulted in less inter-particulate friction and cohesion.
With increasing polymer content, Carr’s index and Hausner’s ratio slightly increased in F4–F6, thereby indicating a marginal decrease in flowability. However, the values remained within acceptable limits, and F5 showed good flow properties (Carr’s index of 8.427% and Hausner’s ratio of 1.094). The angle of repose remained low across this group, thus suggesting that the increase in polymer content did not significantly compromise the flow properties.
Despite having the highest polymer content, F7–F9 maintained good flow properties, although Carr’s index and Hausner’s ratio were slightly higher than those in F1–F3. Moreover, F7 and F9 showed slightly elevated values for Carr’s index (approximately 9.496%) and Hausner’s ratio (approximately 1.098), indicating a minor decrease in flowability. The angle of repose was also slightly higher in these formulations, particularly in F8 (19°), reflecting the impact of increased polymer content on flow properties. Statistical analysis revealed significant differences in these parameters (p < 0.05), thus emphasizing the effects of polymer concentration on pellet flow and handling characteristics. These results suggested that varying the concentrations of Eudragit RSPO and Eudragit L100 had minimal effects on flow properties, because all formulations demonstrated satisfactory flow characteristics.
Drug content
Drug content varied from 92.43% in F4 to 99.12% in F9. The relatively high drug content percentages in F3, F7, and F9 indicated that these formulations achieved efficient drug incorporation and minimal loss during production. Variations in drug content might have resulted from differences in formulation precision, excipient interactions, or processing conditions. The drug content in F1–F3 was high and consistent, ranging from 96.93% in F1 to 98.23% in F3. The relatively lower polymer content probably facilitated the uniformity of drug distribution within the granules. The drug content in batches F4–F6 remained within a slightly lower but still acceptable range, and F4 showed the lowest drug content (92.43%). Increasing polymer concentration might have introduced slight variability in drug distribution; however, the content uniformity remained within satisfactory limits overall. The highest drug content was observed in F7–F9, and that in F9 reached 99.12%. High polymer concentration appeared to enhance drug entrapment and distribution within the granules, thereby increasing content uniformity, particularly in F9 (Table 2). Herein, we observed a higher drug content than that reported for aceclofenac pellets coated with ethyl cellulose N50 and hydroxypropyl methylcellulose [20].
Particle size
Formulations F1–F3 had the lowest concentration of Eudragit RSPO (200 mg). Their particle size distribution was relatively narrow, ranging from 0.4–1.7 mm in F1 to 0.7–2.0 mm in F3 (Table 2). This narrower particle size range suggested a more uniform granule formation, probably because lower polymer content resulted in smaller, more consistent granules. In F4–F6, with a moderate increase in Eudragit RSPO (400 mg), the particle size distribution broadened, ranging from 0.8–1.8 mm in F5 to 1.2–3.2 mm in F4. This increase in particle size indicated that the higher polymer content contributed to the formation of larger granules, possibly because of increased binding and aggregation during granulation.
Batches with the highest Eudragit RSPO concentration (600 mg) (F7–F9) showed the broadest particle size distribution, ranging from 0.1 to 3.2 mm in F9. This wide range suggested that high polymer content led to the formation of both very small and very large granules, thus indicating variability in granule formation, probably because of the higher viscosity of the polymeric solution used during granulation. Statistical analysis (ANOVA) confirmed significant differences in particle size among the batches (p < 0.05), and consequently indicated that polymer amount and type significantly influenced the pellet size distribution.
Among all formulations, F7 was considered optimal because of its balanced particle size range of 0.5–1.5 mm, in accordance with the desired characteristics for optimal drug release and processing. These results were in agreement with those reported by Gupta et al., in which olanzapine matrix pellets demonstrated a particle size range of 1024–1212 μm [49]. F7 had favorable bulk and tapped density, excellent flow properties (as indicated by Carr’s index and Hausner’s ratio), and a good angle of repose. These characteristics are critical for efficient manufacturing processes and ensuring consistent quality of the final product.
The particle size distribution has substantial implications: a uniform and optimal particle size can enhance drug dissolution rates, increase bioavailability, ensure a controlled release profile, and ultimately contribute to the efficacy of sustained-release formulations in managing hypertension. Therefore, the careful selection of polymer concentration and its effects on particle size are crucial for developing high-quality multiparticulate systems for effective drug delivery.
FTIR study of optimized the pellet formulation
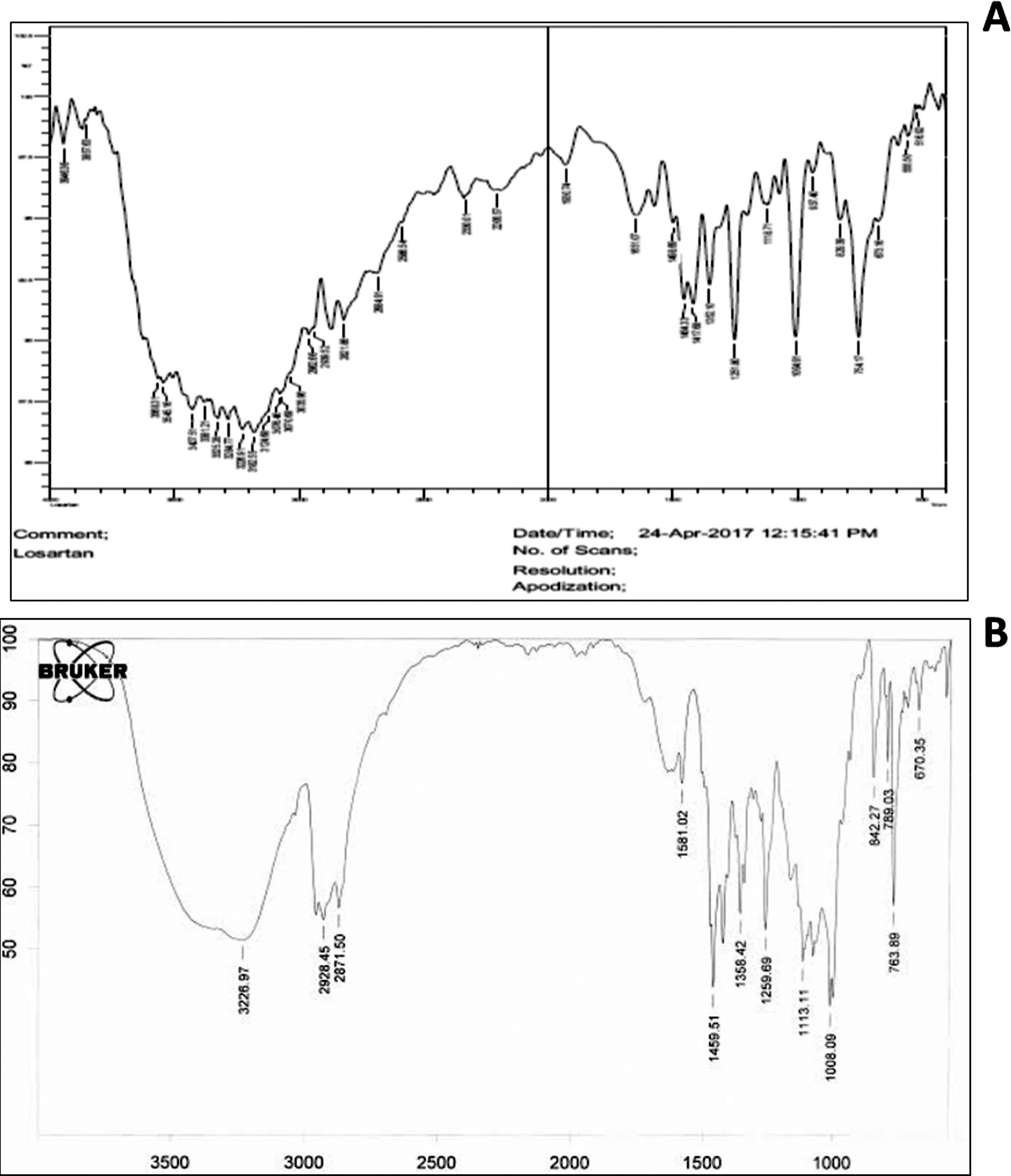
The FTIR spectra showed the characteristic peaks of LP (Figure 1A), and the optimized formulation containing LP along with Eudragit RSPO, Eudragit L100, and triethyl citrate (Figure 1B). The FTIR spectrum of pure LP showed distinct peaks corresponding to functional groups. For instance, a sharp peak around 3325 cm−1 was attributed to N-H stretching vibrations, whereas the peaks observed near 1651 cm−1 and 1454 cm−1 corresponded to C=O stretching and C=C aromatic stretching, respectively. Peaks at 754 and 1498 were attributed to C-Cl stretching and C=N stretching, respectively. Other notable peaks included those around 1256 cm−1 and 1133 cm−1, which were associated with C-O stretching.
Figure 1 FTIR spectra of A) losartan potassium and B) optimized pellet formulation.
In the optimized formulation, the FTIR spectrum showed peaks characteristic of both the drug and the excipients used (Eudragit RSPO, Eudragit L100, and triethyl citrate). Notable shifts or changes in peak intensities indicated potential interactions between LP and the polymers/excipients.
The broad peaks around 2928 cm−1 and 2871 cm−1, associated with C-H stretching vibrations, suggested the presence of aliphatic chains from the Eudragit polymers and possibly triethyl citrate. Additionally, the peaks at 1358 cm−1 and 1113 cm−1 reflected interactions between LP and the ester groups of the polymers. Importantly, the key peaks of losartan, such as those around 1459 cm−1, 763 cm−1, and 1259 cm−1, remained present but showed diminished intensity or slight shifts potentially indicating hydrogen bonding or other non-covalent interactions with the excipients. The absence of major new peaks or shifts suggested that the interactions were likely to be physical rather than chemical, and therefore that the drug’s structure remained intact within the formulation.
FTIR analysis indicated that LP was successfully incorporated into the optimized formulation without undergoing substantial chemical alteration. The observed interactions were primarily physical, and were likely to involve hydrogen bonding or Van der Waals interactions between the drug and the polymer matrix. These interactions beneficially contributed to the drug’s stability and release profile within the final pellet formulation. The presence of the characteristic peaks of both the drug and the excipients in the optimized formulation suggested a well-formed, stable mixture suitable for further pharmaceutical application.
Differential scanning calorimetry of the optimized pellet formulation
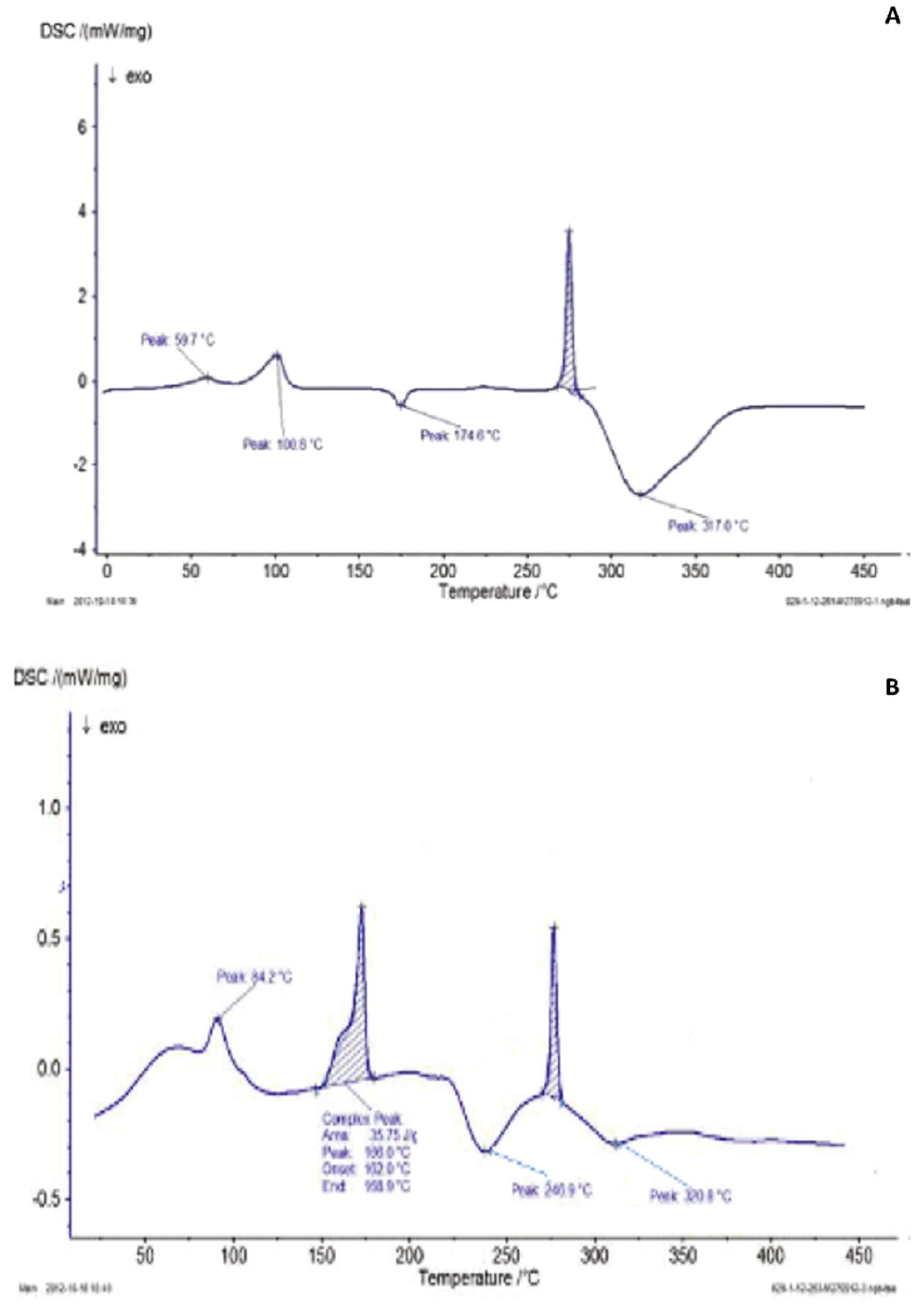
The DSC thermograms for both the pure drug losartan (Figure 1A) and the optimized formulation (Figure 1B) revealed distinct thermal transitions that provided insights into the drug’s behavior in both its pure and formulated states.
In the DSC thermogram of pure losartan (Figure 2A), several thermal events were observed. An initial endothermic peak at 59.7°C corresponded to the loss of absorbed moisture or volatile compounds. This peak was followed by another endothermic peak at 100.8°C, associated with the glass transition temperature of the drug from a glassy to a rubbery state. An exothermic peak at 174.6°C suggested crystallization of the amorphous form of losartan into a more ordered crystalline structure. The sharp endothermic peak at 274.6°C was attributed to the melting point of losartan, thereby indicating its transition from solid to liquid. The final broad endothermic peak at 317.6°C suggested thermal degradation of the drug, thus indicating its decomposition. In contrast, the DSC thermogram for the optimized LP formulation (Figure 2B) displayed a series of peaks slightly differing from those of the pure drug. An endothermic peak at 84.2°C, which was lower than that observed for the pure drug, indicated potential loss of moisture or interactions of losartan with excipients, thereby altering the thermal profile. A peak around 170°C corresponded to a melting event or glass transition of one of the excipients, reflecting changes in the formulation’s thermal stability. The peak at 272°C, similarly to the melting point of pure losartan, indicated that the drug retained some degree of crystallinity within the formulation, although the nature of this crystallinity might differ according to interactions with excipients. Furthermore, these findings confirmed that the drug was uniformly distributed within the pellets. Similar observations have been reported by Gupta et al., in which olanzapine exhibited a sharp endothermic peak at 196.26°C, whereas the endothermic peak in olanzapine-loaded matrix pellets appeared at 193.19°C; this shift in thermal behavior suggests uniform dispersion of the drug throughout the pellet matrix [49].
Figure 2 DSC thermogram of A) losartan potassium and B) optimized pellet formulation.
The exothermic peak at 238°C suggested recrystallization within the drug-excipient mixture, thereby indicating that the formulation underwent a phase transition leading to a more stable crystalline form. Finally, the broad exothermic peak around 320°C indicated thermal decomposition, probably because of breakdown of both losartan and the excipients.
Comparison of the DSC thermograms suggested that the crystallinity of losartan was partially retained in the formulated state but was influenced by the presence of excipients. The lower onset temperatures and shifts in the peaks in the formulation suggest interactions between LP and the excipients, which may have reduced crystallinity or altered the crystalline form. These interactions are crucial for the formulation’s stability, because they might alter drug solubility and bioavailability. The observed shift in melting points and changes in peak intensities suggested that interactions between the drug and the excipients altered the thermal stability and crystallinity.
The presence of a recrystallization peak in the formulation further suggested that the drug might exist in both crystalline and amorphous forms, and excipients might facilitate or stabilize these transitions. The slightly lower melting point in the formulation than the pure drug suggested a decrease in the crystallite size leading to altered thermal behavior. Overall, the DSC analysis indicated that, although losartan retained some degree of crystallinity in the formulated state, its thermal behavior was significantly influenced by the excipients in the formulation.
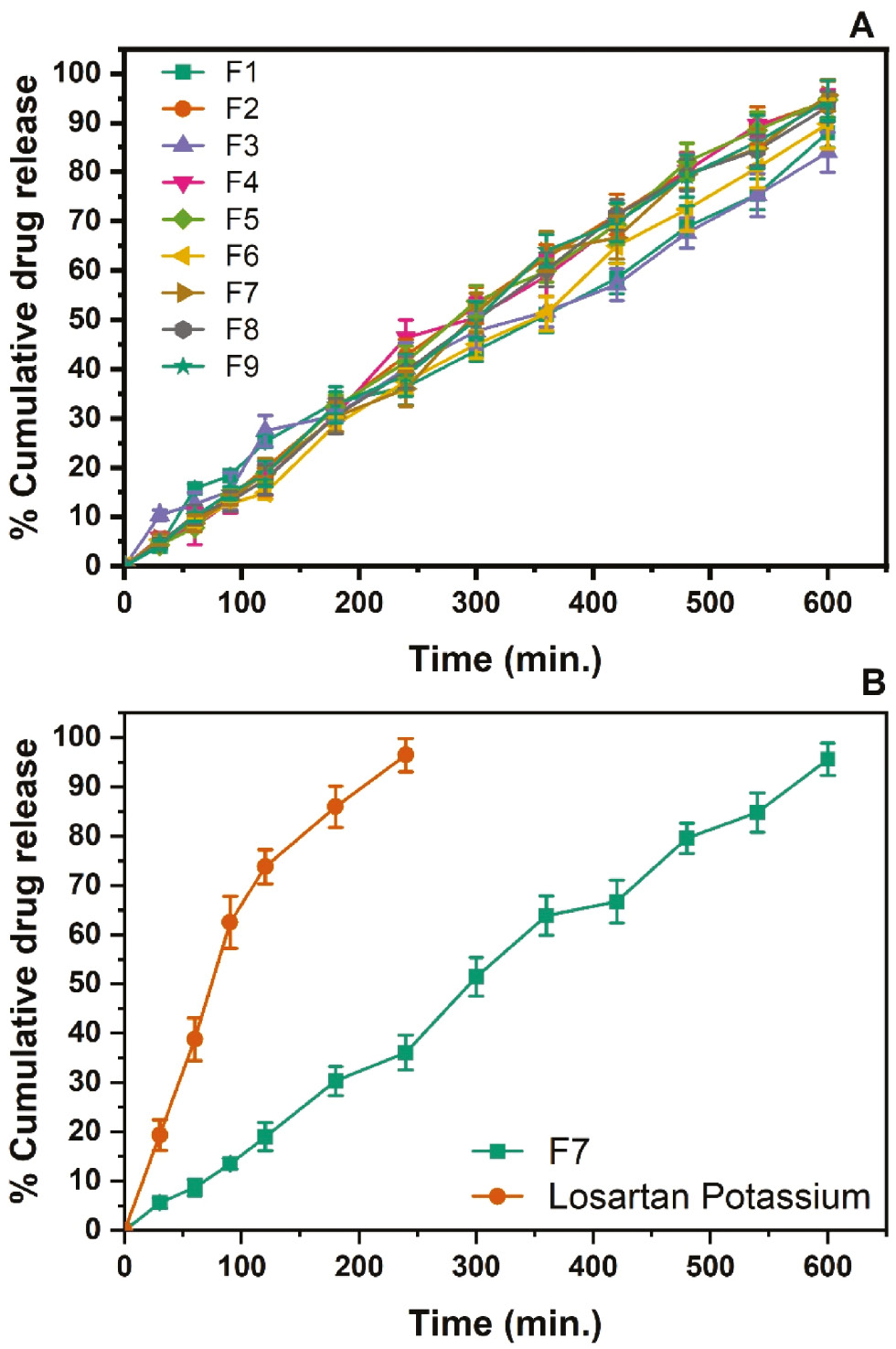
In vitro drug release study
The dissolution study revealed cumulative drug release profiles over time for nine formulations (F1–F9) containing varying concentrations of Eudragit RSPO, Eudragit L100, and triethyl citrate (Table 3). These formulations were evaluated over a 10-hour period (600 minutes), and the results provided insights into how variations in polymer and plasticizer concentrations affected drug release behavior (Figure 3A).
Table 3 Comparison of Cumulative Drug Release at Various Time Intervals Among Developed Formulations and Pure Losartan Potassium (Mean ± SD)
| Time (min) | Cumulative Drug Release (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | LP | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 30 | 4.02 ± 0.1 | 4.48 ± 0.14 | 10.35 ± 1.05 | 5.73 ± 1.05 | 4.33 ± 0.17 | 5.4 ± 0.34 | 5.56 ± 1.02 | 4.64 ± 1.02 | 4.31 ± 0.9 | 19.32 ± 3.14 |
| 60 | 15.8 ± 0.9 | 9.07 ± 0.8 | 12.62 ± 2.36 | 8.08 ± 3.68 | 7.88 ± 0.8 | 8.97 ± 0.9 | 8.7 ± 1.66 | 10.38 ± 1.96 | 10.09 ± 0.98 | 38.78 ± 4.29 |
| 90 | 18.4 ± 0.9 | 14.16 ± 1.2 | 15.25 ± 3.74 | 13.08 ± 2.34 | 14.39 ± 1.2 | 12.72 ± 0.5 | 13.48 ± 1.08 | 13.17 ± 2.05 | 14.84 ± 1.36 | 62.49 ± 5.28 |
| 120 | 25.26 ± 1.2 | 19.89 ± 1.67 | 27.5 ± 3.16 | 17.59 ± 3.19 | 18.79 ± 1.67 | 14.84 ± 1.2 | 18.99 ± 2.88 | 17.56 ± 2.98 | 18.74 ± 2.58 | 73.81 ± 3.47 |
| 180 | 33.26 ± 2.1 | 31.99 ± 2.35 | 30.63 ± 1.05 | 30.73 ± 3.44 | 32.41 ± 2.35 | 28.73 ± 1.6 | 30.32 ± 2.96 | 30.45 ± 3.58 | 32.75 ± 3.68 | 85.98 ± 4.18 |
| 240 | 36.39 ± 3.65 | 42.77 ± 3.24 | 40.13 ± 5.24 | 46.33 ± 3.66 | 41.46 ± 3.23 | 37.52 ± 2.5 | 36.06 ± 3.57 | 39.62 ± 3.47 | 38.73 ± 4.25 | 96.47 ± 3.36 |
| 300 | 43.74 ± 2.14 | 52.95 ± 3.67 | 47.74 ± 3.67 | 50.46 ± 4.02 | 53.8 ± 3.13 | 45.04 ± 2.8 | 51.44 ± 3.94 | 50.07 ± 3.66 | 50.08 ± 3.68 | |
| 360 | 51.07 ± 3.67 | 62.59 ± 2.67 | 51.68 ± 3.15 | 58.89 ± 4.18 | 59.92 ± 2.24 | 51.28 ± 3.47 | 63.88 ± 4.02 | 60.21 ± 3.47 | 64.05 ± 3.285 | |
| 420 | 58.44 ± 3.14 | 71.43 ± 4.09 | 57.2 ± 3.22 | 69.63 ± 4.06 | 69.37 ± 4.11 | 65.05 ± 3.64 | 66.75 ± 4.37 | 71.28 ± 3.12 | 69.82 ± 3.95 | |
| 480 | 68.92 ± 4.28 | 80.33 ± 3.67 | 67.64 ± 3.1 | 80.27 ± 3.57 | 82.17 ± 3.67 | 72.42 ± 4.32 | 79.6 ± 3.08 | 79.44 ± 3.19 | 79.21 ± 4.28 | |
| 540 | 75.51 ± 3.16 | 89.46 ± 3.77 | 75.38 ± 4.36 | 89.55 ± 2.36 | 88.47 ± 3.77 | 80.88 ± 4.06 | 84.8 ± 3.99 | 84.46 ± 3.17 | 86.14 ± 5.36 | |
| 600 | 88.01 ± 3.07 | 94.38 ± 4.05 | 84.05 ± 4.09 | 93.58 ± 3.17 | 94.69 ± 4.09 | 89.79 ± 4.99 | 95.61 ± 3.25 | 93.3 ± 3.08 | 94.49 ± 4.08 | |
LP: losartan potassium.
Figure 3 A) In vitro drug release of F1–F9 pellet formulation batches and B) comparative dissolution profiles of the optimized batch and pure losartan potassium.
Batches F1–F3 contained a fixed amount of Eudragit RSPO (200 mg) but increasing concentrations of Eudragit L100 (200 mg, 400 mg, or 600 mg) and triethyl citrate (20 mg, 40 mg, or 60 mg). The drug release was initially slow: F1 showed 4.02% release at 30 minutes and reached 88.01% release by 600 minutes; F2 and F3 showed similar trends, with slightly higher drug release rates reaching 94.38% and 84.05% by the end of the study. These findings suggested that a moderate increase in Eudragit L100 enhanced the release rate, although the effects were less pronounced because of the lower concentration of triethyl citrate.
Batches F4–F6 contained twice the Eudragit RSPO concentration (400 mg) of F1–F3, and had corresponding increases in Eudragit L100 and triethyl citrate. The cumulative drug release rates in F4, F5, and F6 significantly increased over time, reaching 93.58%, 94.69%, and 89.79%, respectively, at 600 minutes. Notably, F4 showed rapid drug release between 180 minutes (30.73%) and 240 minutes (46.33%), thereby indicating that synergistic effects between higher concentrations of Eudragit L100 and triethyl citrate enhanced the permeability and release rate.
Batches F7–F9 contained the highest concentrations of Eudragit RSPO (600 mg), Eudragit L100, and triethyl citrate. F7 showed 95.61% release by 600 minutes, and F8 and F9 also demonstrated high release percentages of 93.30% and 94.49%, respectively, reflecting a consistent and prolonged release pattern.
Increasing the concentration of Eudragit RSPO from 200 mg (F1–F3) to 400 mg (F4–F6) or 600 mg (F7–F9) resulted in higher cumulative drug release percentages, probably because of interactions between Eudragit RSPO and the other formulation components (Eudragit L100 and triethyl citrate), thereby optimizing the matrix’s permeability and facilitating drug release efficiency.
Higher concentrations of Eudragit RSPO, along with appropriate levels of Eudragit L100 and triethyl citrate, appeared to create a matrix that balanced controlled release with enhanced drug diffusion, and led to an overall increase in drug release. Eudragit L100, a polymer with pH-responsive behavior, influenced drug release in conjunction with Eudragit RSPO.
As the concentration Eudragit RSPO increased across formulations (from 200 mg in F1 to 600 mg in F9), drug release tended to increase, particularly in later stages of the dissolution study. This finding indicated that Eudragit L100 enhanced drug release as it dissolved under the elevated pH levels encountered in late stages of passage through the gastrointestinal tract. The concentration of triethyl citrate, a plasticizer, directly correlated with the flexibility and permeability of the polymeric matrix. Higher concentrations (from 20 mg in F1 to 180 mg in F9) facilitated faster drug release, on the basis of the progressive increase in cumulative drug release observed across batches. Triethyl citrate might have lowered the glass transition temperature of the polymers, thereby increasing their flexibility and the diffusion rate of the drug. The dissolution study results clearly indicated that variations in polymer and plasticizer concentrations significantly influenced the drug release profiles. Formulations with higher concentrations of Eudragit RSPO and triethyl citrate showed more controlled and prolonged release profiles, which were ideal for sustained-release applications. The influence of Eudragit L100 was more pronounced at higher concentrations, particularly when paired with an adequate amount of triethyl citrate, thereby facilitating efficient drug release in late stages of the dissolution process.
The dissolution profiles (Figure 3B) of the optimized batch and pure losartan potassium were compared over 600 minutes. Initially, the pure drug exhibited a significantly higher dissolution rate than the optimized batch. At 30 minutes, 19.32% of the pure drug had dissolved, whereas only 5.56% had dissolved in the optimized batch. This pattern persisted: the pure drug achieved 73.81% dissolution at 120 minutes, compared with 18.99% for the optimized batch.
The low initial release of the optimized batch in the first 2 hours was attributed to the presence of Eudragit L100 and Eudragit RSPO. Eudragit L100, an enteric polymer known for its pH-responsive behavior, remains insoluble in the acidic environment of the stomach but dissolves in the more alkaline environment of the intestines. This characteristic contributed to delayed dissolution for the optimized batch. Eudragit RSPO, a non-ionic polymer, provides additional benefits by enhancing formulation stability and controlling the drug release rate. This polymer forms a barrier that modulates drug release over time and contributes to a sustained release profile.
Over time, the gap between the dissolution rates of the two formulations began to narrow. At 180 minutes, the pure drug showed 85.98% dissolution, whereas the optimized batch achieved 30.32% dissolution. The optimized batch continued to exhibit a sustained release profile, with gradually increasing dissolution. By 300 minutes, 51.44% had dissolved, whereas no dissolution data was available for the pure drug at this time point. By 600 minutes, the optimized batch achieved a dissolution of 95.61%.
Whereas pure losartan potassium demonstrated initially faster and higher dissolution rates, the optimized batch, with sustained release due to the presence of polymer, showed a slower but steady increase in dissolution over time. This sustained release profile suggested that the optimized batch provided prolonged drug release and may potentially offer enhanced therapeutic benefits. However, the results obtained in this study are inferior to those previously reported by Abou Obaid et al., who prepared sustained-release matrix pellets containing solid dispersions of LP prepared with a hydrophilic polymer, PEG 6000, mixed with Avicel PH 101, and achieved less than 20% drug release in 8 hours [50].
Conclusion
This investigation highlights the critical roles of selecting appropriate polymers and drugs in the design and development of multiple unit particulate systems. In this study, LP pellets were successfully formulated with Eudragit RSPO and Eudragit L100 polymers for sustained release, and PVP as a binder, through the matrix pelletization method. Infrared spectroscopy confirmed the compatibility of the polymers (Eudragit RSPO, Eudragit L100, and PVP) and microcrystalline cellulose with LP. The extrusion-spheronization method was demonstrated to enable effective, simple, reliable, and cost-effective preparation of pellets. The flow properties of the formulations were satisfactory, as indicated by a low angle of repose (<20°), low compressibility index (<15), and Hausner’s ratio <1.18, reflecting non-aggregated powder with good flow characteristics. Among formulations, F7 had the best performance, releasing 95.61% of LP in a sustained manner over an extended period as long as 10 hours. The sustained-release formulation of LP might potentially offer clinical benefits including maintenance of consistent plasma levels, decreased dosing frequency, and minimal drug concentration fluctuations, thus increasing patient adherence, decreasing adverse effects, enhancing blood pressure control, and resulting in better therapeutic outcomes and patient compliance in hypertension management. Therefore, the multiple unit particulates of LP developed herein may provide a promising approach for controlled drug delivery systems offering both safety and efficacy. Future studies should focus on optimizing the formulation parameters, investigating long-term stability under various environmental conditions, and conducting in vivo evaluations to confirm the clinical efficacy and safety of the developed pellets. Additionally, the potential for decreased dosing frequency and improved patient convenience might substantially influence quality of life among patients with hypertension and enable better disease management. Exploration of alternative polymers and binders might provide further insights into improving the performance and versatility of the pellet system.
Acknowledgements
None.
Declaration of competing interests
The authors declare no conflicts of interest.
References
- Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol 2020;16:223-37. [PMID: 32024986 DOI: 10.1038/s41581-019-0244-2]
- Heidari B, Avenatti E, Nasir K. Pharmacotherapy for essential hypertension: a brief review. Methodist Debakey Cardiovasc J 2022;18(5):5-16. [PMID: 36561082 DOI: 10.14797/mdcvj.1175]
- Kommana N, Bharti K, Surekha DB, Thokala S, Mishra B. Development, optimization and evaluation of losartan potassium loaded self emulsifying drug delivery system. J Drug Deliv Sci Technol 2020;60:102026. [DOI: 10.1016/j.jddst.2020.102026]
- Ahmad H, Khan H, Haque S, Ahmad S, Srivastava N, et al. Angiotensin-converting enzyme and hypertension: a systemic analysis of various ACE inhibitors, their side effects, and bioactive peptides as a putative therapy for hypertension. J Renin Angiotensin Aldosterone Syst 2023;2023:7890188. [PMID: 37389408 DOI: 10.1155/2023/7890188]
- Khan KA, Ahmad A, Marini C, Nicotra M, Di Cerbo A, et al. Formulation and preparation of losartan-potassium-loaded controlled-release matrices using Ethocel grade 10 to establish a correlation between in vitro and in vivo results. Pharmaceutics 2024;16(2):186. [PMID: 38399247 DOI: 10.3390/pharmaceutics16020186]
- Yasar Ü, Forslund Bergengren C, Tybring G, Dorado P, Llerena A, et al. Pharmacokinetics of losartan and its metabolite E-3174 in relation to the CYP2C9 genotype. Clin Pharmacol Ther 2002;71:89-8. [PMID: 11823761 DOI: 10.1067/mcp.2002.121216]
- Lee CR, Pieper JA, Hinderliter AL, Blaisdell JA, Goldstein JA. Losartan and E3174 pharmacokinetics in cytochrome P450 2C9*1/*1, *1/*2, and *1/*3 individuals. Pharmacotherapy 2003;23(6):720-5. [PMID: 12820813 DOI: 10.1592/phco.23.6.720.32187]
- Yang L, Guo T, Xia DY, Zhao LS. Pharmacokinetics of losartan and its active carboxylic acid metabolite E-3174 in five ethnic populations of China. J Clin Pharm Therapeut 2012;37:226-31.
- Wani TU, Mir KB, Fazli AA, Raza SN, Khan NA. HPMC/Carbopol-based extended-release gastroretentive dosage form of losartan potassium: formulation and in vivo pharmacokinetic evaluation in rabbits. J Drug Deliv Sci Technol 2020;60:102006. [DOI: 10.1016/j.jddst.2020.102006]
- Mokale V, Jitendra N, Yogesh S, Gokul K. Chitosan reinforced alginate controlled release beads of losartan potassium: design, formulation and in vitro evaluation. J Pharm Invest 2014;44(4):243-52. [DOI: 10.1007/s40005-014-0122-7]
- Ferreira JP, Konstam M, Rossignol P, Kiernan MS, Zannad F. High-versus low-dose losartan and serum potassium: an analysis from HEAAL. J Card Fail 2023;29(1):45-52. [PMID: 36244652 DOI: 10.1016/j.cardfail.2022.09.008]
- Luo Q, Zhang Q, Wang P. Hydrochlorothiazide/Losartan potassium tablet prepared by direct compression. Pharmaceutics 2022;14(8):1741. [PMID: 36015367 DOI: 10.3390/pharmaceutics14081741]
- Khan KA, Khan GM, Muzammal M, Al Mohaini M, Alsalman AJ, et al. Preparation of losartan potassium controlled release matrices and in vitro investigation using rate-controlling agents. Molecules 2022;27(3):864. [PMID: 35164127 DOI: 10.3390/molecules27030864]
- Veena G, Saritha M. Effect of losartan potassium on the solubility of hydrochlorothiazide by solid dispersion technique. J Chem Pharm Res 2011;3(4):150-8.
- Qushawy M, Soliman GM, Mortagi Y, El-Sherbiny M, Elsherbiny N. Development, optimization, and assessment of losartan nano-bilosomes to mitigate diabetes-induced microvascular complications in Sprague Dawley rats. J Drug Deliv Sci Technol 2024;92:105295. [DOI: 10.1016/j.jddst.2023.105295]
- Agrawal S, Fernandes J, Shaikh F, Patel V. Quality aspects in the development of pelletized dosage forms. Heliyon 2022;8(2):e08956. [PMID: 35243077 DOI: 10.1016/j.heliyon.2022.e08956]
- Al-Hashimi N, Begg N, Alany RG, Hassanin H, Elshaer A. Oral modified release multiple-unit particulate systems: compressed pellets, microparticles, and nanoparticles. Pharmaceutics 2018;10(4):176. [PMID: 30287798 DOI: 10.3390/pharmaceutics10040176]
- Politis SN, Rekkas DM. Pelletization processes for pharmaceutical applications: a patent review. Recent Pat Drug Deliv Formul 2011;5(1):61-78. [PMID: 21143125 DOI: 10.2174/187221111794109493]
- Muley S, Nandgude T, Poddar S. Extrusion–spheronization: a promising pelletization technique: in-depth review. Asian J Pharm Sci 2016;11(6):684-99. [DOI: 10.1016/j.ajps.2016.08.001]
- Ravella VN, Nadendla RR, Kesari NC. Design and evaluation of sustained release pellets of aceclofenac. J Pharm Res 2013;6(5):525-31. [DOI: 10.1016/j.jopr.2013.04.040]
- Kulkarni S, Londhe VY. Development and evaluation of extended release metformin pellets suspended in teneligliptin jelly for the treatment of diabetes mellitus. J Drug Deliv Sci Technol 2022;70:103192. [DOI: 10.1016/j.jddst.2022.103192]
- Nidhi, Rashid M, Kaur V, Hallan SS, Sharma S, Mishra N. Microparticles as controlled drug delivery carrier for the treatment of ulcerative colitis: a brief review. Saudi Pharm J 2016;24:458-72. [PMID: 27330377 DOI: 10.1016/j.jsps.2014.10.001]
- Tang ES, Chan LW, Heng PW. Coating of multiparticulates for sustained release. Am J Drug Deliv 2005;3(1):17-28. [DOI: 10.2165/00137696-200503010-00003]
- Haritha VVN. Multiparticulate drug delivery system: pelletization. Am J PharmTech Res 2012;2:81-7.
- Sultana S, Khosru KH, Masud AA. Development and evaluation of in vitro release kinetics of sustained release pellets of gliclazide using combinations of cellulose polymers. J Pharm Educ Res 2012;3:1-9.
- Young CR, Koleng JJ, McGinity JW. Production of spherical pellets by a hot-melt extrusion and spheronization process. Int J Pharm 2002;242(1-2):87-92. [PMID: 12176229 DOI: 10.1016/s0378-5173(02)00152-7]
- Khan A, Malviya R, Sharma PK. Multi-unit drug delivery system: a brief review of pelletization technique. World Appl Sci J 2014;31:2137-40.
- Sharma D, Sharma SK, Jaimini M, Kumar A. A review on multiparticulate floating drug delivery system. Int J Pharm Chem Biol Sci 2014;4:201-7.
- Sinha VR, Agrawal MK, Agarwal A, Singh G, Ghai D. Extrusion-spheronization: process variables and characterization. Crit Rev Ther Drug Carrier Syst 2009;26(3):275-331. [PMID: 19799528 DOI: 10.1615/critrevtherdrugcarriersyst.v26.i3.20]
- Varca GH, Lopes PS, Ferraz HG. Development of papain containing pellets produced by extrusion-spheronization: an operational stage approach. Drug Dev Ind Pharm 2015;41(3):430-35. [PMID: 24410044 DOI: 10.3109/03639045.2013.877481]
- Trivedi NR, Rajan MG, Johnson JR, Shukla AJ. Pharmaceutical approaches to preparing pelletized dosage forms using the extrusion-spheronization process. Crit Rev Ther Drug Carrier Syst 2007;24(1):1-40. [PMID: 17430098 DOI: 10.1615/critrevtherdrugcarriersyst.v24.i1.10]
- Karim S, Baie SH, Hay YK, Bukhari NI. Development and evaluation of omeprazole pellets fabricated by sieving-spheronization and extrusion-spheronization process. Pak J Pharm Sci 2014;27(3):425-38. [PMID: 24811797]
- Petrovick GF, Breitkreutz J, Pein-Hackelbusch M. Taste-masking properties of solid lipid-based micropellets obtained by cold extrusion-spheronization. Int J Pharm 2016;506(1-2):361-70. [PMID: 27132502 DOI: 10.1016/j.ijpharm.2016.04.058]
- Jadhav NR, Kambar RS, Patil S, Nadaf SJ. Strength enhancement of talc pellets by incorporation of high percentage of hydroxypropyl methyl cellulose. Der Pharm Lett 2013;5:17-26.
- Nadaf SJ, Jadhav AB, Killedar SG. Mung bean (Vigna radiata) porous starch for solubility and dissolution enhancement of poorly soluble drug by solid dispersion. Int J Biol Macromol 2021;167:345-57. [PMID: 33253744 DOI: 10.1016/j.ijbiomac.2020.11.172]
- Nadaf S, Nnamani P, Jadhav N. Evaluation of Prosopis africana seed gum as an extended-release polymer for tablet formulation. AAPS PharmSciTech 2015;16:716-29. [PMID: 25523143 DOI: 10.1208/s12249-014-0256-y]
- Killedar SG, Nale AB, More HN, Nadaf SJ, Pawar AA, et al. Isolation, characterization, and evaluation of Cassia fistula Linn. seed and pulp polymer for pharmaceutical application. Int J Pharm Investig 2014;4(4):215-25. [PMID: 25426443 DOI: 10.4103/2230-973X.143128]
- Yadav A, Mane S, Nadaf S. Development and evaluation of mucoadhesive bilayer buccal tablet of carvedilol. CIBTech J Pharm Sci 2015;1:51-6.
- Nadaf SJ, Killedar SG. Curcumin nanocochleates: use of design of experiments, solid-state characterization, in vitro apoptosis and cytotoxicity against breast cancer MCF-7 cells. J Drug Deliv Sci Technol 2018;47:337-50. [DOI: 10.1016/j.jddst.2018.06.026]
- Bhagwat DA, Swami PA, Nadaf SJ, Choudhari PB, Kumbar VM, et al. Capsaicin loaded solid SNEDDS for enhanced bioavailability and anticancer activity: in-vitro, in-silico, and in-vivo characterization. J Pharm Sci 2021;110(1):280-91. [PMID: 33069713 DOI: 10.1016/j.xphs.2020.10.020]
- Jadhav NR, Kambar RS, Nadaf SJ, Phadatare PD. Design, development, in vitro and in vivo evaluation of multicomponent tablet formulation for enteral delivery of atorvastatin calcium and felodipine. J Pharm Investig 2015;45:115-30. [DOI: 10.1007/s40005-014-0148-x]
- Killedar S, Pawar A, Nadaf S, Nale A, Tamboli U, et al. Novel analytical method development for some amide group containing drugs using Bougainvillea spectabilis bract extracts. Asian Pac J Trop Med 2014;7(suppl 1):S560-7. [PMID: 25312184 DOI: 10.1016/S1995-7645(14)60290-X]
- Bhagwat D, Kolekar VR, Nadaf SJ, Choudhari PB, More HN, et al. Acrylamide grafted neem (Azadirachta indica) gum polymer: screening and exploration as a drug release retardant for tablet formulation. Carbohydr Polym 2020;229:115357. [PMID: 31826453 DOI: 10.1016/j.carbpol.2019.115357]
- Nadaf SJ, Killedar SG. Implementation of statistical tools and novel approaches to develop and validate the method for the estimation of Pazopanib in bulk, tablets and fabricated nano-sized formulation. Adv Pharm J 2017;2(6):218-27.
- Dias C, Ayyanar M, Amalraj S, Khanal P, Subramaniyan V, et al. Biogenic synthesis of zinc oxide nanoparticles using mushroom fungus Cordyceps militaris: characterization and mechanistic insights of therapeutic investigation. J Drug Deliv Sci Technol 2022:73;103444. [DOI: 10.1016/j.jddst.2022.103444]
- Savekar PL, Nadaf SJ, Killedar SG, Kumbar VM, Hoskeri JH, et al. Citric acid cross-linked pomegranate peel extract-loaded pH-responsive β-cyclodextrin/carboxymethyl tapioca starch hydrogel film for diabetic wound healing. Int J Biol Macromol 2024;274:133366. [PMID: 38914385 DOI: 10.1016/j.ijbiomac.2024.133366]
- Usapkar P, Saoji S, Jagtap P, Ayyanar M, Kalaskar M, et al. QbD-guided phospholipid-tagged nanonized boswellic acid naturosomal delivery for effective rheumatoid arthritis treatment. Int J Pharm X 2024;7:100257. [DOI: 10.1016/j.ijpx.2024.100257]
- Rodrigues K, Nadaf S, Rarokar N, Gurav N, Jagtap P, et al. QbD approach for the development of hesperetin-loaded colloidal nanosponges for sustained delivery: in-vitro, ex-vivo, and in-vivo assessment. OpenNano 2022;7:100045. [DOI: 10.1016/j.onano.2022.100045]
- Gupta VN, Gowda D, Balamuralidhara V, Khan SM. Formulation and evaluation of olanzapine matrix pellets for controlled release. Daru 2011;19(4):249-56. [PMID: 22615665]
- Abou Obaid NI, Al-Jenoobi FI, Ibrahim MA, Alam MA. Losartan potassium sustained release pellets with improved in vitro and in vivo performance. Pharm Dev Technol 2020;25(9):1031-42. [PMID: 32538215 DOI: 10.1080/10837450.2020.1782934]