Exploring the Frontier of Inhalation Therapy: A Review of Dry Powder Inhalers for Precision Management of Inflammatory Lung Diseases
1Bharati Vidyapeeth College of Pharmacy, Palus 416310, Maharashtra, India
2Arvind Gavali College of Pharmacy Jaitapur, Satara, Maharashtra, India
3New Women’s College of Pharmacy, Kolhapur, Maharashtra, India
4Department of Pharmaceutical Chemistry, Modern College of Pharmacy (For Ladies), Moshi 412105, India
5Annasaheb Dange College of Pharmacy, Ashta, Maharashtra, India
*Correspondence to: Mr. Amir R. Tamboli, Bharati Vidyapeeth College of Pharmacy, Palus 416310, Maharashtra, India, Tel.: 9325646566, E-mail: amirrtamboli@gmail.com; Dr. Sameer J. Nadaf, Bharati Vidyapeeth College of Pharmacy, Palus 416310, Maharashtra, India. E-mail: Sam.nadaf@rediffmail.com
Received: July 31 2024; Revised: November 5 2024; Accepted: November 22 2024; Published Online: December 4 2024
Cite this paper:
Tamboli AR, Yadav VD, Nadaf SJ et al. Exploring the Frontier of Inhalation Therapy: A Review of Dry Powder Inhalers for Precision Management of Inflammatory Lung Diseases. BIO Integration 2024; 5: 1–14.
DOI: 10.15212/bioi-2024-0062. Available at: https://bio-integration.org/
Download citation
© 2024 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
Chronic respiratory diseases impose a substantial health burden globally with increasing prevalence and mortality rates, especially in affluent nations. Recent studies underscore the escalating contribution to premature morbidity and mortality, highlighting the critical need for effective interventions. Inflammatory lung diseases, such as asthma, chronic obstructive pulmonary disease (COPD), acute respiratory distress syndrome (ARDS), and idiopathic pulmonary fibrosis (IPF), have significant roles in this burden that are characterized by heightened inflammation affecting the airways or lung parenchyma, leading to obstructive or restrictive lung diseases. These conditions often exhibit acute and chronic components, which complicate disease classification and management. Current treatments for lung illnesses predominantly rely on systemic drug delivery, which may result in poor efficacy and adverse effects on other organs. Inhalation lung delivery presents a promising alternative, offering advantages, such as targeted drug deposition, reduced systemic side effects, and rapid onset of action. Despite these benefits, inhalation drug delivery systems are still in the developmental stage, particularly for targeted local delivery. Dry powder inhalers (DPIs) have emerged as a popular choice due to ease of use, high-dose delivery capability, and breath-activated mechanisms. This review delves into the intriguing world of pulmonary drug delivery, with a spotlight on DPIs. From the fascinating design principles to the potential for precision medicine, DPIs offer a glimpse into the future of respiratory care. By unravelling the mysteries of DPI formulation and performance assessment, this review aimed to propel the field forward, ushering in a new era of personalized and efficacious inhalation therapies for inflammatory lung diseases and beyond. Furthermore, this article delved into the identification of prevalent technologies within the DPI domain while also probing the prospective avenues of emerging development. Such insights aim to assist researchers in making informed decisions regarding relevant research and development pursuits.
Keywords
COPD, DPIs, inhalation device, pulmonary route, respiratory disorders.
Introduction
The 2017 Global Burden of Disease study reports a rise in chronic respiratory diseases that affects 545 million people (7.4% of the global population), highlighting the significant impact on health [1]. Inflammatory lung diseases, such as asthma, chronic obstructive pulmonary disease (COPD), acute respiratory distress syndrome (ARDS), idiopathic pulmonary fibrosis (IPF), significantly contribute to the global disease burden. Recent studies predict an increase in respiratory diseases, mainly due to chronic conditions characterized by high inflammatory cell counts [2]. Dry powder inhaler (DPI) formulations have been studied in clinical trials for treating ARDS associated with COVID-19. Notably, a phase II clinical trial investigated the use of inhaled budesonide delivered via a DPI in patients with COVID-19 who were at an increased risk of developing ARDS. This approach highlights the potential of inhalation therapies in managing respiratory complications related to the virus [3–5]. In recent studies, Lu et al. [6] developed a DPI formulation for salvianolic acid B (Sal B) aimed at treating IPF. Lu et al. [6] findings demonstrated that the Sal B-DPI exhibits promising results, including enhanced lung tissue distribution and effective pharmacokinetics, thereby suggesting the potential of Sal B-DPI as a viable non-invasive treatment option for IPF [6].
Inflammation in these diseases can affect the airways (trachea, bronchi, and bronchioles) and/or lung parenchyma (alveoli). This situation can lead to obstructive lung disease, characterized by increased airflow resistance or restrictive lung disease, in which stiffened lungs lose compliance and gas exchange units [2]. Inflammatory lung diseases can be acute, emerging abruptly due to infections, environmental triggers, or chronic involving persistent and progressive lung impairment, leading to tissue restructuring or fibrosis [7]. Acute diseases often manifest within chronic illnesses, such as acute exacerbations during airway remodelling in severe asthma. There is significant overlap and heterogeneity between diseases like asthma and COPD, with diverse subtypes and phenotypes [8]. Therefore, focusing on shared pathologic mechanisms within the lung, especially pathologic mechanisms at the core of disease pathogenesis, is more effective than concentrating on individual diseases [8, 9]. Lung illnesses are treated with various methods, including bronchodilators, corticosteroids, anti-inflammatories, antibiotics, proteins, peptides, and genetic elements. Oxygen therapy is also used to enhance blood oxygen levels in cases of hypoxemia [10]. Most chemical treatments are administered orally or intravenously, often resulting in poor efficacy and potential side effects on other organs. These treatments typically provide only symptomatic relief rather than a complete cure [11].
The lungs present an attractive route for drug delivery due to the extensive surface area and rich vascularization, facilitating effective therapeutic deposition and systemic absorption. Inhalation delivery bypasses gastrointestinal degradation and first-pass hepatic metabolism, which enhances bioavailability. Despite these benefits, the adoption of systemic inhalation delivery remains limited. Conversely, systemic administration for lung diseases often results in low efficacy and significant adverse effects on other organs. Therefore, direct inhalation delivery of therapeutics to the lungs optimizes treatment efficacy and minimizes off-target effects [12].
In recent years inhalation drug delivery has become a favoured approach for treating lung disorders, although significant advances are still needed. Chronic lung disorders require long-term therapy, often leading to adverse effects from systemic drug administration [13]. While inhalation delivery provides rapid effects, predicting and standardizing inhalation delivery remains challenging [14].
While several inhalation drug delivery devices are already in clinical use, developing efficient systems for targeted inhalation delivery is ongoing. Inhalation delivery offers benefits, such as fewer systemic side effects, effective treatment of respiratory tract issues, and bypassing hepatic metabolism, allowing for lower dosages and reduced patient discomfort. However, lung defence mechanisms, like muco-ciliary clearance and phagocytosis, can hinder medication transport [15].
DPIs are widely used due to numerous advantages: (i) no need for cold chain storage or powder reconstitution; (ii) superior physicochemical stability; (iii) effective pulmonary deposition through patient respiration; and (iv) easy incorporation of high drug masses [16]. The ease of use and capacity of DPIs for high-dose delivery increase adoption. Additionally, DPIs typically feature breath activation, which eliminates the need for coordinating inhalation with device actuation. This review covers the classification of pulmonary drug delivery systems, focusing on the advantages, requirements, current status, and formulation strategies of DPIs, along with various evaluation techniques.
Pulmonary drug delivery systems
Nebulizers
Nebulizers, in use since the mid-nineteenth century, produce aerosols by dissolving or suspending drugs in a suitable solvent. Nebulizers excel at generating fine mists of small droplets, which are effectively absorbed by the lungs. Nebulizers utilize air jets or ultrasonic devices to atomize aqueous-based medicinal solutions and are commonly used in hospitals and ambulatory care for newborns, the elderly, and critically ill patients. Some formulations may contain preservatives to prevent microbial growth [17, 18].
Nebulizers offer several advantages. Nebulizers allow for the use of a greater amount of medicine, accommodate multiple medications in one system, require less patient coordination, and have simple formulation handling. However, nebulizers also have drawbacks. Nebulizers are costly and cumbersome to transport, there is variation in the performance of different nebulizers, and nebulizers typically require an external power source [19].
Pressurized metered dose inhaler (pMDI)
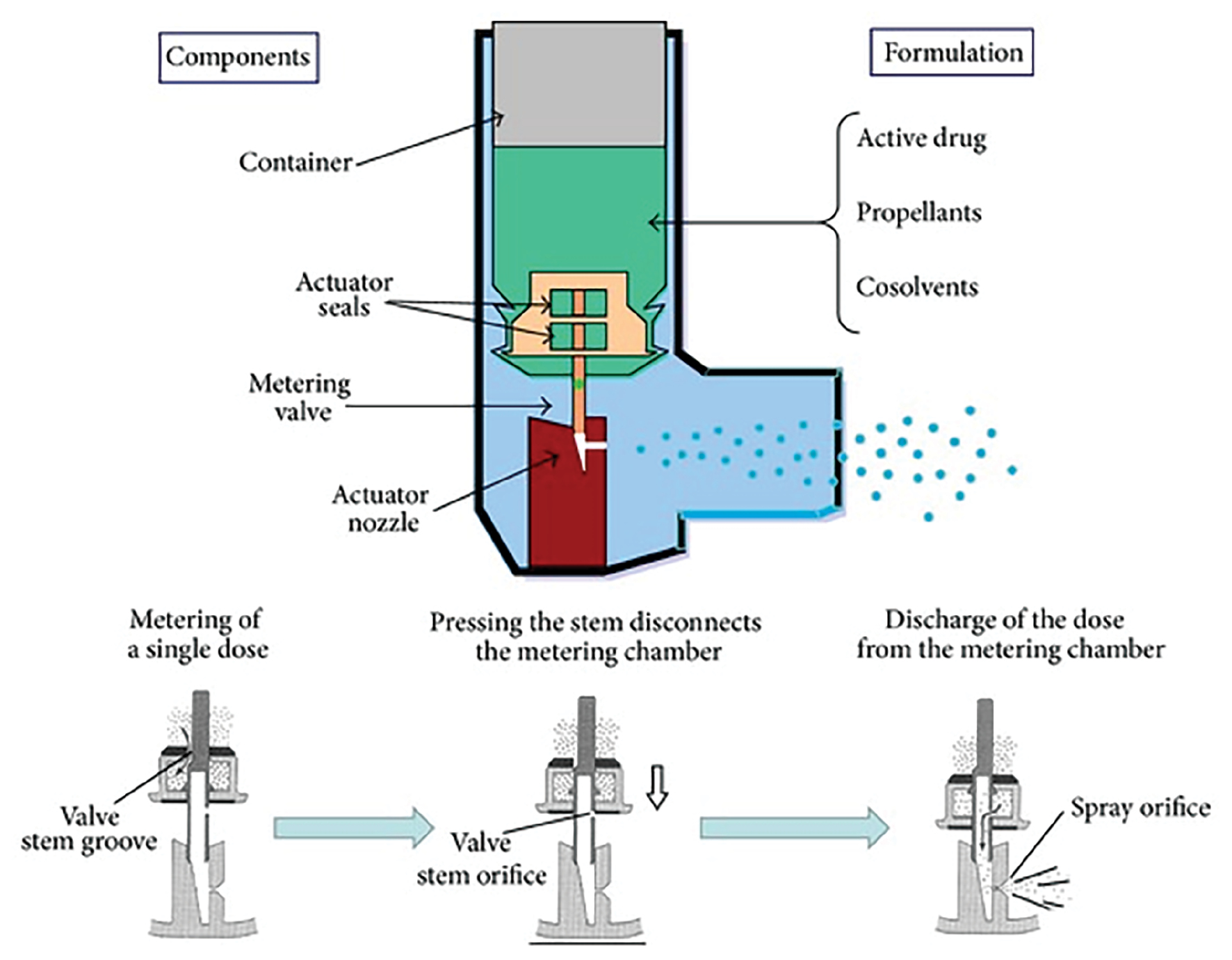
In 1956,the pMDI was approved as the first modern inhaler device. When a pMDI is triggered, medicine is combined with a propellant in a canister, then released in exact regulated amounts. Components of pMDI are shown in Figure 1 [8, 10, 11].
Figure 1 Pressurised metered dose inhaler (adapted from [12]). This work is licensed under a CC BY 4.0.
pMDIs are favoured for simplicity of use, compact convenience, reliability, precise metering performance, and cost-effectiveness [20]. However, pMDIs also have several drawbacks. The rapid rate of dose delivery increases the likelihood of early deposition in the oropharynx, resulting in reduced drug retention in the airways, which limits effectiveness primarily to upper airway disorders with only 10%–15% of the medication reaching the lungs. pMDIs require careful synchronization of inhalation and actuation, and in the case of suspensions, failure to shake the device can complicate dose accuracy. Additionally, pMDIs often contain propellants, like chlorofluorocarbons (CFCs), that harm the ozone layer and can only be used with drugs stable in a propellant environment [21].
Dry powder inhaler (DPI)
A DPI is a device that delivers medication to the airways using dry powder. DPIs are commonly used for managing pulmonary conditions, such as asthma, bronchitis, emphysema, and COPD, and have also been used for diabetes management [22]. DPIs offer an alternative to pMDIs. There are two primary types of DPIs (active and passive). Most DPIs are passive breath-activated devices that rely on inhalation to operate. The patient inhales deeply to access the medication, eliminating the need to coordinate breathing with activation. Pre-metered DPIs, which can be single or multidose, have the dosage pre-measured during the production process. These DPIs use systems, such as blisters or capsules, where the medication is stored in a reservoir and each dose is pre-measured upon activation [8, 20, 21].
The dry powder dais has equipment that produce aerosol directly from pharmaceutical blends or powder with active ingredients in sizes ranging from 1–5 microns. The ingredients utilised in dry powder inhaler transport active pharmaceutical ingredients (APIs). Lactose monohydrate is a common transporter. The need for alternatives to pMDI to limit pollution from ozone-depleting and greenhouse gases hydrofluoroalkanes and chlorofluorocarbons, which are used as propellants, prompted the development of DPIs. The mechanism of dispersion of APIs through DPIs is shown in Figure 2.
Figure 2 Schematics of dry powder inhaler (DPI) dispersion mechanisms (adapted from [13]). This work is licensed under a CC BY 4.0.
Advantages and essential requirements of DPIs
DPIs offer several advantages over pMDIs, which address key needs in respiratory therapy. These benefits stem from the technical requirements and unique design of DPIs, which ensure stability, precise dosing, and enhanced patient convenience.
- Effective Particle Size for Lung Delivery: One major advantage of DPIs is the ability to deliver drug particles in an optimal size range (1–10 μm), which is essential for lung deposition and therapeutic efficacy. Techniques, like micronization, selective precipitation, and spray drying, help produce particles of the right size for effective inhalation [14].
- Content Uniformity and Consistency: DPIs are designed to provide consistent drug doses. In single-dose DPIs, each capsule or blister must contain the same drug quantity, while multi-dose DPIs are engineered to release uniform doses with each use, regardless of respiratory patterns. This property ensures reliable medication delivery across different inhalation rates, which is crucial given the variability in breathing patterns [11].
- Stability Against Environmental Factors: DPIs have a solid formulation that inherently offers greater stability than liquid formulations found in pMDIs. The dry powder design minimizes the risk of degradation caused by temperature or moisture, preserving drug potency, even under varying environmental conditions. To prevent unwanted particle growth, which could affect drug dispersion, lactose carriers are used to protect particle size distribution and DPIs are often stored in specialized packaging for added stability [11].
- Ease of Use with Breath Activation: Unlike pMDIs, which require coordinated inhalation and device actuation, DPIs are breath-activated, meaning the medication is automatically released upon inhalation. This ease of use makes DPIs especially beneficial for patients with limited coordination or dexterity, improving adherence and ensuring proper medication intake [23, 24].
- Environmentally Friendly Design: The propellant-free design of DPIs reduces environmental impact. pMDIs often rely on CFCs or hydrofluoroalkanes, which contribute to ozone depletion and greenhouse gas emissions. DPIs eliminate these propellants, making the DPIs a more sustainable choice for respiratory treatments [24, 25].
- High-dose Delivery Capability: DPIs can deliver high doses with each inhalation, making DPIs suitable for acute exacerbations and chronic management of respiratory diseases. This high-dose delivery ensures that patients receive the required therapeutic dose in a single use, which improves treatment outcomes.
- Customization and Flexibility in Drug Formulation: DPIs allow for flexible drug combinations, including the incorporation of multiple active ingredients in a single device or targeting specific lung areas. This customization capability aligns with the need for personalized treatment approaches, enhancing device versatility and clinical applicability.
The Bevespi Aerosphere® is an example of a DPI that meets these requirements and has these advantages. The Bevespi Aerosphere® combines glycopyrrolate and formoterol fumarate using PulmoSphere® technology. This DPI has shown effective deposition in all lung regions with minimal exhaled fractions, demonstrating its capability for consistent, high-quality drug delivery [26].
Formulation strategies for DPIs
DPI effectiveness is mostly determined by the powder flow principle, which is primarily influenced by solid interparticle forces and results in a cohesive bulk powder agglomeration. Interparticle forces include the capillary force electrostatic force, capillary force, and van der Waals force. When all the particles are close enough to each other (0.2–1.0 nm) and fine enough, the van der Waals force becomes known (≤20 μm). Geometrical structure, surface roughness, and particle deformation all impact the van der Waals force. The potential difference between particles with distinct job tasks may create electrostatic force when in contact. The powder sticks together because of the Coulomb attraction. Condensation of fluid in the gaps between particles in close proximity causes capillary force, which results in creation of liquid bridges between the particles. Electrostatic force, which decreases as the moisture levels rise, is sacrificed by high capillary force [27].
Various types of DPI formulation techniques to overcome these complications are described below.
Carrier free
APIs can involve a single chemical, a multi-compound, or encapsulated particles in a carrier-free environment. Only a handful of the production processes accessible include crystallisation and supercritical fluid, spray drying, and milling. Because APIs do not create amorphous material escape, optimum particle shape, limited particle size distribution, reduced surface energy, crystallisation and milling appear to be insufficient for the formulation of pulmonary drugs. The aerodynamic particle size of the inhalational medication must be <5 μm [16, 27–29].
Drug carrier
Dispensing 1 g to 1 mg dosages of medication through tiny DPI blisters is difficult. In addition, the ideal particles are between 1 and 5 μm, which makes straining powder by inhalation difficult. As a result, to improve the flow property as well as the volume of each dose, drug molecules are combined with larger particles. Between 50 and 100 μm is the size range of these carrier particles. Indeed, it is easier to distribute smaller dosages when the dose volume is larger. The downside of this approach is that carriers tend to settle into the oral cavity, where various dose particles stick to the carriers, resulting in poor drug concentrations in the lungs. Drugs with low particle separation from carrier particle surface result in low delivery ability [16, 27–29].
Drug additive
The fluidization quality of fine pharmaceutical powders could be enhanced by adding fine particles. Because Van der Waals forces of attraction are mostly dependent on particle-particle space, increasing separation distance lowers the adherent force and enhances the fluidization and flow properties of microscopic particles. Some of the additives used include aerosol 200 (12 nm), alumina (29 nm), and submicron silica (0.5–3 weight percent) [16, 27–29].
Drug carrier additive
To increase medicine distribution, it is possible that a new particle type will be introduced to the mix. These additives might be small particles that function as physical spacers, with tiny particles having the same composition as the carrier or high-energy locations, such as fissures in carrier surface. This type of technology is demonstrated by incorporating fine lactose into a lactose carrier system. Improved separation of medicine particles from carrier particles has been achieved by increasing the proportion of lactose fine particles [16, 27–29].
Manufacturing of DPIs
3 steps are involved in the formulation of DPIs.
API production
In the case of DPIs, particle size is a critical requirement of APIs. The particle size must be <5 microns. However, the particle size usually ranges from 0.5–5 μm. Examples of the processes for lowering size include supercritical fluid extraction, milling, and spray drying. Many kinds of mills are utilized to reduce the size of pharmaceuticals but only a few mills, such as high-peripheral-speed mills like the ball and pin mills, jet mills, and fluid-energy mills, are appropriate for DPIs to reduce the size range to 0.5–5 μm. High-velocity particle-particle impacts in a jet mill reduce particle size. Particles that have not been milled are carried into the milling space. The solid particles are accelerated to sonic velocities by high-pressure nitrogen delivered from nozzles. Collisions and crashes occur as the particles collide. As the particles fly around the mill, larger particles are driven into the outside perimeter of that region due to stronger centrifugal forces. The little particles exit the mill via the middle discharge stream [17, 18].
Preparation of APIs without or with carriers
The purpose of carriers in DPIs is to increase aerosol performance of cohesive medications and fine lactose by improving powder flow characteristics. The drug and carrier(s) are then transferred to appropriate forms and mixed. Inadequate mixing might result in inconsistent dosages. The amount of time required to blend cannot always compensate for poor mixing. Blend consistency is affected by optimization restrictions, such as rotation speed, fill level, capacity, and blender selection. Depending on the tensions that exist in between particles, powders might have varying blending characteristics. Less concentration (drug-to-carrier ratio) mixes require pre-blending geometric dilutions [19]. The resulting high-energy active area on the surface of the coarse carrier particles cause the drug particles to adhere firmly to the coarse carrier particles (particle size <20 μm). The active sites of coarse carrier particles are saturated by fine carrier particle enlargement (10 μm), which are then connected to micronized medicine. As a result, the drug sticks to passive sites or sites with low energy and aid in the disaggregation of the micronized drug during inhalation, resulting in an improved respirable fraction [11, 19, 30].
Incorporation of the formulation into the device
The combination is packed in reservoirs, multi-dose blisters, and capsules for use with an inhaler machine. Filling is automatic and controlled by metering system features [30].
Polymeric DPIs
Polymers are commonly utilized in the production of DPIs to allow continuous release of the dosage and prevention from enzymatic degradation of active chemicals [20]. First-line polymeric DPIs were created to alter the features of quick drug release in water-soluble formulations. Because of the low toxicity, biocompatible polymers, like polylactic-co-glycolic acid (PLGA) and polyvinyl alcohol (PVA), are frequently utilised as controlled agents for drug release [21, 31, 32]. Other polymers used in the production of polymeric DPIs include polylactide (PLA), poly-caprolactone (PCL), hydroxyl propyl methyl cellulose (HPMC), chitosan, gelatine, hyaluronic acid (HA), and locust bean gum [33]. Long-term lung exposure to a higher polymer concentration, which is required to achieve the appropriate dose proportion in the target area, might cause fibrosis and/or respiratory inflammation [34, 35].
Natural polymers, rather than manmade polymers, are increasingly being used to address this problem [36, 37]. Furthermore, nanoparticle-based powder drug formulations are more stable than liposomal formulations and prevent the active ingredient against mucociliary evacuation and phagocytosis, which are both airway defensive processes [37]. Nevertheless, there is still a concern about the toxicity of inhalable nanoparticles. Andrade et al. [38, 39] developed and tested polymeric micelles containing insulin that self-assemble as DPIs using thin-film hydration and freeze-drying processes. In vitro aerosolization and sedimentation characteristics were estimated using the instrument Andersen cascade impactor of these particles at a flow rate of 28.3 L/min and a 4 L air pass state. Using a Rotahaler®, it was shown that median mass aerodynamic diameter (MMAD) was <5.1 μm and the fine particle fraction (FPF) was >44% [38]. Rezazadeh et al. used spray drying to make paclitaxel-containing polymeric micelles as DPIs. Local application likely alleviated systemic side effects in the investigation.
Polymeric micelles were made with tocopheryl succinate and polyethylene glycol. The Andersen cascade impactor with Spinhaler® equipment was utilized to examine the aerodynamic assets of the resulting DPIs with a MMAD, FPF, and emitted dosage of 4 μm, 60.11 ± 0.23%, and 89.84%, respectively [40]. Inhalers made of polymeric dry powder can be made in a variety of methods. Farhangi et al. [41] used a spray drying technique to create ciprofloxacin-loaded polymeric nano-micelles utilising chitosan-lipid conjugates. The formulations exhibited a much stronger antibacterial action when compared to free ciprofloxacin. The FPF of the preparation and mean volume diameter were 60% and 1.7 μm, respectively [41].
Lipid-based DPIs
Liposomes
Liposomes are vesicles that can be utilised to deliver medications. Due to a lack of stability, use of liposomes is restricted. Liposomes, commonly described as lipospheres or pro-liposomes, are solid liposomes that were established to solve this problem. Pro-liposomes are granular materials consisting of dry powder and phospholipid precursors that can be hydrated to create liposomes before or after drug administration [42, 43]. Liposomes, which are made from phospholipids found in pulmonary surfactant, are biologically compatible, biodegradable, and non-toxic materials. Both hydrophilic and lipophilic medicines can be carried in liposomes. Furthermore, these liposomes can administer cytotoxic, anti-asthmatic, anti-microbial, and anti-viral active ingredients effectively and systemically. Excipients used to make pro-liposomes have been demonstrated to impact not just the aerosolization capability but also the drug release profile [44, 45].
Li et al. [46] used an injectable technique to make liposomal and rographolide DPIs to cure bacterial pneumonia and achieved a 23.03% FPF and 4.87-μm MMAD. Chennakesavulu et al. [47] developed liposomal DPIs containing colchicine and budesonide to cure IPF. Liposomes with a mean range <100 nm were developed using the thin layer film hydration method. The liposomes were freeze-dried with mannitol before analysis using an Andersen cascade impactor to create a dry powder composition. The MMAD and FPF were 45–50% <5 μm, respectively. Different liposomal compositions with different aerodynamic properties may be generated [47].
Solid-lipid nanoparticles (NPs)
Traditional colloidal systems, such as emulsions and liposomes, can be replaced by solid lipid nanoparticles (SLNs). SLNs may be mass-produced in huge numbers and loaded with a wide range of active chemicals (prednisolone, diazepam, and camptothecin) [48]. Rosière et al. [49] developed chitosan derivative-coated SLNs with paclitaxel targeting lung cancer cells. Spray drying procedures and nano-precipitation were used to make these NPs. A size 3 hydroxy propyl methylcellulose (HPMC) capsule filled with 20 mg of powder (100 L/min for 2.4 sec) and a multi-stage fluid impinger (Axahaler®) to investigate in vitro aerosolization qualities. Furthermore, when compared to a commercially available paclitaxel formulation (taxol) FPF, the human ovarian HeLa cell line was used in an in vitro cell viability study with a 34% increase in anti-cancer efficacy [49]. Bakhtiary et al. developed DPIs to cure non-small cell lung cancer using erlotinib-loaded SLNs. Spray drying was utilised to make DPIs with and without mannitol after producing 100 nm SLNs. The next generation impact or aerodynamic parameters were examined. The emanated dosage, geometric standard deviation, MMAD, and FPF were 87.16 ± 0.16%, 24.25 ± 0.72%, 2.582 ± 06 μm, and 5.528 ± 0.47 μm, respectively, without mannitol DPIs [46]. The geometric standard deviation, emitted dose, FPF, MMAD, and geometric standard deviation for mannitol DPIs were 94.91 ± 0.15%, 30.98 ± 0.87%, 3.931 ± 0.31 μm, and 4.339 ± 0.07μm, respectively. Based on the the results, excipients used in the manufacturing stage, such as carriers and cryoprotectants, can amend the aerodynamic characteristics of DPIs [50].
Solid-lipid microparticles (SLMs)
SLMs are a type of DPI preparation that enables medicine release control. SLMs are identical to o/w emulsions because oils are used that are solid at room temperature. A surfactant stabilises the microparticles and traps the hydrophobic active component inside the oil droplet. The drug is dissolved in melted oil, then blended with the aqueous phase and homogenised to make droplets as small as possible. The device is cooled to create SLMs after reaching the right droplet size [51]. Scalia et al. used the phase inversion technique to make quercetin-containing microscopic particles utilising an o/w emulsification technique. The lipid component was tristearin and the emulsifier used was phosphatidylcholine. The FPF value was determined to be 20.5 ± 3.3% with the next-generation impactor, despite the formulation having an aerodynamic diameter <5 μm [52]. This finding emphasises the need of taking radiated dosage into account when calculating the FPF parameter. SLMs containing rifampicin were produced by Maretti et al. to direct alveolar macrophages in curing tuberculosis. The tapped density, bulk density, porosity, and apparent density 0.161 ± 0.020 g/cm3, 070 ± 0.002 g/cm3, 80.44 ± 0.05%, and 1.058 ± 0.010 g/cm3, respectively. The predicted aerodynamic diameter of SLMs was 0.51 ± 0.08 μm, even though the mean particle size diameter was 1.15 ± 0.25 μm [53]. The volume diameter appears to be larger than the aerodynamic diameter as the porosity of particles is enhanced and the density is decreased.
Nanostructured lipid carriers
A solid lipid core with a surfactant to keep it stable makes up nanostructured lipid carriers. This method may distribute both lipophilic and hydrophilic medicines. High-drug loading capacity, controlled drug release ability, biodegradability, and biocompatibility are some of the advantages of this system in addition to long-term stability and the potential to scale-up [54]. Patil-Gadhe et al. used melt-emulsification, ultra sonication, and lyophilization techniques to make rosuvastatin-loaded nanostructured lipid transporters with or without L-leucin as DPIs. A Westech 8-stage, non-viable cascade impactor was utilised to evaluate the aerosolization properties at fluid velocities of 30 and 60 L/min. The aerodynamic properties were shown to improve in the presence of leucine. The MMAD and geometric standard deviation (GSD) decreased as the flow rate increased from 30-to-60 L/min, whereas the FPF increased. In vitro aerosolization studies yielded a MMAD <3 μm and a FPF >90% FPF at a flow rate of 60 L/min. The inhalation rate had the ability to affect the aerodynamic properties independently of the formulations [55].
Lipid-polymer NPs
Lipid–polymer NP systems enhance bioavailability by combining the features of nano-systems, polymers, and liposomes. A lipid layer is applied to the polymer NP core to produce these lipid-polymer NP systems. The lipid-to-polymer ratio is critical because the lipid-to-polymer ratio directly influences the active substance encapsulation efficiency and release qualities. The polymer used is poly (lactic-co-glycolic acid) and the lipid source contains lecithin, which is often used in lipid–polymer NP-based DPIs. To accomplish effective aerosolization, NPs <1 μm are converted to microscale configurations during breathing [56–58].
Micro-particulate DPIs
Microparticles are microsphere or microcapsule complexes made from hydrophilic and lipophilic medicines with particle sizes ranging from 1–999 μm. The aerodynamic dimensions should be in the range of 1–5 μm for microparticles formulated as DPIs. The active ingredients in solids, solutions, suspensions, and emulsions can be encapsulated within the particles. By altering the process parameters, microparticles of the necessary size, shape, and porosity may be manufactured. Microparticles delivered via the lungs to alveolar macrophages in diseases, such as tuberculosis, are additionally successful in providing high quantities of active substance to cells. Alveolar macrophages should be avoided in the treatment of various disorders to enhance the bioavailability and alveolar half-life of the active drug by inhibiting pulmonary clearance. To prepare micro-particulate DPIs for the specified purpose, microparticles without carriers and large porous microparticles can be used.
Carrier-free microparticles
Excipients are used in inhaler formulations to increase the micronized medication flow and aerosolization characteristics. However, there are only few excipients that are permitted for inhalation and adding a high amount of excipient to a preparation reduces the amount of active drug in the formulation, limiting DPI formulations to μg levels. Because introducing a transport to a powder formulation increases the formulation volume, carriers are rarely used with medications given in large doses, such as antibiotics. As a result, research has been conducted to develop compositions which do not need use of a transporter to increase micronized medicine flow. Because the compositions contain minute quantities of the medication, commercial formulations, like as Bricanyl® (terbutaline) and Pulmicort® (budesonide) DPIs, have been developed without using carriers [59]. Yazdi et al. created carrier-less ibuprofen DPIs using an air-jet milling process. A next generation impactor (HPMC capsule comprising 10, 25, or 50 mg of drug) was used to examine aerosolization features. The emitted dosage, FPF, and MMAD were 69–73%, 72–80%, and 2.6-2.9 μm, respectively [60]. This finding demonstrates that DPIs can be made utilising simple, one-step processes that are suited for scale-up and industrial production. Similarly, using the spray drying process, Cayli et al. synthesised levofloxacin hemihydrate and ciprofloxacin hydrochloride as carrier-free DPI microparticles with or without dornase alpha or N-acetylcysteine. Although all formulations had MMAD values between 2 and 3 μm, the levofloxacin hemihydrate and N-acetylcysteine combination DPIs [61] had the highest FPF (85%). This finding shows that DPIs are formulations that can be used in combination with other drugs.
Large porous microparticles
Particles having a low density have smaller aerodynamic dimensions than the volume diameters. Because of the low density, large particles are often used, as in manufacturing of DPIs. Furthermore, the large volume diameters are associated with excellent flow properties, while low density results in good aerosolization. Using doxorubicin-loaded highly porous large PLGA microparticles, Kim et al. developed a sustained-release inhalation device. Although the MMAD was 3.6 ± 0.4 μm, the volume diameter was 14.1 ± 2.1 μm. Furthermore, the aerodynamic diameter was smaller than the volume diameter, as predicted [62]. DPI formulations can also be used for long-term release according to one study. However, with long-acting formulations, it is vital to consider the lung clearance process.
NP-based DPIs
The pulmonary route is used to give NP-based DPIs [63]. NPs with a modest particle size are not well-adapted for lung accretion and are expelled. Spray freeze-drying or spray-drying [56, 64, 65] NPs with leucine, PVA, or mannitol [66, 67] and co-administering carrier particles with lactose [68] may help solve this problem. NPs increase the solubility of liquid medicines and decrease the mucociliary clearance, so respirable NP preparations are favoured [20]. NPs may also be administered to specific target sites, such as malignancies, in addition to these qualities [63, 69]. Other DPI production technologies, such as nanocomposites, deliberately target respirable NPs, inorganic nanocarriers, nanoaggregates, and nanocrystals are available. NPs made of polymeric and lipid-based materials are also available. However, the deposition of lipid based-material are mostly influenced by the aerodynamic diameter of inhaled droplets (Figure 3).
Figure 3 Schematic summary of AD-dependent deposition and distribution mechanisms of inhaled lipid nanoparticle aggregates in the airways (adapted from [65]). This work is licensed under a CC BY 4.0.
Inorganic nanocarriers
Because of the advantageous physicochemical features, inorganic carriers are used for producing DPIs. Due to the biocompatibility and biodegradability of amorphous silica, silica NPs are often utilised to make DPIs. Furthermore, organic-based delivery of drugs techniques is less persistent than silica NPs. Silica particles may change shape and the surfaces can be painted [69]. Cheow et al. used an experimental design method to improve spray drying procedure parameters and make cylindrical clumps of biocompatible silica NPs. Small geometric and aerodynamic sizes were attained using the spray drying process at pH and feed concentrations. Cheow et al. were able to acquire particles to achieve consistent particle size and dose distribution while boosting aerosolization. A geometric diameter was chosen that was larger than the aerodynamic diameter [70]. This finding showed that hollow particles may be made by fine-tuning the parameters of the spray-drying technique, which is widely used in DPI manufacturing. The density is lowered, while also improving the aerodynamic properties.
Nanocrystals
The Nanocrystal® milling process (Elan Pharmaceutical Technologies, city, state, USA) lowers particle size to <400 nm. Wet milling can be used with the ball mill technology. Because the synthesis takes place in an aqueous medium, the amorphous areas of the particles recrystallize. Wet milling, as opposed to dry milling, develops more homogeneous crystal structure and more stable moisture-resistant particles. PVP and lecithin are used as excipients to offer the active component physical stability and prevent NP aggregation. The nanocrystalline technique has two major drawbacks (the process period may be extended and unclear extended toxicity in the respiratory system) [71]. To develop a DPI formulation, Hu et al. created curcumin nanocrystals. Hu et al. used a mixing of spray drying and wet milling techniques to achieve this DPI formulation. Using a next generation impactor, the effects of varying milling periods on aerosolization qualities were also examined. FPF increases from 62.4%-to-72.3% when the milling time increases from 10 min-to-40 min, indicating that the volume of medication predicted to pass to the lung increases [72]. Production conditions have a significant impact on the aerosolization qualities of DPI formulations.
Nanocomposites and nanoaggregates
Nanocomposites are materials made up of NPs and a carrier material, like sugar or a polymer. Physical factors, such as van der Waals forces [73], hold large porous or hollow NP together to create nanoaggregates. Mucociliary clearance, macrophage-mediated phagocytosis, and enzymatic degradation are all efficiently blocked by DPI nanocomposites and nanoaggregates without producing exhalation issues [74]. Kaur et al. were able to attain an aerodynamic diameter of <4 μm using a spray drying approach to make DPIs from isoniazid and rifampicin nanoaggregates for both isoniazid and rifampicin-containing DPIs [75]. Using the same manufacturing technique proved that numerous active compounds may be synthesised as DPI formulations.
Cyclodextrin complexes
Cyclodextrins (CDs) are cyclic oligosaccharides with hydroxyl groups that are hydrophilic on the outside and lipophilic on the inside. By generating an inclusion complex, the active compounds are linked to CDs. Because of the cavity size, complexing activity, and low cost, β-CD is one of the most extensively utilised CDs in the pharmaceutical industry [75]. CDs shield the active ingredient from enzymatic degradation and offer a long-lasting release, which enhances drug bioavailability and facilitates ideal release patterns [76]. Mohtar et al. used spray drying in the attendance of ethanol to create dry powder CD complexes for respiratory injection of fisetin, achieving an FPF of 75.83 ± 3.34%, an efficient delivery (ED) of 97.31 ± 0.74% ED, a MMAD of 2.0–2.5 μm, and a fine particle dose (FPD) of 7.06 ± 0.30 mg, all of which indicated successful aerodynamic behaviour. The FPF increased 2-fold when ethanol was used in the spray-drying stage and the FPF increased 2.3-fold when leucine was added to the formulation [77]. According to the study, excipients used in the spray-drying process or preparation affect the amount of medicine delivered to the deep lungs. Kinnarinen et al. [78] used a dry mixing approach to create a budesonide/-CD complex as a DPI. Lactose was used as the carrier. An Andersen cascade impactor was used to examine aerosolization qualities before and after 1 month of storage in a relative humidity of 75% at 40°C. The budesonide/-CD combination had a respirable percentage of 35% before storage and 31% after storage [78]. Aerodynamic qualities can vary during storage, as illustrated in Kinnarinen et al. [78] study.
Evaluation
In vitro testing of DPIs
A medicine must be able to reach the target in a reasonable concentration and with a level of contamination that is acceptable to be safe and effective. The quantity of medication given and the aerodynamic particle diameter range being supplied should be considered for inhalation dose forms. This element is determined by the amount of medicine delivered to the respiratory tract within a specific size range [79]. DPIs and median dose inhalers (MDIs) are the most frequent moveable devices utilized to transport drugs into the lungs. Because the operative methods of these two distribution mechanisms were so dissimilar, in vitro approaches for describing these dosage forms were taken into account [80]. Many pharmaceutical companies use the pMDI design strategy, which includes the container, surfactant, actuator, propellant, micronized drug, and metering valve. The high vapour pressure propellant flowing from the tiny departure hole in the valve stem delivers medicine to the patient in a disaggregated form. As a result, the medicine administered to the patient is mostly unaffected by the pace at which the patient breathes [81]. All pharmaceutical dose forms are necessary to guarantee that the substance supplied is safe and effective. Furthermore, the in vitro test must be created in such a way that the test simulates patient utility [82]. Due to the uniqueness of specific respiratory dosage forms, further testing is essential to advance and critically assess a guaranteed product quality. The correct drug is present with a reasonable number of contaminants according to product safety tests. A list of tests that are typically performed as part of product safeguarding is provided under the following: appearance; identity (chromatography and spectroscopy); microbial limits; extractives; respirable dose/particle size analysis; water content; patient use that is simulated (through the usage of a gadget, parameters and parallelisms of the patient, the flow rate, amount of air inhaled, andenvironmental considerations); drug-related impurities; reusable versus disposable testing for reliability; and drug content per unit dose/dose delivery).
Toxicity assessment
The biosafety of DPIs hinges on the toxicity profile. A considerable proportion of formulations are recognized for inducing local toxicity manifestations, such as lung irritation and oedema. In vitro cytotoxicity investigations stand out as the principal non-invasive method for appraising toxicity profiles. Furthermore, these studies serve to ascertain the LD50 (median lethal dose) for inhaled formulations, thereby aiding in the anticipation of appropriate dosages for subsequent investigations [83].
Current advances
Significant advances in DPI drug delivery systems have ushered in the development of cutting-edge platforms, including liposomes, nanocomposites, solid lipid NPs, polymeric micro-NPs, and microspheres. These innovative systems aim to surpass the limitations of traditional carrier-based DPIs, with several already in existence. The latest generation of DPIs, termed “active” or power-assisted DPIs, achieve drug dispersion at low flow rates by harnessing vibrating piezoelectric crystals powered by batteries. As the demand for inhalation vaccines rises, there is a burgeoning focus on crafting single-use DPI systems tailored for vaccination purposes. Moreover, peptides, proteins, genes, and viruses are increasingly integrated into inhalation powders, capitalizing on the multifaceted advantages offered by DPIs for delivering safe and efficacious treatments across a spectrum of diseases [84]. In response to patient needs, digitalized DPIs are also emerging as frontrunners among DPIs [85]. Current advances in nano-formulation-based DPIs are summarized in Table 1.
Table 1 Inhalable Nano-formulations under Clinical Application or Development for Respiratory Disease
| Nano-formulations | API | Implications | Key findings | Reference |
|---|---|---|---|---|
| Liposomes | Colchicine and budesonide | Idiopathic pulmonary fibrosis | Sustained drug release at the targeted site, effectively reducing systemic exposure. | [47] |
| Solid-lipid sanoparticles (SLNs) | Polyphenol and doxofylline | Asthma | In vivo studies in a murine asthma model demonstrated significant reductions in the serum bicarbonate level and eosinophil count, along with improvements in respiratory flow rate. | [87] |
| Nanostructured lipid carriers | Montelukast and mannitol | Allergic asthama | Enhanced bioavailability, prolonged drug residence time in the lungs. and targeting factor of 11.76 compared to montelukast aqueous solution | [88] |
| Lipid polymer hybrid nanoparticles | Ivermectin | Lung cancer | Direct lung deposition for rapid onset of action and increased efficacy at lower dose | [89] |
| Nanocrystals | Curcumin | COPD and asthma | Improved curcumin delivery to deep lung regions | [90] |
| Nano-composite and nano-aggregate | Rifampicin and xyclodextrin | Treatment of tuberculosis | Successful delivery of drug to the site of infection, while providing both immediate and sustained release effects | [91] |
| DPIs | Budesonide and arformoterol | COPD and asthma | Improved therapeutic efficacy than plain drug | [92] |
| Liposomes | Azithromycin | Chronic lower respiratory tract infection | Enhanced activity | [93] |
Challenges
Despite these advantages, DPIs also have some limitations. Specifically, DPIs require patients to generate sufficient inspiratory flow to disperse the powder into small particles for inhalation. This may be challenging for some patients, particularly patients with severe respiratory impairment. Additionally, DPIs may not be suitable for delivering some types of medications, such as medications with poor flow properties or medications that require precise dosing. Safety risks from excipients may impede use in pulmonary drug delivery. While high in vitro drug delivery typically indicates effective in vivo lung deposition, this correlation may be unreliable due to unrealistic throat models and individual breath pattern variations [86].
Future prospects
Future studies could focus on developing DPIs with improved inhalation mechanisms, such as devices that require less forceful inhalation or devices that can adapt to patients with varying levels of respiratory impairment. Additionally, research into innovative breath-activated mechanisms could further simplify the inhalation process for patients. Novel formulation strategies can be undertaken to optimize properties of the powdered medication, including particle size, flowability, and aerodynamic behaviour to enhance drug delivery efficiency. Such novel formulation strategies might involve the development of engineered particles with tailored characteristics to improve lung deposition and maximize therapeutic efficacy.
To address usability challenges associated with DPIs, future studies could focus on designing devices that are more intuitive and user-friendly, particularly for elderly or paediatric patients. Human factor research could inform the development of DPIs with ergonomic designs, clear instructions, and features that facilitate proper technique and dosage administration. Additionally, incorporating patient feedback and preferences into device design could improve overall adherence to treatment regimens. DPIs offer the potential for delivering multiple medications simultaneously, making the DPIs suitable for combination therapy in the treatment of complex respiratory conditions. Future studies could explore the development of DPIs capable of delivering tailored combinations of medications to target specific disease phenotypes or patient populations. Furthermore, advances in personalized medicine could enable the customization of DPI formulations to match individual patient profiles, optimizing treatment outcomes and minimizing adverse effects.
Research on overcoming biological barriers in lungs through innovative drug delivery strategies, such as the use of mucoadhesive formulations or NP-based carriers would be beneficial. Additionally, advances in imaging techniques could facilitate the visualization and assessment of drug deposition within the lungs, aiding in the optimization of DPI performance.
Overall, the future of DPIs holds great promise for addressing current challenges and advancing respiratory drug delivery. By addressing issues related to the inhalation technique, formulation optimization, patient-centred design, combination therapy, and drug delivery optimization, future studies have the potential to significantly enhance the efficacy, usability, and personalized nature of DPIs, ultimately improving patient outcomes in the management of respiratory diseases.
Clinical implications of DPI development
The industrial and clinical translation of DPIs for treating inflammatory lung disorders, such as asthma and COPD, has made significant strides in recent years. Advances in formulation techniques have improved the stability and bioavailability of inhaled therapies, allowing for targeted drug delivery to the lungs. Clinical trials have demonstrated that DPIs enhance patient compliance due to ease of use and breath-activated mechanisms. Future directions include the development of personalized DPI formulations that account for individual patient variability in disease presentation and drug response. Additionally, incorporating smart technologies, such as inhaler monitoring systems, could provide real-time feedback to patients and healthcare providers, improving treatment adherence and outcomes. Research into combination therapies that leverage the synergistic effects of multiple drugs within a single DPI formulation may further enhance therapeutic efficacy for managing inflammatory lung diseases. Overall, continued innovation in DPI design and formulation will be crucial for optimizing treatment strategies and improving the quality of life for patients with these chronic conditions.
Conclusion
DPIs have emerged as a popular choice due to ease of use, high-dose delivery capability, and breath-activated mechanisms. Over the years, DPI development has witnessed significant scientific advancements and innovations within the realm of pulmonary drug delivery. This technology has demonstrated effective utility in treating several pulmonary disorders using single or multiple drugs in combination formulations. However, persistent technical hurdles and gaps in fundamental understanding pertaining to process formulation and development remain to be addressed. Future prospects for pulmonary drug delivery systems include enhancing the efficiency and specificity of DPIs for local targeted inhalation delivery. This involves further research into formulation strategies to optimize drug delivery and overcome biological barriers. Additionally, the development of novel evaluation techniques for DPIs will facilitate the clinical implementation and ensure safety and efficacy. Furthermore, advances in personalized medicine are promising for tailoring inhalation therapies to individual patient needs, optimizing treatment outcomes while minimizing adverse effects. With continued research and innovation, the field of pulmonary drug delivery is poised to usher in a new era of personalized and efficacious inhalation therapies for inflammatory lung diseases and beyond, ultimately improving patient outcomes and enhancing the quality of life.
Abbreviations
DPI, Dry powder inhaler; COPD, Chronic obstructive pulmonary disease; pMDI, Pressurised meter dose inhaler; API, Active pharmaceutical ingredients; CFCs, Chlorofluorocarbons; PLGA, Polylactic-co-glycolic acid; PVA, Polyvinyl alcohol; PLA, Polylactide; PCL, Poly-caprolactone; HPMC, Hydroxypropyl methyl cellulose; HA, Hyaluronic acid; LBG, Locust bean gum; MMAD, Median mass aerodynamic diameter; FPF, Fine particle fraction; SLN, Solid-lipid nanoparticle; NPs, Nanoparticles; SLMs, Solid-lipid micro particles; GSD, Geometric standard deviation; CD, Cyclodextrin; FPD, Fine particle dose; ED, Efficient delivery; RH, Relative humidity
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Soriano JB, Kendrick PJ, Paulson KR, Gupta V, Abrams EM, et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med 2020;8(6):585-96. [PMID: 3252618 DOI: 10.1016/S2213-2600(20)30105-3]
- Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 2016;138(1):16-27. [PMID: 27373322 DOI: 10.1016/j.jaci.2016.05.011]
- Kim J, De Jesus O. Medication routes of administration. Treasure Island, FL: StatPearls Publishing; 2021.
- Fei Q, Bentley I, Ghadiali SN, Englert JA. Pulmonary drug delivery for acute respiratory distress syndrome. Pulm Pharmacol Ther 2023;79:102196. [PMID: 36682407 DOI: 10.1016/j.pupt.2023.102196]
- Ramakrishnan S, Nicolau DV, Langford B, Mahdi M, Jeffers H, et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med 2021;9(7):763-72. [PMID: 33844996 DOI: 10.1016/S2213-2600(21)00160-0]
- Lu P, Li J, Liu C, Yang J, Peng H, et al. Salvianolic acid B dry powder inhaler for the treatment of idiopathic pulmonary fibrosis. Asian J Pharm Sci 2022;17(3):447-61. [PMID: 35782322 DOI: 10.1016/j.ajps.2022.04.004]
- Racanelli AC, Kikkers SA, Choi AM, Cloonan SM. Autophagy and inflammation in chronic respiratory disease. Autophagy 2018;14(2):221-32. [PMID: 29130366 DOI: 10.1080/15548627.2017.1389823]
- Pilicheva B, Katsarov P, Kassarova M. Flowability evaluation of dry powder inhalation formulations intended for nasal delivery of betahistine dihydrochloride. Sikk Manipal Univ Med J 2015;2(1):77-90.
- Leung JM, Sin DD. Asthma-COPD overlap syndrome: pathogenesis, clinical features, and therapeutic targets. Br Med J 2017;358:j3772. [PMID: 28947632 DOI: 10.1136/bmj.j3772]
- Yadav N, Lohani A. Dry powder inhalers: a review. Indo Global J Pharm Sci 2013;3(2):142-55.
- Telko MJ, Hickey AJ. Dry powder inhaler formulation. Respir Care 2005;50(9):1209-27. [PMID: 16122404]
- Lavorini F. The challenge of delivering therapeutic aerosols to asthma patients. ISRN Allergy 2013;2013:102418. [PMID: 23984095 DOI: 10.1155/2013/102418]
- Zheng Z, Leung SSY, Gupta R. Flow and particle modelling of dry powder inhalers: methodologies, recent development and emerging applications. Pharmaceutics 2021;13(2):189. [PMID: 33535512 DOI: 10.3390/pharmaceutics13020189]
- Sahane S, Nikhar A, Bhaskaran S, Mundhada D. Dry powder inhaler: an advance technique for pulmonary drug delivery system. Int J Pharm Chem Sci 2012;1:1376-83.
- Dal Negro RW. Dry powder inhalers and the right things to remember: a concept review. Multidiscip Respir Med 2015;10(1):13. [PMID: 25878791 DOI: 10.1186/s40248-015-0012-5]
- Shah DS, Moravkar KK, Jha DK, Lonkar V, Amin PD, et al. A concise summary of powder processing methodologies for flow enhancement. Heliyon 2023;9(6):e16498. [PMID: 37292344 DOI: 10.1016/j.heliyon.2023.e16498]
- Tanaka R, Takahashi N, Nakamura Y, Hattori Y, Ashizawa K, et al. Performance of an acoustically mixed pharmaceutical dry powder delivered from a novel inhaler. Int J Pharm 2018;538(1-2):130-8. [PMID: 29341919 DOI: 10.1016/j.ijpharm.2018.01.001]
- Kadu P, Kendre P, Gursal K. Dry powder inhaler: a review. J Adv Drug Deliv 2016;3(3):42-52.
- Alagusundaram M, Deepthi N, Ramkanth S, Angalaparameswari S, Saleem TM, et al. Dry powder inhalers: an overview. Int J Res Pharm Sci 2010;1(1):34-42.
- Zhang J, Wu L, Chan H-K, Watanabe W. Formation, characterization, and fate of inhaled drug nanoparticles. Adv Drug Deliv Rev 2011;63(6):441-55. [PMID: 21118707 DOI: 10.1016/j.addr.2010.11.002]
- Salama RO, Traini D, Chan H-K, Sung A, Ammit AJ, et al. Preparation and evaluation of controlled release microparticles for respiratory protein therapy. J Pharm Sci 2009;98(8):2709-17. [PMID: 19130607 DOI: 10.1002/jps.21653]
- Setter SM, Levien TL, Iltz JL, Odegard PS, Neumiller JJ, et al. Inhaled dry powder insulin for the treatment of diabetes mellitus. Clin Ther 2007;29(5):795-813. [PMID: 17697900 DOI: 10.1016/j.clinthera.2007.05.015]
- Bhalekar T, Sable K, Mehetre J, Dhamak K. Dry powder inhalers: a critical evaluation of their effectiveness and accessibility. Int J Pharm Sci 2023;1(7):288-301. [DOI: 10.5281/zenodo.8173889]
- Malcolmson RJ, Embleton JK. Dry powder formulations for pulmonary delivery. Pharm Sci Technol Today 1998;1(9):394-8. [DOI: 10.1016/S1461-5347(98)00099-6]
- Crompton GK. Dry powder inhalers: advantages and limitations. J Aerosol Med 1991;4(3):151-6. [PMID: 10147676 DOI: 10.1089/jam.1991.4.151]
- D’Urzo AD, Cazzola M, Hanania NA, Buhl R, Maleki-Yazdi MR. New developments in optimizing bronchodilator treatment of COPD: a focus on glycopyrrolate/formoterol combination formulated by co-suspension delivery technology. Int J Chron Obstruct Pulmon Dis 2018;13:2805-19. [PMID: 30233171 DOI: 10.2147/COPD.S113306]
- Daniher DI, Zhu J. Dry powder platform for pulmonary drug delivery. Particuology 2008;6(4):225-38. [DOI: 10.1016/j.partic.2008.04.004]
- Peng T, Lin S, Niu B, Wang X, Huang Y, et al. Influence of physical properties of carrier on the performance of dry powder inhalers. Acta Pharm Sin B 2016;6(4):308-18. [PMID: 27471671 DOI: 10.1016/j.apsb.2016.03.011]
- Hamishehkar H, Rahimpour Y, Javadzadeh Y. The role of carrier in dry powder inhaler. In: Sezer AD, editor. Recent advances in novel drug carrier systems; 2012.
- Hebbink GA, Jaspers M, Peters HJ, Dickhoff BH. Recent developments in lactose blend formulations for carrier-based dry powder inhalation. Adv Drug Deliv Rev 2022;189:114527. [PMID: 36070848 DOI: 10.1016/j.addr.2022.114527]
- Roberts RA, Shen T, Allen IC, Hasan W, DeSimone JM, et al. Analysis of the murine immune response to pulmonary delivery of precisely fabricated nano-and microscale particles. PLoS One 2013;8(4):e62115. [PMID: 23593509 DOI: 10.1371/journal.pone.0062115]
- Dailey L, Jekel N, Fink L, Gessler T, Schmehl T, et al. Investigation of the proinflammatory potential of biodegradable nanoparticle drug delivery systems in the lung. Toxicol Appl Pharmacol 2006;215(1):100-8. [PMID: 16551473 DOI: 10.1016/j.taap.2006.01.016]
- Miranda MS, Rodrigues MT, Domingues RM, Torrado E, Reis RL, et al. Exploring inhalable polymeric dry powders for anti-tuberculosis drug delivery. Mater SciEng C Mater Biol Appl 2018;93:1090-103. [PMID: 30274040 DOI: 10.1016/j.msec.2018.09.004]
- Tomashefski Jr JF, Cohen AM, Doershuk CF. Longterm histopathologic follow-up of bronchial arteries after therapeutic embolization with polyvinyl alcohol (Ivalon) in patients with cystic fibrosis. Hum Pathol 1988;19(5):555-61. [PMID: 3371980 DOI: 10.1016/s0046-8177(88)80204-1]
- Hitzman CJ, Elmquist WF, Wattenberg LW, Wiedmann TS. Development of a respirable, sustained release microcarrier for 5-fluorouracil I: in vitro assessment of liposomes, microspheres, and lipid coated nanoparticles. J Pharm Sci 2006;95(5):1114-26. [DOI: 10.1002/jps.20591]
- Fiegel J, Fu J, Hanes J. Poly(ether-anhydride) dry powder aerosols for sustained drug delivery in the lungs. J Control Release 2004;96(3):411-23. [PMID: 15120898 DOI: 10.1016/j.jconrel.2004.02.018]
- Sivadas N, Cryan S-A. Inhalable, bioresponsive microparticles for targeted drug delivery in the lungs. J Pharm Pharmacol 2011;63(3):369-75. [PMID: 21749384 DOI: 10.1111/j.2042-7158.2010.01234.x]
- Andrade F, Fonte P, Costa A, Reis CC, Nunes R, et al. Pharmacological and toxicological assessment of innovative self-assembled polymeric micelles as powders for insulin pulmonary delivery. Nanomedicine 2016;11(17):2305-17. [PMID: 27487859 DOI: 10.2217/nnm-2016-0045]
- Kuchekar AB, Pawar AP. Screening of factors using Plackett Burman design in the preparation of capecitabine-loaded nano polymeric micelles. Int J Pharm Pharm Sci 2014;6(5):489-96.
- Rezazadeh M, Davatsaz Z, Emami J, Hasanzadeh F, Jahanian-Najafabadi A. Preparation and characterization of spray-dried inhalable powders containing polymeric micelles for pulmonary delivery of paclitaxel in lung cancer. J Pharm Pharm Sci 2018;21(1s):200s-14s. [PMID: 30321135 DOI: 10.18433/jpps30048]
- Farhangi M, Mahboubi A, Kobarfard F, Vatanara A, Mortazavi SA. Optimization of a dry powder inhaler of ciprofloxacin-loaded polymeric nanomicelles by spray drying process. Pharm Dev Technol 2019;24(5):584-92. [PMID: 30431373 DOI: 10.1080/10837450.2018.1545237]
- Elhissi A, Taylor K. Delivery of liposomes generated from proliposomes using air-jet, ultrasonic, and vibrating-mesh nebulisers. J Drug Deli Sci Technol 2005;15(4):261-5. [DOI: 10.1016/S1773-2247(05)50047-9]
- Song K-H, Chung S-J, Shim C-K. Preparation and evaluation of proliposomes containing salmon calcitonin. J Control Release 2002;84(1-2):27-37. [PMID: 12399165 DOI: 10.1016/s0168-3659(02)00238-9]
- Rojanarat W, Changsan N, Tawithong E, Pinsuwan S, Chan H-K, et al. Isoniazid proliposome powders for inhalation – preparation, characterization and cell culture studies. Int J Mol Sci 2011;12(7):4414-34. [PMID: 21845086 DOI: 10.3390/ijms12074414]
- Patil-Gadhe A, Pokharkar V. Single step spray drying method to develop proliposomes for inhalation: a systematic study based on quality by design approach. Pulm Pharmacol Ther 2014;27(2):197-207. [PMID: 23916767 DOI: 10.1016/j.pupt.2013.07.006]
- Li M, Zhang T, Zhu L, Wang R, Jin Y. Liposomal andrographolide dry powder inhalers for treatment of bacterial pneumonia via anti-inflammatory pathway. Int J Pharm 2017;528(1-2):163-71. [PMID: 28583330 DOI: 10.1016/j.ijpharm.2017.06.005]
- Chennakesavulu S, Mishra A, Sudheer A, Sowmya C, Reddy CS, et al. Pulmonary delivery of liposomal dry powder inhaler formulation for effective treatment of idiopathic pulmonary fibrosis. Asian J Pharm Sci 2018;13(1):91-100. [PMID: 32104382 DOI: 10.1016/j.ajps.2017.08.005]
- Müller RH, Mäder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the art. Eur J Pharm Biopharm 2000;50(1):161-77. [PMID: 9704911 DOI: 10.1016/s0939-6411(97)00150-1]
- Rosière R, Amighi K, Wauthoz N, Dalby R, Byron PR, et al. New dry powders for inhalation containing chitosan derivative-coated solid lipid nanoparticles for targeted delivery to lung cancer cells. RDD Europe 2015;2015:447-52.
- Bakhtiary Z, Barar J, Aghanejad A, Saei AA, Nemati E, et al. Microparticles containing erlotinib-loaded solid lipid nanoparticles for treatment of non-small cell lung cancer. Drug Dev Ind Pharm 2017;43(8):1244-53. [PMID: 28323493 DOI: 10.1080/03639045.2017.1310223]
- Mezzena M, Scalia S, Young PM, Traini D. Solid lipid budesonide microparticles for controlled release inhalation therapy. AAPS J 2009;11(4):771-8. [PMID: 19908147 DOI: 10.1208/s12248-009-9148-6]
- Scalia S, Haghi M, Losi V, Trotta V, Young PM, Traini D. Quercetin solid lipid microparticles: a flavonoid for inhalation lung delivery. Eur J Pharm Sci 2013;49(2):278-85. [PMID: 23541500 DOI: 10.1016/j.ejps.2013.03.009]
- Maretti E, Rossi T, Bondi M, Croce MA, Hanuskova M, et al. Inhaled solid lipid microparticles to target alveolar macrophages for tuberculosis. Int J Pharm 2014;462(1-2):74-82. [PMID: 24374224 DOI: 10.1016/j.ijpharm.2013.12.034]
- Moreno-Sastre M, Pastor M, Esquisabel A, Sans E, Viñas M, et al. Pulmonary delivery of tobramycin-loaded nanostructured lipid carriers for Pseudomonas aeruginosa infections associated with cystic fibrosis. Int J Pharm 2016;498(1-2):263-73. [PMID: 26705155 DOI: 10.1016/j.ijpharm.2015.12.028]
- Patil-Gadhe A, Pokharkar V. Pulmonary targeting potential of rosuvastatin loaded nanostructured lipid carrier: optimization by factorial design. Int J Pharm 2016;501(1-2):199-210. [PMID: 26844785 DOI: 10.1016/j.ijpharm.2016.01.080]
- Wang Y, Kho K, Cheow WS, Hadinoto K. A comparison between spray drying and spray freeze drying for dry powder inhaler formulation of drug-loaded lipid–polymer hybrid nanoparticles. Int J Pharm 2012;424(1-2):98-106. [PMID: 22226876 DOI: 10.1016/j.ijpharm.2011.12.045]
- Yang Y, Cheow WS, Hadinoto K. Dry powder inhaler formulation of lipid-polymer hybrid nanoparticles via electrostatically-driven nanoparticle assembly onto microscale carrier particles. Int J Pharm 2012;434(1-2):49-58. [PMID: 22634138 DOI: 10.1016/j.ijpharm.2012.05.036]
- Bhardwaj A, Mehta S, Yadav S, Singh SK, Grobler A, et al. Pulmonary delivery of antitubercular drugs using spray-dried lipid-polymer hybrid nanoparticles. Artif Cells Nanomed Biotechnol 2016;44(6):1544-55. [PMID: 26178768 DOI: 10.3109/21691401.2015.1062389]
- Pilcer G, Amighi K. Formulation strategy and use of excipients in pulmonary drug delivery. Int J Pharm 2010;392(1-2):1-19. [PMID: 20223286 DOI: 10.1016/j.ijpharm.2010.03.017]
- Yazdi AK, Smyth HD. Carrier-free high-dose dry powder inhaler formulation of ibuprofen: physicochemical characterization and in vitro aerodynamic performance. Int J Pharm 2016;511(1):403-14. [PMID: 27349791 DOI: 10.1016/j.ijpharm.2016.06.061]
- Akdag Cayli Y, Sahin S, Buttini F, Balducci AG, Montanari S, et al. Dry powders for the inhalation of ciprofloxacin or levofloxacin combined with a mucolytic agent for cystic fibrosis patients. Drug Dev Ind Pharm 2017;43(8):1378-89. [PMID: 28420285 DOI: 10.1080/03639045.2017.1318902]
- Yang M-S, Kang J-H, Kim D-W, Park C-W. Recent developments in dry powder inhalation (DPI) formulations for lung-targeted drug delivery. J Pharm Investig. 2024;54(2):113-130. [DOI: 10.1007/s40005-023-00635-w]
- Sham JO-H, Zhang Y, Finlay WH, Roa WH, Löbenberg RJ. Formulation and characterization of spray-dried powders containing nanoparticles for aerosol delivery to the lung. Int J Pharm 2004;269(2):457-67. [DOI: 10.1016/j.ijpharm.2003.09.041]
- Yamasaki K, Kwok PC, Fukushige K, Prud’homme RK, Chan HK. Enhanced dissolution of inhalable cyclosporine nano-matrix particles with mannitol as matrix former. Int J Pharm 2011;420(1):34-42. [PMID: 21864662 DOI: 10.1016/j.ijpharm.2011.08.010]
- Leong EWX, Ge R. Lipid nanoparticles as delivery vehicles for Inhaled therapeutics. Biomid 2022;10:2179. [DOI: 10.3390/biomedicines10092179]
- D’Addio SM, Chan JG, Kwok PC, Benson BR, Prud’homme RK, Chan HK. Aerosol delivery of nanoparticles in uniform mannitol carriers formulated by ultrasonic spray freeze drying. Pharm Res 2013;30:2891-901. [PMID: 23893019 DOI: 10.1007/s11095-013-1120-6]
- Cheow WS, Ng ML, Kho K, Hadinoto K. Spray-freeze-drying production of thermally sensitive polymeric nanoparticle aggregates for inhaled drug delivery: effect of freeze-drying adjuvants. Int J Pharm 2011;404(1-2):289-300. [PMID: 21093560 DOI: 10.1016/j.ijpharm.2010.11.021]
- Valero J, Egea MA, Espina M, Gamisans F, Garcia MJ. Effect of polymerization coadjuvants on nanocapsule elaboration and triamcinolone entrapment. Drug Dev Ind Pharm 1996;22(2):167-73. [DOI: 10.3109/03639049609041987]
- Feng SS, Mu L, Chen BH, Pack DW. Polymeric nanospheres fabricated with natural emulsifiers for clinical administration of an anticancer drug paclitaxel (Taxol®). Mater Sci Eng C 2002;20(1-2):85-92. [DOI: 10.1016/s0928-4931(02)00017-6]
- Cheow WS, Li S, Hadinoto K. Spray drying formulation of hollow spherical aggregates of silica nanoparticles by experimental design. Chem Eng Res Des 2010;88(5-6):673-85. [DOI: 10.1016/j.cherd.2009.11.012]
- Chan HK, Chew NY. Novel alternative methods for the delivery of drugs for the treatment of asthma. Adv Drug Deliv Rev 2003;55(7):793-805. [PMID: 12842601 DOI: 10.1016/s0169-409x(03)00078-4]
- Hu L, Kong D, Hu Q, Gao N, Pang S. Evaluation of high-performance curcumin nanocrystals for pulmonary drug delivery both in vitro and in vivo. Nanoscale Res Lett 2015;10(1):381. [PMID: 26428016 DOI: 10.1186/s11671-015-1085-y]
- Al-Hallak MK, Sarfraz MK, Azarmi S, Roa WH, Finlay WH, et al. Pulmonary delivery of inhalable nanoparticles: dry powder inhalers. Ther Deliv 2011;2(10):1313-24. [PMID: 22826885 DOI: 10.4155/tde.11.100]
- Abdelaziz HM, Gaber M, Abd-Elwakil MM, Mabrouk MT, Elgohary MM, et al. Inhalable particulate drug delivery systems for lung cancer therapy: nanoparticles, microparticles, nanocomposites and nanoaggregates. J Control Release 2018;269:374-92. [PMID: 29180168 DOI: 10.1016/j.jconrel.2017.11.036]
- Kaur R, Garg T, Das Gupta U, Gupta P, Rath G, et al. Preparation and characterization of spray-dried inhalable powders containing nanoaggregates for pulmonary delivery of anti-tubercular drugs. Artif Cells Nanomed Biotechnol 2016;44(1):182-7. [PMID: 24992699 DOI: 10.3109/21691401.2014.930747]
- Duchêne D, Wouessidjewe D, Ponchel G. Cyclodextrins and carrier systems. J Control Release 1999;62(1-2):263-8. [PMID: 10518659 DOI: 10.1016/s0168-3659(99)00046-2]
- Mohtar N, Taylor KM, Sheikh K, Somavarapu S. Design and development of dry powder sulfobutylether-β-cyclodextrin complex for pulmonary delivery of fisetin. Eur J Pharm Biopharm 2017;113:1-10. [PMID: 27916704 DOI: 10.1016/j.ejpb.2016.11.036]
- Kinnarinen T, Jarho P, Järvinen K, Järvinen T. Pulmonary deposition of a budesonide/γ-cyclodextrin complex in vitro. J Control Release 2003;90(2):197-205. [PMID: 12810302 DOI: 10.1016/s0168-3659(03)00176-7]
- Zeng XM, Martin GP, Marriott C, Pritchard J. The influence of carrier morphology on drug delivery by dry powder inhalers. Int J Pharm 2000;200(1):93-106. [PMID: 10845690 DOI: 10.1016/s0378-5173(00)00347-1]
- Norwood DL, Prime D, Downey BP, Creasey J, Sethi SK, et al. Analysis of polycyclic aromatic hydrocarbons in metered dose inhaler drug formulations by isotope dilution gas chromatography/mass spectrometry. J Pharm Biomed Anal 1995;13(3):293-304. [PMID: 7619890 DOI: 10.1016/0731-7085(95)01273-n]
- de Boer AH, Hagedoorn P, Grasmeijer F. Dry powder inhalation, part 2: the present and future. Expert Opin Drug Deliv 2022;19(9):1045-59. [PMID: 35984322 DOI: 10.1080/17425247.2022.2112570]
- Dalby R, Suman J. Inhalation therapy: technological milestones in asthma treatment. Adv Drug Deliv Rev 2003;55(7):779-91. [PMID: 12842600 DOI: 10.1016/s0169-409x(03)00077-2]
- Shahin HI, Chablani L. A comprehensive overview of dry powder inhalers for pulmonary drug delivery: challenges, advances, optimization techniques, and applications. J Drug Deli Sci Technol 2023;84:104553. [DOI: 10.1016/j.jddst.2023.104553]
- Wang J, Kong X, Hu L, Hu Y. Dry powder inhalers: a patent review. J Drug Deli Sci Technol 2022;74:103540. [DOI: 10.1016/j.jddst.2022.103540]
- Xiroudaki S, Schoubben A, Giovagnoli S, Rekkas DM. Dry powder inhalers in the digitalization era: current status and future perspectives. Pharmaceutics 2021;13(9):1455. [PMID: 34575530 DOI: 10.3390/pharmaceutics13091455]
- Ye Y, Ma Y, Zhu J. The future of dry powder inhaled therapy: promising or discouraging for systemic disorders? Int J Pharm 2022;614:121457. [PMID: 35026316 DOI: 10.1016/j.ijpharm.2022.121457]
- Pareek A, Kothari R, Pareek A, Ratan Y, Kashania P, et al. Development of a new inhaled swellable microsphere system for the dual delivery of naringenin-loaded solid lipid nanoparticles and doxofylline for the treatment of asthma. Eur J Pharm Sci 2024;193:106642. [PMID: 37977235 DOI: 10.1016/j.ejps.2023.106642]
- Patil-Gadhe A, Kyadarkunte A, Patole M, Pokharkar V. Montelukast-loaded nanostructured lipid carriers: part II Pulmonary drug delivery and in vitro – in vivo aerosol performance. Eur J Pharm Biopharm 2014;88(1):169-77. [PMID: 25078860 DOI: 10.1016/j.ejpb.2014.07.007]
- Kassaee SN, Ayoko GA, Richard D, Wang T, Islam N. Inhaled ivermectin-loaded lipid polymer hybrid nanoparticles: development and characterization. Pharmaceutics 2024;16(8):1061. [PMID: 39204406 DOI: 10.3390/pharmaceutics16081061]
- Emami S, Hemmati Z, Yaqoubi S, Hamishehkar H, Alvani A. Nanocrystal agglomerates of curcumin prepared by electrospray drying as an excipient-free dry powder for inhalation. Adv Pharmacol Pharm Sci 2024;2024(1):6288621. [PMID: 39281030 DOI: 10.1155/2024/6288621]
- Freeman MT, Shen J, Meenach SA. An aerosol nanocomposite microparticle formulation using rifampicin-cyclodextrin inclusion complexes for the treatment of pulmonary diseases. Int J Pharm 2024;665:124755. [DOI: 10.1016/j.ijpharm.2024.124755]
- Oh DW, Choi JH, Yu GH, Kim BK, Cho SM, et al. Formulation and evaluation of carrier-based dry powders containing budesonide and arformoterol for inhalation therapy. Pharm Dev Technol 2024:1-10. [PMID: 39422557 DOI: 10.1080/10837450.2024.2413145]
- Bashi YH, Ali A, Al Ayoub Y, Assi KH, Mairs R, et al. Inhaled dry powder liposomal azithromycin for treatment of chronic lower respiratory tract infection. Int J Pharm 2024;653:123841. [PMID: 38266939 DOI: 10.1016/j.ijpharm.2024.123841]