Unlocking the Potential of Receptor-Based Approaches in Diabetes Treatment
1Luqman College of Pharmacy, Gulbarga, Karnataka, India
2Honorary Faculty Member at Novel Global Community Educational Foundation, Australia
3Research Scholar, Department of Pharmacy, Raffles University, Neemrana, Alwar, Rajasthan 301705, India
4Department of Pharmacology, Mohan Babu University, MB School of Pharmaceutical Sciences (Erstwhile Sree Vidyanikethan College of Pharmacy), Tirupati, India
5Department of Pharmacy, TRR pharmacy College, Pragathi Colony, Meerpet, Pragathi Colony, Hyderabad, Telangana 500097, India
6Department of Pharmaceutics, Santhiram College of Pharmacy, Andhra Pradesh, India
7Department of Pharmacy Practice, St. Peter’s Institute of Pharmaceutical Sciences, Hanamkonda, Telangana 506331, India
8Department of Pharmacy, University College of Technology, Osmania University, Amberpet, Hyderabad, Telangana 500007, India
9Department of Chemistry, Raffles University, Neemrana, Alwar, Rajasthan 301705, India
aThese authors contributed equally to this work.
*Correspondence to: Luqman College of Pharmacy, Gulbarga, Karnataka, India. E-mail: mohsina.patwekar@gmail.com; Department of Chemistry, Raffles University, Neemrana, Alwar, Rajasthan 301705, India. E-mail: selvarrraj@gmail.com
Received: July 31 2024; Revised: October 4 2024; Accepted: November 22 2024; Published Online: January 28 2025
Cite this paper:
Patwekar M, Patwekar F, Kumar JP et al. Unlocking the Potential of Receptor-Based Approaches in Diabetes Treatment. BIO Integration 2025; 6: 1–20.
DOI: 10.15212/bioi-2024-0047. Available at: https://bio-integration.org/
Download citation
© 2025 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
Diabetes, a common metabolic condition, poses a substantial health burden worldwide. To revolutionize diabetes management, enhance glycemic control, and decrease the risk of complications, recent research has revealed innovative targets and therapeutic options. A thorough examination of modern drugs that target particular receptors and metabolic pathways for glucose and fat metabolism is presented. Recent research has revealed innovative targets and therapeutic options. Liraglutide, a GLP-1 receptor agonist, has been shown to effectively lower glucagon levels and promote weight loss. Empagliflozin is a sodium-glucose co-transporter 2 (SGLT2) inhibitor with substantial promise in decreasing blood glucose levels, and providing cardiovascular and renal advantages. Pegbelfermin (BMS-986036), a fibroblast growth factor 21 (FGF21) analogue, is being investigated for its ability to regulate glucose and lipid metabolism, and potentially enhance glycemic control and lipid profiles. Additionally, G-protein-coupled receptor (GPCR) agonists and adenosine monophosphate-activated protein kinase (AMPK) activators are emerging as potential medicines to improve insulin sensitivity, glucose uptake, and insulin signaling pathways. Despite being in early research stages, bile acid receptor agonists and mitochondrial uncouplers have promising potential for modifying lipid and glucose metabolism. The long-acting insulin analogue insulin glargine, which replaces basal insulin, continues to be a cornerstone of advanced diabetes management. In the future, these medications are expected to be improved through the use of combination therapy and personalized, precision medicine. Gene therapies show promise as novel strategies to address genetic defects and provide potential treatments. Additionally, patient monitoring, adherence, and self-management will be greatly aided by the integration of digital health technology, telemedicine, and artificial intelligence (AI), thus leading to better treatment outcomes and patient quality of life. Healthcare professionals, researchers, politicians, and patients working together will pave the way to substantial improvements in the management of metabolic disorders including diabetes. In conclusion, hope for more efficient, individualized, and secure therapies may come from continuing research and breakthroughs in novel diabetes treatment targets. These developments are at the forefront of offering people with diabetes and related metabolic disorders a better and healthier future, by revolutionizing diabetes management.
Keywords
Diabetes, GLP-1 agonists, metabolic disorders, receptors, SGLT2 inhibitors, therapeutic targets.
Introduction
Diabetes, often known as diabetes mellitus, is a long-term metabolic disease characterized by consistently high blood glucose levels. Impaired insulin action, inadequate insulin synthesis, or a combination thereof are the causes of this syndrome. The pancreas secretes the hormone insulin, which is essential for controlling the body’s metabolism of glucose. Insulin helps cells absorb glucose for immediate use or store it for later use. In hyperglycemia, glucose accumulates in the bloodstream because of impaired insulin synthesis or activity. Two main forms of diabetes exist: type 1 diabetes, in which the pancreas produces little to no insulin, and type 2 diabetes, in which the body either develops resistance to insulin or is unable to make sufficient amounts of insulin. Prolonged hyperglycemia, if left untreated, can cause major problems affecting the heart, kidneys, nerves, eyes, and other organs and systems. Therefore, controlling blood glucose levels well is essential to avoiding these negative effects [1–3].
Receptors and insulin action
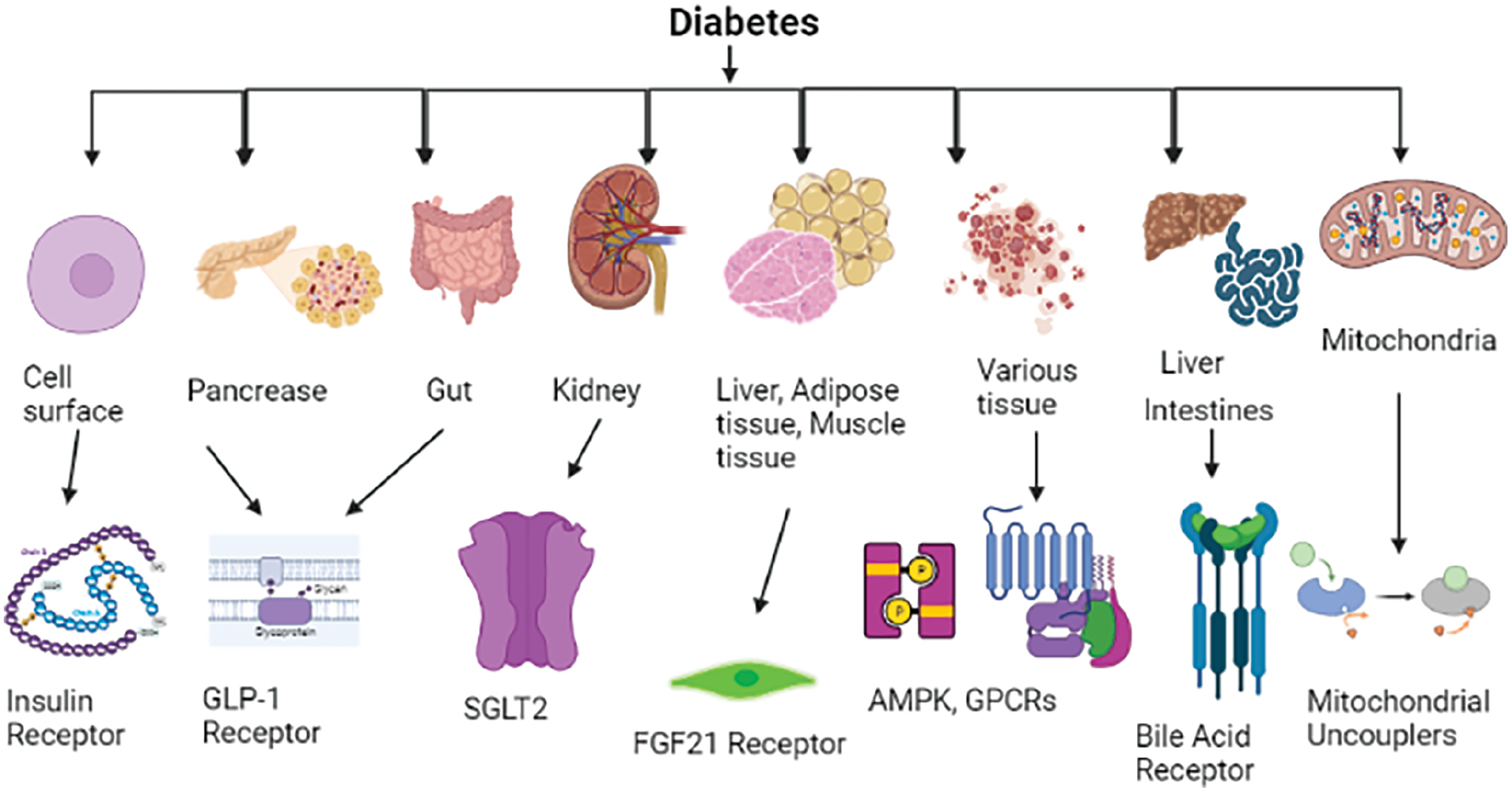
Target cells are affected by insulin’s binding to certain receptors on their surface. These insulin receptors are found in the muscle, liver, adipose tissue, and central nervous system, among other organs. When a ligand binds an insulin receptor, a cascade of intracellular signaling events is triggered, and causes a variety of cellular reactions controlling glucose metabolism. The receptor tyrosine kinase (RTK) family includes the transmembrane protein known as the insulin receptor. RTKs comprise two transmembrane beta subunits and two extracellular alpha subunits connected by disulfide bonds (Table 1) [4]. The tyrosine kinase activity of the beta subunits is activated when insulin binds the receptor’s alpha subunits and causes a conformational shift. Insulin receptor substrate proteins and other intracellular substrates, such as other insulin receptors, are phosphorylated at certain tyrosine residues when insulin receptors are activated. These phosphorylated insulin receptor substrate proteins serve as docking sites for downstream signaling molecules, such as phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinases (MAPKs). Phosphatidylinositol (3,4,5)-trisphosphate (PIP3) is produced as a result of PI3K activation, and PKB/Akt is subsequently activated. Via translocation of the glucose transporter type 4 (GLUT4) to the cell membrane, Akt promotes glucose absorption in muscle and adipose tissues, and consequently modulates the metabolic effects of insulin [5–8]. This process increases the uptake of glucose into the cells from the bloodstream and consequently lowers blood glucose levels. Additionally, Akt activation decreases the liver’s ability to produce glucose, thereby aiding in regulation of blood glucose levels. Furthermore, the effects of insulin on lipid metabolism, protein synthesis, and cell proliferation are also influenced by insulin signaling pathways, thus demonstrating the pleiotropic effects of the hormone on numerous physiological processes. In conclusion, insulin’s interaction with its receptors is a crucial biochemical process that controls glucose metabolism and maintains proper blood glucose levels. In the onset and development of diabetes, dysregulation of insulin action—whether elicited by receptor deficiencies, decreased receptor sensitivity, or aberrant downstream signaling—plays a crucial part. Understanding the subtleties of insulin receptor signaling and how it affects glucose homeostasis provides important information for prospective therapeutic approaches to treat diabetes and its complications. More effective new treatments for diabetes are being developed as a result of ongoing study of these pathways (Figure 1) [9, 10]. Receptor-based therapies aim to modulate important physiological pathways involved in metabolic regulation by targeting certain cell receptors. Through activating receptors associated with insulin sensitivity, lipid metabolism, energy expenditure, and glucose uptake, these treatments can alter essential metabolic balance processes. When treating complicated metabolic illnesses such as diabetes, in which dysregulation of these pathways leads to hyperglycemia, insulin resistance, and aberrant lipid profiles, this focused approach is particularly pertinent. These treatments seek to restore metabolic balance by activating or inhibiting receptors, and consequently might provide more accurate and efficient diabetic treatment approaches. The classification of targets of conventional and novel treatments is shown in Table 2.
Table 1 Details of Receptors, Each Target’s Role in Modulating Physiological Pathways Associated with Glucose Uptake, Insulin Sensitivity, Lipid Metabolism, and Energy Expenditure, Highlighting Relevance in Managing Metabolic Diseases Such as Diabetes
| Target Receptor | Location | Primary Function | Relevance in Diabetes Management |
|---|---|---|---|
| Insulin Receptor | Cell surface | Facilitates glucose uptake by cells, and mediates insulin signaling | Enhances glucose uptake, which is crucial for decreasing blood glucose levels |
| GLP-1 Receptor | Pancreas, gut | Increases insulin secretion, suppresses glucagon release, enhances satiety, and slows gastric emptying | Improves insulin secretion and controls appetite, thus aiding in glycemic control and weight management |
| SGLT2 | Kidneys | Inhibits renal glucose reabsorption, thus increasing glucose excretion in urine | Decreases blood glucose levels by promoting glucose excretion; helps manage hyperglycemia |
| FGF21 Receptor | Liver, adipose tissue, muscles | Regulates lipid and glucose metabolism, thus improving insulin sensitivity | Enhances insulin sensitivity, promotes lipid metabolism, and aids in overall metabolic health |
| AMPK | Various tissues | Manages cellular energy balance and stimulates glucose uptake | Increases glucose uptake and energy expenditure, thereby supporting insulin sensitivity and glucose homeostasis |
| GPCRs | Various tissues | Modulates pathways associated with insulin signaling and glucose homeostasis | Enhances insulin action and improves glucose utilization |
| Bile Acid Receptor (FXR, TGR5) | Liver, intestines | Regulates lipid and glucose metabolism through bile acid signaling | Enhances lipid and glucose metabolism, thus supporting improved glycemic control and metabolic balance |
| Mitochondrial Uncouplers | Mitochondria | Increases energy expenditure and modulates glucose homeostasis by uncoupling oxidative phosphorylation | Promotes energy expenditure and improves glucose metabolism, thus offering potential benefits in weight and glucose control |
Figure 1 Receptor, position, and function of diabetes.
Table 2 Conventional and Novel Diabetes Treatment Targets, According to Mechanisms of Action, Receptor Involvement, and Therapeutic Efficacy
| Target Type | Target Name | Primary Function | Mechanism of Action | Examples of Drugs | Therapeutic Efficacy |
|---|---|---|---|---|---|
| Conventional Targets | Insulin receptor | Glucose transport | Facilitates glucose uptake by promoting GLUT4 translocation | Insulin glargine (long-acting insulin) | Highly effective in lowering blood glucose; essential for type 1 diabetes and some patients with type 2 diabetes |
| PPARs (peroxisome proliferator-activated receptors) | Lipid metabolism and insulin sensitivity | Regulates lipid metabolism, insulin sensitivity, and glucose homeostasis | Pioglitazone, rosiglitazone | Improves insulin sensitivity, beneficial in type 2 diabetes; risk of weight gain and fluid retention | |
| Sulfonylurea receptor | Insulin secretion | Stimulates pancreatic insulin release by closing K_ATP channels | Glimepiride, glyburide | Effective in increasing insulin release; risk of hypoglycemia and weight gain | |
| GLP-1 receptor (glucagon-like peptide-1) | Glucose homeostasis and appetite regulation | Increases insulin secretion, suppresses glucagon, slows gastric emptying | Liraglutide, semaglutide | Effective in improving glycemic control and promoting weight loss; low risk of hypoglycemia | |
| DPP-4 (dipeptidyl peptidase-4) | Glp-1 activity prolongation | Inhibits GLP-1 breakdown, thus enhancing insulin secretion | Sitagliptin, linagliptin | Moderately effective in lowering blood glucose; minimal hypoglycemia risk | |
| Novel Targets | SGLT2 (sodium-glucose co-transporter 2) | Glucose transport | Decreases glucose reabsorption in the kidney, thus increasing urinary glucose excretion | Empagliflozin, dapagliflozin | Effective in lowering blood glucose; also has cardiovascular and renal benefits |
| FGF21 receptor (fibroblast growth factor 21) | Glucose and lipid metabolism | Enhances insulin sensitivity and promotes lipid metabolism | Pegbelfermin (BMS-986036) | Promise in improving glycemic control, lipid profile, and weight loss; still in development | |
| AMPK (Adenosine monophosphate-activated protein kinase) | Energy balance and insulin sensitivity | Improves insulin sensitivity by regulating cellular energy balance | Metformin (indirect activator), AICAR | Highly effective as a first-line agent in type 2 diabetes; improves insulin sensitivity | |
| GPCRs (G-protein coupled receptors) | Glucose homeostasis | Modulates insulin signaling and glucose homeostasis | Agonists under investigation | Potentially improves insulin signaling; efficacy under clinical evaluation | |
| Bile acid receptors (FXR, TGR5) | Lipid and glucose metabolism | Regulates glucose, lipid metabolism, and energy expenditure | Obeticholic acid (FXR agonist) | Potential benefits in glycemic control and lipid metabolism; FXR agonists still under study | |
| Mitochondrial uncouplers | Energy expenditure | Increases energy expenditure by uncoupling oxidative phosphorylation | DNP (toxic); safer alternatives in development | Promising for weight management and metabolic improvement; safety issues with DNP, safer options needed | |
| GLUT4 enhancers | Glucose transport | Enhances glucose uptake in muscle and fat cells | Under investigation | Potentially enhances insulin sensitivity; preclinical and early-stage clinical trials | |
| DGAT1 (diacylglycerol acyltransferase-1) inhibitors | Lipid metabolism | Decreases triglyceride synthesis, thereby improving insulin sensitivity | None approved to date | Promise in improving insulin sensitivity; no approved drugs to date | |
| GLP-1 and GIP dual agonists | Enhanced insulin secretion | Targets both GLP-1 and GIP receptors, thus achieving synergistic effects on insulin secretion | Tirzepatide | Highly effective in improving glycemic control and promoting weight loss; emerging dual-target approach | |
| FGFR1c (fibroblast growth factor receptor 1c) inhibitors | Insulin sensitivity | Improves insulin sensitivity through FGF21 signaling | None approved to date | Promise in enhanced insulin sensitivity; currently in development |
Novel targets for diabetes treatment
Several targets have been identified to address various diabetes pathophysiology-related issues, thus providing new opportunities for creating more efficient and specialized treatments. These innovative targets include the following.
Glucagon-like peptide-1 (GLP-1) receptor agonists
Type 2 diabetes mellitus is managed with a class of drugs known as GLP-1 receptor agonists, which imitate the effects of GLP-1, a hormone that is naturally produced in the intestines, and is essential for maintaining glucose homeostasis and metabolism. The enteroendocrine L-cells in the gut release GLP-1 when glucose is consumed. GLP-1 then exerts its effects on particular receptors known as GLP-1 receptors, which are found primarily in the pancreas, brain, and gastrointestinal tract. The beta cells in the pancreas, which secrete insulin, express GLP-1 receptors that bind GLP-1 [11, 12]. A signaling cascade activated by this binding subsequently results in the release of insulin into the bloodstream. The hormone insulin lowers blood glucose levels by facilitating the transfer of glucose from the blood into cells. The pancreatic alpha cells that secrete the hormone glucagon, which raises blood glucose levels, are likewise inhibited by GLP-1. GLP-1 aids in glycemic management by suppressing glucagon secretion and preventing the liver from producing excessive glucose. The brain’s GLP-1 receptors play roles in controlling hunger and fullness. Receptor activation results in greater sensations of satiety or fullness, and consequently can lead to decreased food intake and weight loss. GLP-1 slows the rate at which food travels from the stomach to the small intestine, and therefore affects the digestive system. Because of the delayed nutritional absorption caused by prolonged stomach emptying, post-meal glucose excursions are better controlled [13, 14].
Mechanism of action
GLP-1 receptor agonists consist of synthetic compounds that act as agonists by binding and activating the GLP-1 receptors. The enzyme dipeptidyl peptidase-4 (DPP-4) quickly breaks down natural GLP-1. However, medications in this category are designed to withstand enzymatic degradation, so that their effects persist. Subcutaneously administered GLP-1 receptor agonists (often in the form of injections) enter the circulation and bind GLP-1 receptors in several organs. This binding initiates several intracellular signaling cascades, most notably the cyclic AMP (cAMP) pathway; subsequently, beta cells in the pancreas that express GLP-1 receptors are activated in response to elevated blood glucose levels. Because GLP-1 receptor agonists prevent alpha cells from secreting glucagon, the liver decreases its glucose production. Decreased food intake results from an increase in fullness and a decrease in hunger elicited by the activation of GLP-1 receptors in the brain. Antagonizing the GLP-1 receptor slows the emptying of the stomach and consequently maintains steady blood glucose levels after eating. Because of their multifaceted effects on glucose metabolism and weight control, GLP-1 receptor agonists have emerged as valuable therapeutic options for the treatment of type 2 diabetes. They are particularly helpful for people with obesity and diabetes, because they can be administered alone or in conjunction with other antidiabetic drugs to enhance glycemic control and potentially result in weight loss [14–16].
SGLT2 inhibitors
Inhibitors of sodium-glucose co-transporter 2 (SGLT2) increase glucose excretion in the urine by lowering renal glucose reabsorption. These medications further improve the heart and kidneys while lowering blood glucose levels. Type 2 diabetes mellitus is treated with a class of drugs known as SGLT2 inhibitors, which specifically target SGLT2, a protein found primarily in the proximal tubules of the kidneys. SGLT2 facilitates glucose reabsorption from the glomerular filtrate back into the bloodstream. By blocking SGLT2, these medications lead to glucosuria, a condition involving elevated glucose excretion through the urine [17, 18].
Mechanism of action
In healthy people, glucose freely filters into the renal tubules as blood passes through the glomeruli in the kidneys. SGLT2 is essential for reabsorbing filtered glucose into the bloodstream in the proximal tubules. The co-transport of sodium ions and glucose molecules from the tubular fluid into the cells of the proximal tubules is part of the energy-dependent reabsorption process. Small compounds known as SGLT2 inhibitors prevent SGLT2 from functioning in the proximal tubules. These medications halt the co-transport of sodium and glucose, and consequently prevent the absorption of glucose back into the bloodstream. The mechanism involves binding and inhibition of SGLT2. With SGLT2 inhibition, more glucose remains in the tubular fluid, and the amount of glucose in the urine consequently increases [19–21]. The kidneys are unable to reabsorb all the filtered glucose back into the bloodstream if the glucose concentration reaches the renal threshold for glucose, which is typically 180 mg/dL. Consequently, the excess glucose leaks into the urine and causes glucosuria. The amount of glucose circulating in the bloodstream is directly decreased by the increased excretion of glucose in the urine. Over time, this process decreases blood glucose levels, a beneficial effect for people with diabetes, by facilitating glycemic control. SGLT2 inhibitors also have cardiovascular and renal advantages in addition to decreasing blood glucose levels. In individuals with type 2 diabetes and established cardiovascular disease, clinical trials have demonstrated that SGLT2 inhibitors lower the risk of cardiovascular events such as heart attack, stroke, and heart failure. In patients with diabetes and renal impairment, SGLT2 inhibitors have been found to decrease the progression of chronic kidney disease and the likelihood of unfavorable kidney outcomes. Although the precise processes underlying these cardio-renal benefits are not fully understood, they are believed to be linked to improved hemodynamic variables, decreased deposition of intrarenal fat, and changes in several biomarkers associated with heart and kidney function. In general, SGLT2 inhibitors work by preventing the kidneys from reabsorbing glucose, thereby increasing the amount of glucose excreted in the urine and lowering blood glucose levels. Because of their effectiveness in lowering blood glucose and other cardio-renal advantages, these medications have emerged as essential therapeutic alternatives for individuals with type 2 diabetes with cardiovascular or renal problems [22, 23].
FGF21 analogues
Analogues of fibroblast growth factor 21 (FGF21) are being investigated for their ability to modulate lipid and glucose metabolism, and consequently enhance glycemic control and lipid profiles. The fibroblast growth factor (FGF) family of hormones includes FGF21, which is essential in controlling the body’s metabolism of lipids and glucose. Synthetic versions of this hormone, known as FGF21 analogues, are being studied for their potential therapeutic advantages in treating type 2 diabetes and related metabolic diseases. In the liver, adipose tissue, and pancreas, FGF21 is produced and released primarily in response to several metabolic stimuli, such as fasting, nutrition intake, and cold exposure. Peroxisome proliferator-activated receptor alpha (PPAR) and other transcription factors control FGF21 release [24–26].
Mechanisms of action of FGF21 analogues
The goal of FGF21 analogues is to mimic the natural activity of FGF21, a hormone essential for controlling the metabolism of fats and carbohydrates. These analogues increase insulin sensitivity by binding FGF receptors, including FGFR1 and the co-receptor Klotho. Subsequently, the amount of glucose that tissues can absorb from the bloodstream increases, thereby improving glycemic control and lowering blood glucose levels. These analogues also halt glycogenolysis and gluconeogenesis, and consequently lower the amount of glucose produced by the liver. FGF21 stimulates lipolysis, which breaks down triglycerides, or stored fat, in adipose tissue, then releases free fatty acids that the muscles and liver can use to produce energy through beta-oxidation. FGF21 also aids in the transformation of white fat into beige fat, the latter of which has greater potential for thermogenesis, thus increasing energy expenditure and decreasing fat storage. Additionally, FGF21 has anti-inflammatory and cardiovascular properties including lowering blood pressure and decreasing atherosclerosis. Consequently, FGF21 analogues show promise as a treatment for metabolic diseases and diabetes [26–29].
Adenosine monophosphate-activated protein kinase (AMPK) activators
AMPK activators are being studied as potential treatments to improve glucose absorption and insulin sensitivity. The enzyme AMPK plays a crucial role in controlling the homeostasis of cellular energy. Because it detects changes in the ratio of adenosine monophosphate (AMP) to adenosine triphosphate (ATP), two essential molecules involved in cellular energy transfer, it is frequently referred to as a cellular energy sensor. AMPK is activated under energy deficiency (high AMP:ATP ratio), such as during exercise, nutrient restriction, or cellular stress. AMPK is a heterotrimeric enzyme complex made up of catalytic, regulatory, and regulatory subunits. AMPK’s activation status is controlled by the four AMP, ADP, and ATP binding sites found in the subunit [30–32].
Mechanism of activation
The AMP:ATP ratio rises when cellular energy levels are low, such as during prolonged activity or fasting. AMP subsequently binds the subunit and activates AMPK allosterically. The subunit of AMPK is also phosphorylated and activated by liver kinase B1 (LKB1) and calcium/calmodulin-dependent protein kinase kinase (CaMKK). Once activated, AMPK controls a variety of cellular functions, thereby balancing energy, and facilitating the uptake and utilization of glucose. In glucose metabolism, AMPK activation substantially affects insulin-sensitive tissues such as skeletal muscle, liver, and adipose tissue. By encouraging the translocation of glucose transporter type 4 (GLUT4) to the cell membrane, AMPK activation improves the absorption of glucose into cells. By allowing cells to absorb more glucose from the bloodstream, this enhanced GLUT4 translocation lowers blood glucose levels. In muscle and other tissues, AMPK promotes glycolysis, the breakdown of glucose into energy (ATP). When cellular energy levels are low, this mechanism aids in the production of ATP. The liver’s ability to produce glucose from non-carbohydrate precursors is inhibited by AMPK activation. AMPK, by lowering gluconeogenesis, aids in preventing excessive glucose release into the bloodstream [33]. Additionally, AMPK affects lipid metabolism by suppressing lipogenesis and increasing fatty acid oxidation (beta-oxidation), thus decreasing cholesterol buildup and increasing insulin sensitivity. Beyond glucose metabolism, AMPK activation promotes the development of new mitochondria, the organelles within cells that produce energy. Increased energy production and improved cellular function result. Autophagy, a cellular process that aids in the removal of damaged organelles and proteins, supports the management and preservation of cellular quality. AMPK activates autophagy. AMPK activators are being investigated as potential treatments to improve glucose uptake and insulin sensitivity. These substances control glucose metabolism, increase glucose absorption, and enhance insulin sensitivity in diverse tissues via activation of AMPK. Modulation of AMPK activation is a promising therapeutic approach for treating metabolic diseases including insulin resistance and diabetes [30, 34].
G-protein-coupled receptor (GPCR) agonists
As possible targets for diabetes therapy, particular GPCRs implicated in insulin signaling and glucose homeostasis are being investigated. The broad family of cell surface receptors known as GPCRs is crucial in cell signaling and communication. By altering numerous intracellular signaling pathways, activation of these GPCRs increases glucose uptake, insulin sensitivity, and glycemic management. GPCRs on the surfaces of many cells, including muscle, adipose tissue, and liver cells, have been implicated in insulin signaling. Insulin triggers downstream signaling pathways after binding its receptor, thus causing the physiological reactions necessary for glucose uptake and metabolism [35–37].
Mechanisms of action of GPCR agonists
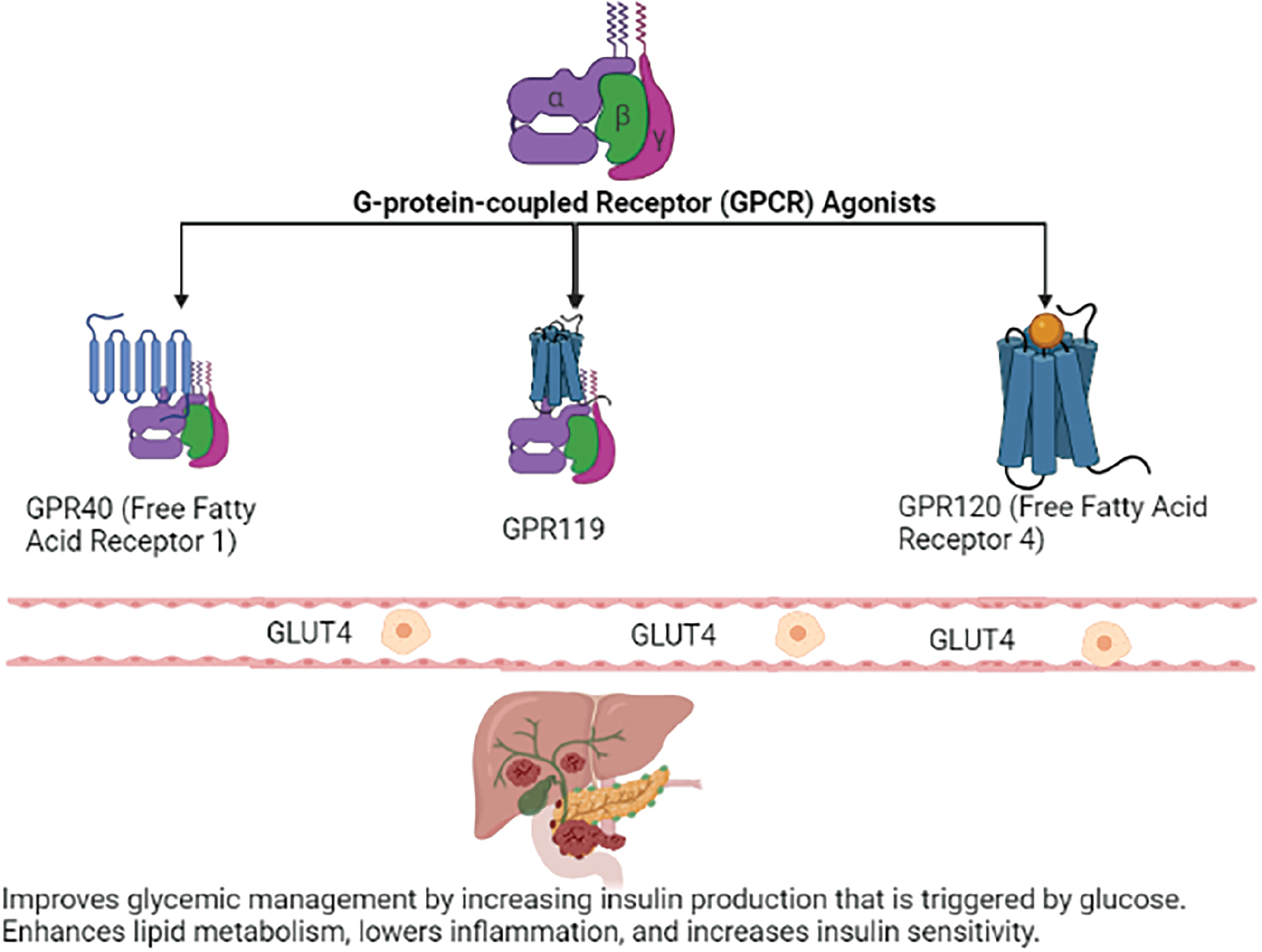
GPCR agonists function similarly to the ligands that bind GPCRs in nature. These agonists activate GPCRs and initiate a chain of intracellular processes that alter cellular function (Figure 2). GPCR agonists have several effects on glucose homeostasis that are being investigated as potential targets for diabetes treatment: The activation of specific GPCRs increases the translocation of glucose transporter type 4 (GLUT4) to the cell membrane. Consequently, muscles and adipose tissue, two tissues that respond to insulin, absorb glucose from the bloodstream more effectively. Some GPCR agonists improve insulin sensitivity by increasing the responsiveness of the target tissues to the effects of insulin. Consequently, glucose absorption and utilization become more effective. By suppressing gluconeogenesis, the process through which the liver manufactures glucose from non-carbohydrate sources, GPCR agonists might also influence the amount of glucose produced by the liver. Several GPCRs are being studied as potential diabetes therapy targets (Table 3). Examples are described below.
Figure 2 Mechanisms of action of GPCR agonists.
Table 3 GPCRs Targeted for Diabetes Treatment
| GPCR | Location of Expression | Mechanism of Action | Effects on Glucose and Lipid Metabolism |
|---|---|---|---|
| GPR40 (Free Fatty Acid Receptor 1) | Pancreas, gastrointestinal tract | Binds free fatty acids and encourages pancreatic beta cells to secrete insulin | Improves glycemic management by increasing insulin production that is triggered by glucose |
| GPR119 | Pancreas, gastrointestinal tract | Increases GLP-1 and GIP release, and activates cAMP signaling, thereby increasing insulin secretion | Increases glucose-dependent insulin release and stimulates insulin secretion |
| GPR120 (Free Fatty Acid Receptor 4) | Adipose tissue, macrophages | Binds free fatty acids and activates signaling pathways downstream | Enhances lipid metabolism, lowers inflammation, and increases insulin sensitivity |
GPR40 (Free Fatty Acid Receptor 1)
In response to increased amounts of free fatty acids, GPR40, which is expressed in pancreatic beta cells, regulates insulin secretion. GPR40 antagonists increase insulin secretion triggered by glucose and promote glycemic control.
GPR119
The pancreas and gastrointestinal tract both express GPR119. Activation of the GPR119 receptor increases glucose-dependent insulin release and induces insulin secretion.
GPR120 (Free Fatty Acid Receptor 4)
Both macrophages and adipose tissue express GPR120. Activation of GPR120 increases insulin sensitivity and has anti-inflammatory effects, which may be advantageous for people with type 2 diabetes and inflammation elicited by obesity [38, 39].
Developing GPCR agonists as therapeutic drugs for diabetes treatment has potential yet also poses challenges. To prevent off-target effects and potential negative reactions, specificity and selectivity for the target GPCR are essential. Additionally, agonists’ pharmacokinetic properties must be optimized for clinical application to be successful. GPCR agonists show substantial promise as treatments for metabolic illnesses such as diabetes. These agonists have the potential to provide unique and focused methods for enhancing insulin sensitivity and glycemic management by altering important elements of insulin signaling and glucose homeostasis. As possible targets for diabetes therapy, particular GPCRs implicated in insulin signaling and glucose homeostasis are being investigated. GPCR agonists activate these receptors and modify several intracellular signaling pathways, thereby improving insulin sensitivity and glycemic management while increasing glucose absorption. Given the ongoing research and development in this field, new therapeutic alternatives hold promise in supplementing current diabetes treatments and addressing the disease’s complicated pathophysiology [37, 40].
Bile acid receptor agonists
The possible effects of bile acid receptor antagonists on lipid and glucose metabolism are being investigated. A family of substances known as bile acid receptor agonists target particular receptors involved in bile acid signaling. The potential effects of these agonists on lipid and glucose metabolism, as well as their implications in diabetes and related metabolic disorders, are being investigated. Takeda G protein-coupled receptor 5 (TGR5) and the farnesoid X receptor (FXR) are the main bile acid receptors of interest. Bile acids are substances made from cholesterol that are produced in the liver and stored in the gallbladder. They are highly important in the breakdown and absorption of dietary lipids. Bile acids are released into the intestines after food is consumed, where they help emulsify and solubilize lipids, thus enabling breakdown and subsequent absorption. Bile acids play multiple roles in the body’s tissues, including the breakdown of fat, but they also function as signaling molecules by interacting with certain receptors, including FXR and TGR5 [41, 42] (Table 4).
Table 4 FXR and TGR5 Locations, Mechanisms of Action, and Metabolic Activities
| Receptor | Location of Expression | Mechanism of Action | Metabolic Activity |
|---|---|---|---|
| FXR (Farnesoid X Receptor) | Liver, intestines, adipose tissue, others | Forms a heterodimer with RXR and subsequently binds target gene promoter FXR response elements (FXREs) | Inhibits gluconeogenesis, thus controlling glucose metabolism; decreases lipogenesis and liver fat synthesis; encourages bile acid production and release |
| TGR5 (Takeda G protein-coupled receptor 5) | Intestines, brown adipose tissue, macrophages | G-proteins are used to activate intracellular signaling cascades | Enhances muscle and adipose tissue glucose absorption by increasing insulin sensitivity; increases caloric expenditure by causing thermogenesis in brown adipose tissue; macrophages display anti-inflammatory properties |
FXR
The liver, intestines, adipose tissue, and other organs involved in metabolism are the primary sites expressing the nuclear receptor FXR. When activated, the FXR forms a heterodimer with retinoid X receptor (RXR) and binds specific DNA sequences called FXR response elements (FXREs) in the promoters of target genes [43].
Mechanism of action of FXR
Gluconeogenesis, the process through which the liver manufactures glucose, is suppressed after FXR activation in the liver. FXR activation lowers blood glucose levels by preventing gluconeogenesis. FXR activation downregulates genes involved in triglyceride storage and lipogenesis (the synthesis of fatty acids), and consequently decreases liver fat buildup. Bile acid equilibrium is aided by FXR activation, which encourages bile acid production and secretion, and increases bile acid flux and flow [44, 45].
TGR5
TGR5 is a G-protein-coupled receptor found primarily in macrophages, brown adipose tissue, and the intestines. Activation of TGR5 differs from that of FXR in that it involves intracellular signaling cascades through G-proteins rather than direct DNA binding [46].
Mechanism of action of TGR5
TGR5 activation increases insulin sensitivity in adipose tissue and muscle, thereby encouraging glucose absorption into these tissues. Thermogenesis, the process of producing heat, is induced by TGR5 activation in brown adipose tissue. This process increases energy expenditure and aids in weight management. The anti-inflammatory effects of TGR5 activation in macrophages might lessen the inflammation and insulin resistance associated with obesity. The effects of bile acid receptor agonists on lipid and glucose metabolism are currently being investigated. These agonists influence several metabolic pathways, including glucose control, lipid metabolism, and energy expenditure, through their interactions with the bile acid receptors FXR and TGR5. Bile acid receptor agonists have potential as innovative therapeutics for diabetes and metabolic illnesses in this developing field of study [46, 47].
Mitochondrial uncouplers
The potential for improving energy expenditure and glucose homeostasis by modulating mitochondrial uncoupling is an exciting approach to treating diabetes. Mitochondrial uncouplers affect how the mitochondria, the energy-producing organelles in cells, function. These substances influence mitochondrial uncoupling, the breakage of the connection between ATP synthesis and electron transport, thereby increasing energy expenditure and potentially improving glucose homeostasis. Because of its ability to address both energy metabolism and glucose regulation, this strategy is considered an exciting path for diabetes treatment. Uncoupling of the mitochondria and energy production ATP (adenosine triphosphate), the main form of energy used by cells, is achieved by mitochondria during normal cellular respiration through oxidative phosphorylation. Protons (H+) are pumped out of the mitochondrial matrix and into the intermembrane space during this process by electrons moving through the electron transport chain [48, 49]. This process creates a proton gradient, and the passage of protons through ATP synthase back into the mitochondrial matrix powers the production of ATP. By interfering with the proton gradient, mitochondrial uncouplers prevent the electron transport chain and ATP production from being coupled. They perform the function of protonophores, by allowing protons to flow easily back into the mitochondrial matrix without producing ATP. Consequently, the proton gradient dissipates, and the effectiveness of ATP production decreases. Mitochondrial uncouplers increase proton flow back into the matrix, a process that releases heat, by decoupling oxidative phosphorylation, and is referred to as uncoupling-induced thermogenesis. Increased caloric expenditure and decreased energy storage as fat are two effects that increase energy expenditure, primarily in the form of heat production. Uncoupling-induced thermogenesis increases insulin sensitivity in a variety of tissues, including skeletal muscle and adipose tissue, thus facilitating glucose uptake and utilization. Increased lipolysis, or the breakdown of stored fats, as a result of uncoupling in adipocytes, might improve insulin sensitivity and decrease fat storage in adipose tissue. Because more glucose is used to produce energy, increased energy expenditure from mitochondrial uncoupling might cause blood glucose levels to decrease [50, 51].
Although preclinical studies on mitochondrial uncouplers have shown promise, several obstacles hinder their clinical implementation, including the potential for adverse effects, particularly those involving thermogenesis and heat production. Increased body temperature could result from overstimulation of uncoupling-induced thermogenesis, an effect that might not be safe or well-tolerated in all people. The development of mitochondrial uncouplers that specifically target tissues and mitochondria involved in glucose and energy metabolism without having negative consequences remains under investigation. An intriguing approach to treating diabetes involves modulating mitochondrial uncoupling, because it has the potential to increase energy expenditure and improve glucose homeostasis. Mitochondrial uncouplers increase thermogenesis and energy expenditure by breaking the link between ATP generation and electron transport, and might potentially improve insulin sensitivity and glucose control [48, 51]. In preclinical research, mitochondrial uncouplers, which increase energy expenditure by reducing the proton gradient in mitochondria, have been studied for their potential to treat metabolic disorders including diabetes. A promising uncoupler called BAM15 has shown efficacy in rodent models by lowering blood glucose, increasing insulin sensitivity, and decreasing body weight without producing major adverse effects such as hyperthermia—a major drawback of previous uncouplers such as dinitrophenol (DNP). In preclinical studies, the mitochondrial uncoupler CP-316819 has also shown comparable advantages, by enhancing metabolic results and decreasing animal fat mass. Although investigations remain in their infancy, the findings imply that mitochondrial dissociation might provide a unique approach to treating diabetes and associated metabolic conditions. However, clinical trials in humans remain necessary to confirm these benefits and assess long-term safety [48–51].
GLUT4 enhancers
To encourage glucose uptake in insulin-sensitive organs, researchers are searching for GLUT4 enhancers. These compounds are being studied for their ability to increase GLUT4 transporter translocation and activity in tissues sensitive to insulin, such as skeletal muscle and adipose tissue. In reaction to insulin, the glucose transporter protein GLUT4 facilitates glucose entry into cells from the bloodstream. Targeting GLUT4 activity for diabetes treatment might be promising, through improving glucose absorption and utilization. The facilitative glucose transporter family includes GLUT4, which is expressed primarily in tissues that are sensitive to insulin. Only a minor portion of GLUT4 is found on the cell surface under normal conditions, because it is mostly contained within intracellular vesicles. After a meal, elevated blood glucose levels cause the release of insulin, which binds its receptor on the cell surface and starts a chain reaction of intracellular signaling processes [52–54].
Mechanisms of action of GLUT4 enhancers
To increase the number of transporters accessible for glucose absorption, GLUT4 enhancers encourage the translocation of GLUT4 from intracellular vesicles to the cell membrane through several mechanisms, including the activation of GLUT4 translocation-associated signaling pathways by GLUT4 enhancers. For instance, certain substances cause the phosphorylation of proteins involved in the translocation of GLUT4 by activating the insulin signaling pathway downstream of the activation of the insulin receptor. AMPK, an enzyme that measures cellular energy levels, might also become more active as a result of enhancers. Independently of insulin signaling, AMPK activation promotes GLUT4 translocation and consequently helps improve glucose absorption. In tissues resistant to insulin, several GLUT4 enhancers have been found to increase insulin sensitivity. These substances promote the activities of insulin in stimulating GLUT4 translocation and glucose absorption by increasing insulin sensitivity [54]. The adipokine adiponectin, which is released by adipose tissue, promotes GLUT4 translocation and glucose absorption in muscle cells. In response to specific GLUT4 enhancers, adiponectin levels may rise, or cells may become more sensitive to its actions, thus increasing glucose absorption. GLUT4 enhancers increase glucose absorption into tissues that are susceptible to insulin by increasing the activity of GLUT4 and encouraging its translocation to the cell membrane; subsequently, blood glucose levels are lowered, and glycemic management is improved. The increases in glucose absorbed by muscle cells can be used to produce energy or stored as glycogen. Increased glucose absorption in adipose tissue can help prevent fat buildup. A promising strategy for treating diabetes, particularly insulin-resistant diseases such as type 2 diabetes, is increasing GLUT4 activity. GLUT4 enhancers have been found to increase insulin sensitivity, lower blood glucose levels, and lessen the demand on pancreatic beta cells to create insulin, by encouraging glucose absorption in insulin-sensitive tissues [54, 55]. The potential of GLUT4 enhancers to promote glucose absorption in insulin-sensitive tissues is being studied. These substances enhance glucose absorption and GLUT4 transporter translocation and activity, and consequently improve glycemic control and confer potential advantages in diabetes treatment. Future therapeutic applications in diabetes treatment and related metabolic disorders show promise in further research and the creation of particular, secure GLUT4 enhancers [55, 56].
DGAT1 inhibitors
Inhibitors of diacylglycerol acyltransferase 1 (DGAT1) are promising targets for treating diabetes-related insulin resistance and lipid metabolism. DGAT1 inhibitors are a group of drugs that work by inhibiting the DGAT1 enzyme. One of the two diacylglycerol acyltransferase isoforms, DGAT1, functions primarily in the liver, adipose tissue, and intestines, where it catalyzes the last stage of triglyceride production. Inhibiting DGAT1 activity has been proposed as a viable therapeutic approach to control lipid metabolism and insulin resistance in diabetes. Triglycerides, the body’s primary form of stored energy, are produced largely via DGAT1, which plays a crucial role in this process. Three fatty acid molecules are joined to a glycerol backbone to form triglycerides. Triglycerides are created when diacylglycerol is esterified with a third fatty acid, through DGAT1 [57, 58].
Mechanisms of action of DGAT1 inhibitors
Inhibiting DGAT1’s enzymatic activity causes DGAT1 inhibitors to prevent the completion of triglyceride synthesis. These inhibitors decrease triglyceride production by blocking DGAT1. Consequently, triglycerides accumulate less in a variety of tissues, such as the liver and adipose tissue. Diminished hepatic lipogenesis can result from the liver’s decreased ability to synthesis new triglycerides from fatty acids under DGAT1 inhibitor treatment. Subsequently, the risk of fatty liver, type 2 diabetes, and insulin resistance, problems affecting many people, might be lessened. Inhibitors of DGAT1 might decrease the storage of extra fatty acids as triglycerides in adipose tissue, thus possibly decreasing excessive fat deposition and inflammation. DGAT1 inhibitors affect dietary fat absorption in the intestines and consequently decrease the amount of triglycerides transported into the bloodstream. DGAT1 inhibitors might increase insulin sensitivity, because they decrease fat accumulation in insulin-sensitive organs such as the muscle and liver. Insulin resistance is associated with intracellular lipid buildup, particularly in muscle and liver cells [59]. The insulin sensitivity and glucose absorption of these organs might be improved by DGAT1 inhibitors, through decreasing fat accumulation in these tissues. To produce synergistic benefits, DGAT1 inhibitors can also be taken with other diabetes drugs. For instance, using DGAT1 inhibitors in conjunction with insulin sensitizers or glucose-lowering medications might result in more thorough cholesterol and glucose management. Potential targets for reducing insulin resistance and lipid metabolism in diabetes include DGAT1 inhibitors. These substances affect lipid metabolism, lessen intracellular fat buildup, and might possibly enhance insulin sensitivity by decreasing DGAT1 and lowering triglyceride production. Future clinical uses of DGAT1 inhibitors as innovative therapeutic options for diabetes and related metabolic disorders offer promise for further study and development [60, 61].
GLP-1 and GIP dual receptor agonists
The synergistic effects on glycemic control of dual agonists that target both GLP-1 and glucose-dependent insulinotropic peptide (GIP) receptors are being studied. The gastrointestinal tract secretes the incretin hormones GLP-1 and GIP in response to food intake [62, 63].
Mechanisms of action of dual receptor agonists
The GLP-1 and GIP receptors can be activated simultaneously by synthetic drugs called dual receptor agonists. These agonists achieve both additive and synergistic effects on insulin secretion and glucose homeostasis, because they act on both receptors. Beta cells in the pancreas activate GLP-1 and GIP receptors, thus increasing intracellular cyclic AMP (cAMP). Subsequently, protein kinase A (PKA) activation leads to increased insulin synthesis and exocytosis of vesicles carrying insulin. Adiponectin and skeletal muscle are two examples of insulin-responsive tissues that benefit from GLP-1 and GIP’s ability to increase glucose uptake. Adipocytes increase glucose absorption in response to GIP, whereas muscle cells increase insulin-independent glucose uptake in response to GLP-1. Glycemic control and blood glucose lowering are both facilitated by this two-pronged approach [64]. The effects of GLP-1 and GIP on insulin secretion are additive. Whereas GIP amplifies this action by making beta cells more sensitive to glucose, GLP-1 predominantly promotes insulin production in response to increased blood glucose levels. Dual agonists concurrently activate both receptors and cause an additive or synergistic rise in insulin secretion and more effective glucose clearance. Additionally, GLP-1 inhibits pancreatic alpha cell secretion of glucagon, thereby decreasing hepatic glucose synthesis and further aiding in glucose regulation. However, the total effect on glucagon levels is dependent on blood glucose levels. GIP, in contrast, promotes glucagon secretion. In hyperglycemia, GLP-1 inhibits glucagon secretion to a greater extent than GIP stimulates it, thus resulting in a net decrease in glucagon output. GLP-1 is well known for delaying stomach emptying and causing satiety, thereby limiting food intake and positively influencing weight loss. Although GIP also has some satiety benefits, it is less effective. Dual receptor agonists might elicit improved satiety effects and help individuals with diabetes or obesity regulate their weight. Dual receptor agonists that target both the GLP-1 and the GIP receptors might have additive benefits in glycemic control [65]. These agonists provide a thorough strategy for controlling blood glucose levels in people with diabetes by improving insulin production, encouraging glucose absorption, and controlling glucagon secretion. Additionally, because of their effects on satiety and weight management, they are intriguing candidates for addressing the complicated metabolic issues associated with diabetes and obesity [66].
PPAR modulators
The effects of PPAR agonists and modulators on controlling lipid and glucose metabolism are being studied. Nuclear receptors known as PPARs are critical regulators of lipid and glucose metabolism. The therapeutic potential of PPAR agonists and modulators, which target these receptors, has been thoroughly investigated for the management of diabetes and dyslipidemia [67].
Types of PPARs
PPAR-alpha, PPAR-delta (sometimes referred to as PPAR-beta), and PPAR-gamma are the three primary subtypes of PPARs. Each subtype has unique roles in metabolism and is expressed in various organs (Table 5).
Table 5 Comparison of PPAR Types: PPAR-Alpha, PPAR-Delta, and PPAR-Gamma
| PPAR Subtype | Tissue Expression | Main Function | Role in Glucose Metabolism | Role in Lipid Metabolism | Other Functions and Effects |
|---|---|---|---|---|---|
| PPAR-alpha | Liver, kidney, heart | Fatty acid oxidation | Regulates gluconeogenesis | Increases fatty acid oxidation | Regulation of lipoprotein metabolism |
| Triglyceride metabolism | Decreases triglyceride synthesis | Anti-inflammatory effects | |||
| Lipoprotein metabolism | Enhances lipoprotein clearance | ||||
| PPAR-delta | Ubiquitous | Fatty acid metabolism | Enhances glucose uptake | Regulates fatty acid metabolism | Role in energy homeostasis |
| Energy homeostasis | Anti-inflammatory effects | ||||
| Glucose uptake in muscles | |||||
| PPAR-gamma | Adipose tissue | Adipocyte differentiation | Improves insulin sensitivity | Increases fatty acid uptake | Anti-inflammatory effects |
| Macrophages | Lipid storage | Enhances glucose uptake in adipose tissue and muscles | Increases triglyceride synthesis | Regulation of immune response | |
| Other tissues | Glucose metabolism regulation |
PPAR-alpha
PPAR-alpha, which is largely expressed in the liver, controls lipid metabolism, including fatty acid oxidation (also known as beta-oxidation) and triglyceride metabolism.
PPAR-delta
Skeletal muscle, adipose tissue, and the heart are some of the many tissues that express PPAR-delta, which affects energy homeostasis and fatty acid metabolism.
PPAR-gamma
The expression of PPAR-gamma is observed primarily in adipose tissue, where it controls adipocyte development and lipid synthesis. Additionally, it affects insulin sensitivity and glucose metabolism [68–70].
Mechanisms of Action of PPAR Agonists and Modulators
PPAR agonists and modulators interact via the ligand-binding domain of PPARs, and thereby cause conformational changes and activation of these receptors. After activation, PPARs form heterodimers with the RXR and bind specific DNA sequences, known as PPAR response elements, in target gene promoters. PPAR agonists and modulators affect glucose metabolism in several ways. Thiazolidinediones (TZDs), PPAR-gamma agonists, improve insulin sensitivity in adipose tissue and skeletal muscle. They enhance insulin sensitivity and encourage adipocyte development, thus increasing triglyceride storage in adipose tissue while decreasing excessive fat buildup in other insulin-sensitive tissues. The control of gluconeogenesis, the process through which the liver manufactures glucose, is influenced by PPAR-alpha and PPAR-delta [68]. Activation of these PPAR subtypes might result in less hepatic glucose generation and improve glycemic management. PPAR-delta agonists have been demonstrated to promote skeletal muscle glucose uptake and utilization, and hence achieve better blood glucose clearance. The expression of genes involved in fatty acid oxidation is boosted by PPAR-alpha agonists, thus increasing the breakdown of fatty acids for energy production. PPAR agonists and modulators are important in lipid metabolism. The expression of triglyceride metabolism genes is regulated by the activation of PPAR-alpha and PPAR-delta, which influence the production, storage, and utilization of triglycerides in different tissues. The metabolism of lipoproteins, such as very-low-density lipoprotein and high-density lipoprotein, which are essential for carrying lipids in the bloodstream, are affected by PPAR-alpha agonists. By inhibiting the production of pro-inflammatory cytokines and encouraging the expression of anti-inflammatory molecules, PPAR agonists, particularly PPAR-gamma agonists, have anti-inflammatory effects. Compounds that target PPARs, a family of nuclear receptors important in controlling lipid and glucose metabolism, are known as PPAR agonists and modulators. These substances have shown promise in the treatment of dyslipidemia and diabetes by increasing insulin sensitivity, modulating the metabolism of lipids and sugars, and demonstrating anti-inflammatory properties. A comprehensive strategy for addressing the metabolic abnormalities associated with diabetes and related illnesses is provided by PPAR-targeted medicines [70, 71].
FGFR1c Inhibitors
Potential targets for enhancing insulin sensitivity include inhibitors of fibroblast growth factor receptor 1c (FGFR1c). Inhibitors of FGFR1c, a subtype of the fibroblast growth factor receptor (FGFR) family, are a group of drugs that specifically target the FGFR1c isoform. FGFR1c plays a critical role in controlling glucose and lipid metabolism and is expressed primarily in metabolic tissues such as adipose, liver, and skeletal muscle tissue. Inhibition of FGFR1c activity is being studied as a potential method to increase insulin sensitivity and control metabolic diseases including type 2 diabetes. The metabolic effects of FGFs, particularly FGF21 and FGF19, are mediated by FGFR1c. These FGFs bind FGFR1c and activate downstream signaling pathways that affect fat and glucose metabolism [72].
Mechanisms of Action of FGFR1c Inhibitors
By selectively inhibiting FGFR1c activity, FGFR1c inhibitors interfere with the signaling pathways activated by FGF21 and FGF19. This inhibition can have a variety of effects on glucose metabolism and lipids, primarily via improving insulin sensitivity. FGFR1c inhibitors have several actions that increase insulin sensitivity; for example, increased insulin signaling occurs in target tissues such adipose tissue and skeletal muscle when FGFR1c is inhibited. Subsequently, the absorption and utilization of glucose in these tissues, as well as the response of insulin receptors, increase. FGFR1c inhibitors decrease hepatic synthesis of glucose, which is necessary for keeping blood glucose levels within a normal range. Secretion of adiponectin, an adipokine with anti-inflammatory properties that improves insulin sensitivity, increases after FGFR1c inhibition. Additionally, FGFR1c inhibitors have been found to enhance insulin sensitivity, thereby affecting lipid metabolism [73]. These inhibitors lessen ectopic lipid accumulation, which is associated with insulin resistance, by altering lipid metabolism in tissues such as the liver and adipose tissue. Endocrine hormones FGF21 and FGF19 are produced by several organs, including the liver and adipose tissue. FGF21 binds FGFR1c and subsequently affects the metabolism of lipids and glucose. Blood glucose levels are decreased by FGF21, which increases the absorption and utilization of glucose in adipose tissue and skeletal muscle. To increase energy expenditure and decrease lipid buildup, FGF21 stimulates fatty acid oxidation and promotes lipolysis, the breakdown of stored lipids [74–76]. FGF21 improves glucose homeostasis by increasing insulin sensitivity in a variety of tissues. Because of their ability to enhance insulin sensitivity and control glucose and lipid metabolism, FGFR1c inhibitors hold promise as possible therapeutic agents for diabetes and related metabolic disorders. These inhibitors provide a novel method for treating insulin resistance and hyperglycemia by specifically targeting FGFR1c. However, further investigation is required before FGFR1c inhibitors can be implemented in clinical practice for diabetes treatment, to completely comprehend their safety, effectiveness, and long-term implications. FGFR1c inhibitors specifically target the FGFR1c isoform, which is essential in modulating the metabolic effects of FGF21 and FGF19. These inhibitors increase insulin sensitivity, control glucose and lipid metabolism, and present a possible therapeutic strategy for treating insulin resistance and diabetes by decreasing FGFR1c activity. As this field of study develops, FGFR1c inhibitors have potential as cutting-edge therapies to address the complex metabolic dysregulation associated with metabolic diseases such as diabetes. However, the development of new medications necessitates extensive clinical trials and research to confirm their utility and safety [77–79].
Drugs used in treatments with novel technologies
Liraglutide
The GLP-1 receptor agonist liraglutide is used in the more sophisticated management of diabetes, particularly in people with type 2 diabetes. Improved glycemic control and overall diabetes care are essential. GLP-1 receptors, which are found primarily in pancreatic beta cells and the gastrointestinal tract, are activated by liraglutide. GLP-1 is an incretin hormone that increases insulin release when blood glucose levels are high by stimulating insulin secretion in a glucose-dependent manner. Liraglutide improves insulin production by triggering GLP-1 receptors in pancreatic beta cells, thus promoting better glucose utilization in peripheral tissues and lowering blood glucose rises after meals. Liraglutide inhibits the release of glucagon from pancreatic alpha cells via activating GLP-1 receptors [80]. The hormone glucagon encourages the liver to release glucose and subsequently increases blood glucose levels. Inhibition of glucagon secretion enables better glycemic control and helps prevent excessive glucose synthesis. Liraglutide slows stomach emptying, and consequently enables a more progressive digestion and absorption of food. To avoid postprandial (after-meal) blood glucose spikes, this treatment leads to slow and persistent release of glucose into the bloodstream. Liraglutide has been demonstrated to increase satiety and lessen appetite, and consequently might decrease caloric intake and provide weight loss advantages. For people with type 2 diabetes, losing weight can be helpful, by enhancing insulin sensitivity and metabolic health in general [81]. Clinical studies have shown that liraglutide has positive effects on the heart. In people with type 2 diabetes and pre-existing cardiovascular disease, this drug has been demonstrated to lower the risk of serious cardiovascular events such heart attacks and strokes. Once-daily and once-weekly versions of liraglutide are available. The stress of repeated injections is lessened by the once-weekly formulation, which provides improved convenience and supports adherence. For complete glucose control, liraglutide can be used alone or in conjunction with other diabetic drugs, such as metformin, sulfonylureas, or insulin [82]. Liraglutide, a GLP-1 receptor agonist, is important in the treatment of advanced diabetes, because it increases insulin secretion, decreases glucagon release, slows stomach emptying, increases satiety, and might facilitate weight loss. Its cardiovascular advantages also make it effective in controlling type 2 diabetes [83–85].
Empagliflozin
The SGLT2 inhibitor empagliflozin is used to treat type 2 diabetes. By focusing on the kidneys’ mechanism for reabsorbing glucose, this treatment is essential in the management of diabetes and has several positive effects on glycemic control and cardiovascular health in general. Renal SGLT2 is inhibited by empagliflozin. The reabsorption of glucose into the bloodstream from the glomerular filtrate is performed by SGLT2. Empagliflozin increases glucose excretion in the urine via decreasing glucose reabsorption through inhibiting SGLT2. Empagliflozin increases the quantity of glucose discharged in the urine as a result of SGLT2 inhibition [86]. Lower blood glucose levels result, because this treatment lowers the amount of glucose in the bloodstream. Empagliflozin aids in lowering blood glucose levels by increasing glucose excretion, particularly after meals, when blood glucose levels are elevated. Some people with type 2 diabetes might experience modest weight reduction because of the enhanced glucose excretion caused by empagliflozin [87]. This effect is aided by the calories lost as a result of glucose excretion. Empagliflozin increases insulin sensitivity in liver and muscle tissues. Consequently, cells become more receptive to insulin, thereby promoting the uptake and utilization of glucose. Clinical studies have shown that empagliflozin offers important cardiovascular advantages. In individuals with type 2 diabetes and established cardiovascular disease, this drug has been demonstrated to lower the risk of significant cardiovascular events, such as heart attacks, strokes, and cardiovascular mortality [88]. Because of its renoprotective properties, Empagliflozin aids in preventing the harmful effects of diabetes on the kidneys. It can lessen the chances of unfavorable kidney-related outcomes and decrease the advancement of diabetic renal disease. To provide thorough glucose control and improve treatment results, empagliflozin can be taken alone or in conjunction with other diabetic drugs, such as metformin or insulin. As an SGLT2 inhibitor, empagliflozin is essential in the treatment of type 2 diabetes. This treatment improves glycemic management, promotes weight loss, and has positive effects on the cardiovascular system by blocking glucose reabsorption in the kidneys and increasing glucose excretion. Empagliflozin is a beneficial alternative for patients with type 2 diabetes and kidney problems, because of its additional renoprotective benefits [89].
Pegbelfermin
A new FGF21 counterpart, also known as BMS-986036, is being studied as a potential treatment for several metabolic diseases, including type 2 diabetes and non-alcoholic steatohepatitis (NASH). Pegbelfermin functions as an analogue of FGF21, a hormone with strong metabolic effects that is produced by the liver and adipose tissue. Pegbelfermin is a favorable candidate for treating metabolic disorders, because FGF21 is crucial in controlling lipid and glucose metabolism. Enhancing insulin sensitivity in peripheral tissues such as skeletal muscle and adipose tissue is one of FGF21’s main effects. Pegbelfermin might improve glucose absorption and utilization by increasing insulin sensitivity, thus improving glycemic control in people with type 2 diabetes [90]. Clinical investigations on pegbelfermin have demonstrated that it lowers hepatic fat levels. In people with obesity and type 2 diabetes, non-alcoholic fatty liver disease (NAFLD) is a frequent illness, and lowering hepatic fat buildup is essential for enhancing liver function. Pegbelfermin, a derivative of FGF21, has anti-inflammatory properties. Chronic inflammation has a crucial role in the emergence and development of metabolic diseases such as NASH. Pegbelfermin might potentially preserve the liver and other metabolic tissues by lowering inflammation. Pegbelfermin, like FGF21, might aid in weight loss and have positive benefits on controlling body weight. Insulin sensitivity, lipid metabolism, and general metabolic health can all benefit from weight loss. The severe variant of NAFLD known as NASH is characterized by liver damage and inflammation. Because of its ability to lower hepatic fat and inflammation, pegbelfermin is being studied as a viable therapeutic option for NASH, thus offering a holistic strategy to treat this condition. Pegbelfermin might have broader cardiometabolic advantages in addition to its effects on glucose and lipid metabolism, including potential enhancements in blood pressure, cholesterol levels, and overall cardiovascular health. Pegbelfermin (BMS-986036) is an FGF21 analogue with promise for treating metabolic diseases including NASH and type 2 diabetes. Pegbelfermin offers a multimodal strategy for addressing the intricate metabolic abnormalities associated with these illnesses, through its effects on insulin sensitivity, hepatic fat reduction, anti-inflammatory characteristics, and weight management. Its safety and efficacy profile will be clarified by ongoing research and clinical studies, which might lead to its approval as a treatment option for individuals with metabolic disorders [91–93].
Metformin
Metformin is a medication frequently used to treat type 2 diabetes. Its mechanism of action involves AMPK, which affects several insulin and glucose metabolism-associated receptors and pathways [94]. The main mechanism of action of metformin is the activation of AMPK, an important regulator of cellular energy homeostasis. Increased fatty acid oxidation, decreased glucose synthesis in the liver, and increased glucose absorption in skeletal muscle are all effects of AMPK activation. In people with type 2 diabetes, these actions improve insulin sensitivity and glycemic management [95–98]. Metformin increases insulin sensitivity in peripheral tissues and has indirect effects on the insulin receptor. Metformin increases insulin responsiveness and enhances glucose absorption in tissues that are sensitive to insulin by activating AMPK. Although its effects on this receptor are not as immediate as those of GLP-1 receptor agonists, metformin has been demonstrated to have some effects on GLP-1 receptor signaling. Metformin has been hypothesized to increase GLP-1 production from the gut, thus potentially explaining how it improves glucose metabolism [99–103]. The main mechanism of action of metformin differs from that of SGLT2 inhibitors such as empagliflozin. Metformin functions primarily through AMPK activation, and affects hepatic glucose synthesis and peripheral glucose uptake, in contrast to SGLT2 inhibitors, which prevent glucose reabsorption in the kidneys. The direct effects of metformin on the FGF21 receptor are not well known. However, several metabolic effects of FGF21 analogues, such as enhanced insulin sensitivity and decreased hepatic fat content, might overlap with metformin’s downstream effects on AMPK activation. The effects of metformin on GPCRs are not as immediate as those of particular GPCR agonists. However, the downstream effects of AMPK activation on several GPCR signaling pathways involved in insulin signaling and glucose homeostasis can have effects. The direct effects of metformin on bile acid receptors have not been thoroughly investigated. However, through influencing AMPK activation and hepatic glucose generation, it might indirectly affect bile acid metabolism. Mitochondrial uncouplers are not directly targeted by metformin. Instead of interfering with mitochondrial function, its main mode of action is linked to AMPK activation and regulation of glucose and lipid metabolism. Through its activation of AMPK, metformin plays a crucial part in the treatment of type 2 diabetes by improving insulin sensitivity and glucose metabolism. Although various receptors and pathways involved in the control of insulin and glucose are indirectly affected, AMPK activation is the key mechanism underlying the treatment effects. The wide-ranging and complex processes of metformin make it an effective and widely used drug for treating type 2 diabetes and associated metabolic diseases. Although metformin is a foundational drug, its combination with novel therapies continues to be explored in innovative ways. For example, metformin combined with SGLT2 inhibitors (such as empagliflozin) or GLP-1 receptor agonists remains an important part of modern treatment regimens for type 2 diabetes. The use of metformin in dual or triple therapy with these newer agents continues to evolve, to enable novel strategies in diabetes care. Beyond its traditional use in diabetes, metformin is being studied in new areas such as cancer metabolism and aging-associated conditions. These new applications of metformin in metabolic syndrome or conditions adjacent to diabetes are areas of active, innovative research [104–106].
Semaglutide
The GLP-1 receptor agonist semaglutide is used to treat type 2 diabetes. This treatment imitates the effects of GLP-1, an incretin hormone that controls insulin secretion and glucose metabolism. In a glucose-dependent manner, semaglutide increases insulin production by activating GLP-1 receptors on pancreatic beta cells, thereby maintaining healthful blood glucose levels after meals. Semaglutide prevents the hormone glucagon, which boosts blood glucose levels, from being released into the body. Moreover, it aids in preventing excessive glucose synthesis in the liver by lowering glucagon secretion. Because semaglutide slows stomach emptying, glucose is released into the bloodstream after meals more gradually and continuously, and the likelihood of postprandial blood glucose increases is lessened. Semaglutide decreases hunger and aids in weight loss. People with type 2 diabetes and obesity would benefit from this effect [107, 108].
Obeticholic acid
NASH, a severe form of NAFLD, is treated with obeticholic acid, a synthetic bile acid analogue. FXR, a bile acid receptor involved in the control of bile acid production and metabolism, is activated by obeticholic acid. Obeticholic acid facilitates liver health in NASH by decreasing the buildup of fat in the liver by activating the FXR. Because of its anti-inflammatory properties, obeticholic acid lessens liver inflammation and stops the evolution of NASH [109, 110].
DNP
The mitochondrial uncoupler DNP has major safety issues that prevent its use in medicine. The electron transport chain and ATP production in mitochondria are not properly coupled under DNP treatment. This decoupling causes energy to be lost as heat, thereby raising the metabolic rate and energy expenditure. DNP’s uncoupling action increases energy consumption and might theoretically result in weight loss. Although not limited to fat tissue, this effect can cause dangerous hyperthermia and other serious adverse effects. Therefore, DNP is unsafe and inappropriate for use in medicine. DNP has a small safety margin, and is extremely poisonous and hazardous. Its use for medical purposes has not received regulatory approval, and ingesting it can have serious, perhaps fatal adverse effects such as organ failure, overheating, and even death. Using DNP for medical or weight-loss purposes is strongly advised against and generally prohibited to [111–114]. The list of drugs used in the treatments with novel technology are tabulated in Table 6.
Table 6 Drugs Used in Treatments with Novel Technology
| Targeted Drug | Target Receptor/Pathway | Mechanism of Action | Clinical Application |
|---|---|---|---|
| Liraglutide | GLP-1 receptor | Agonist of the GLP-1 receptor that increases insulin secretion while decreasing glucagon production | Helps patients with type 2 diabetes with glucose management |
| Empagliflozin | SGLT2 transporter | SGLT2 inhibitor that prevents the kidneys from reabsorbing glucose, thus increasing the amount of glucose excreted in the urine | Used to lower type 2 diabetes blood glucose levels and lower cardiovascular risk |
| Pegbelfermin (BMS-986036) | FGF21 receptor | Improves glucose and lipid metabolism by activating FGF21 receptors in recombinant form | Under study for their potential to treat metabolic diseases such as diabetes |
| Metformin | AMPK activation | Promotes the absorption and utilization of glucose through AMP-activated protein kinase (AMPK) activation | First-line therapy for type 2 diabetes |
| Semaglutide | GPCR agonist (GLP-1R, GIPR, GCG) | Agonist for the GLP-1 receptor that also affects other GPCRs important for glucose control | Helps patients with type 2 diabetes with glucose management |
| Obeticholic acid | Bile acid receptor | Antagonist that affects the metabolism of lipids and glucose by attacking bile acid receptors | Under study for possible effects on the regulation of cholesterol and glucose levels |
| Dinitrophenol | Mitochondrial uncoupling | Mitochondrial uncoupler that interferes with ATP production and increases energy expenditure | Under evaluation for their potential in diabetes treatment |
Limitations
Current diabetic drugs work well in many situations but have several drawbacks that affect their overall effectiveness, patient compliance, and long-term results. Insulin therapy and oral hypoglycemic medications (such as metformin and sulfonylureas) are examples of conventional treatments that target blood glucose control but frequently ignore the underlying metabolic dysfunctions associated with diabetes. Whereas certain oral medications may lose their effectiveness over time or require complicated dose regimes, thus affecting patient compliance, insulin therapy can cause weight gain and hypoglycemia. Furthermore, many treatments do not address the related metabolic problems that are major causes of diabetic complications, such as insulin resistance, lipid abnormalities, and chronic inflammation. By directly addressing particular physiological pathways implicated in glucose absorption, lipid metabolism, insulin sensitivity, and energy expenditure, receptor-based medicines present a potential benefit that might result in more thorough metabolic control. Finding the right receptors and decreasing off-target effects are two difficulties in the development of such treatments. Further study in this field might lead to new therapies that address the wider metabolic abnormalities associated with diabetes in addition to glucose management.
Future perspectives
The area of diabetes care remains developing, and continuing research is aimed at providing safer, more individualized, and more effective treatments. Some possible future developments for the various targeted treatments and strategies are described below.
Targeted medications and receptor-based therapies
More studies on GLP-1 receptor agonists, SGLT2 inhibitors, FGF21 analogues, GPCRs, and bile acid receptor agonists are likely to reveal more advantages, thereby leading to a broader range of uses in the treatment of metabolic disorders and diabetes. Future research may examine the use of numerous targeted drugs in combination therapy to create synergistic effects and enhance overall glycemic control and metabolic outcomes [115].
AMPK activation and insulin receptor agonists
Research is currently attempting to find and create new AMPK activators with enhanced specificity and safety profiles, thereby improving insulin sensitivity and glucose absorption without causing any negative adverse effects. Advanced insulin receptor agonists: New formulations of insulin receptor agonists with quicker onset and longer durations of action, as well as enhanced delivery techniques, might become available [116].
GPCRs and mitochondrial uncouplers
Research efforts might identify novel GPCRs implicated in glucose homeostasis and insulin signaling, thus offering prospective targets for upcoming drugs. To benefit from higher energy expenditure without the risky adverse effects associated with DNP, non-toxic and tailored mitochondrial uncouplers are being developed [116].
Pegbelfermin (BMS-986036) and other emerging therapies
Pegbelfermin (BMS-986036) might obtain regulatory approval for the management of metabolic disorders, including NASH and type 2 diabetes, thus providing additional therapeutic alternatives for patients. FDA Approval and Expanded Indications: To fix genetic deficiencies and potentially provide treatments for some metabolic disorders, gene therapies and gene-editing methods that target metabolic pathways might receive FDA approval in the future [117].
General perspectives
The application of AI and machine learning algorithms to the management of diabetes might achieve enhanced accuracy in illness prediction, individualized treatment regimens, and improved patient outcomes. Regarding nanotechnology and drug delivery, new developments might completely transform how drugs are delivered, thus enabling targeted and controlled drug release, lowering adverse effects, and enhancing therapeutic efficacy. A greater emphasis on patient education, empowerment, and self-management could enhance adherence to treatment plans and lifestyle changes, and aid in better disease control. Telemedicine and Remote Monitoring: Devices for telemedicine and remote monitoring might become crucial in the management of chronic disease, enhancing patient access to care, and enhancing adherence to therapy. Safety and Regulatory Approval: Before widespread implementation of novel medications, strict regulatory reviews will be required to ensure patient safety and efficacy, and risk-benefit analyses must be performed. Treatment for metabolic disorders and diabetes has a bright future, owing to cutting-edge research, new technologies, and a patient-centered, all-encompassing approach. Although advancements are imminent, patience and vigilance will be necessary as new discoveries pass through clinical trials and receive regulatory authorization. Healthcare professionals, researchers, politicians, and patients working together will be essential to achieving substantial breakthroughs in the management of metabolic disorders and diabetes [117–119].
Conclusion
The review provided a thorough investigation of new therapeutic targets for the management of metabolic illnesses such as diabetes. These innovative therapeutic strategies hold promise for revolutionizing diabetes management, enhancing glycemic control, and lowering the likelihood of complications associated with the condition. The use of drugs that target particular receptors and pathways, such as AMPK activators, GLP-1 receptor agonists, SGLT2 inhibitors, FGF21 analogues, and GPCRs, has been shown to improve insulin sensitivity, encourage glucose uptake, and control lipid metabolism. Additionally, the effects of mitochondrial uncouplers and bile acid receptor agonists on the control of glucose and lipids are being studied. The discussion of potential future directions highlights ongoing investigations and developments in the field of diabetes treatment. Gene treatments, precision medicine, and combination therapies have the potential to offer more individualized and potent treatment alternatives. Improved patient monitoring, adherence, and self-management of metabolic illnesses might result from the combination of digital health technology, telemedicine, and AI-driven techniques, thus improving treatment outcomes and patient quality of life. Additionally, the development of cutting-edge treatments such as pegbelfermin (BMS-986036) and gene-editing methods offers hope for treating diseases including NASH and investigating prospective treatments.
Summary
The review examined several potential targets for the treatment of metabolic diseases including diabetes, and demonstrated the substantial advancements made in comprehending the complex mechanisms underlying glucose and lipid metabolism. The utility of GLP-1 receptor agonists, SGLT2 inhibitors, FGF21 analogues, AMPK activators, and GPCRs in enhancing glycemic management, insulin sensitivity, and lipid profiles has been established. Additionally, the possible effects of mitochondrial uncouplers and bile acid receptor agonists on the control of glucose and lipids are being investigated. A key component of cutting-edge diabetes care is insulin glargine, a long-acting insulin analogue that supplies consistent basal insulin replacement. An effective GLP-1 receptor agonist, liraglutide, has been shown to increase insulin production, decrease glucagon release, and aid in weight loss. An SGLT2 inhibitor called empagliflozin has shown potential in lowering blood glucose levels, encouraging weight loss, and providing cardiovascular and renal advantages. The future is likely to bring a dynamic environment with ongoing research and innovations attempting to improve existing medications, create new therapies, and personalize diabetes management. Realizing the full potential of these novel methods will require collaboration among healthcare professionals, researchers, policymakers, and patients, to enhance outcomes and quality of life for people with diabetes and metabolic disorders.
Acknowledgement
We hereby acknowledge that we used ChatGPT [https://chat.openai.com/] to correct grammar and perform language editing.
Conflict of Interest
The authors have no competing interest to declare.
References
- Kaul K, Tarr JM, Ahmad SI, Kohner EM, Chibber R. Introduction to diabetes mellitus. In: Ahmad SI, editor. Diabetes: an old disease, a new insight. New York, NY: Springer; 2013. pp. 1-1. [DOI: 10.1007/978-1-4614-5441-0_1]
- Yedjou CG, Grigsby J, Mbemi A, Nelson D, Mildort B, et al. The management of diabetes mellitus using medicinal plants and vitamins. Int J Mol Sci 2023;24(10):9085. [PMID: 37240430 DOI: 10.3390/ijms24109085]
- Quazi A, Patwekar M, Patwekar F, Alghamdi S, Rajab BS, et al. In vitro alpha-amylase enzyme assay of hydroalcoholic polyherbal extract: proof of concept for the development of polyherbal teabag formulation for the treatment of diabetes. Evid Based Complement Alternat Med 2022;2022:1577957. [PMID: 35600963 DOI: 10.1155/2022/1577957]
- Saraon P, Pathmanathan S, Snider J, Lyakisheva A, Wong V, et al. Receptor tyrosine kinases and cancer: oncogenic mechanisms and therapeutic approaches. Oncogene 2021;40(24):4079-93. [PMID: 34079087 DOI: 10.1038/s41388-021-01841-2]
- Saltiel AR. Insulin signaling in health and disease. J Clin Invest 2021;131(1):e142241. [PMID: 33393497 DOI: 10.1172/JCI142241]
- Wang D, Zhou W, Chen J, Wei W. Upstream regulators of phosphoinositide 3-kinase and their role in diseases. J Cell Physiol 2019;234(9):14460-72. [PMID: 30710358 DOI: 10.1002/jcp.28215]
- White MF, Kahn CR. Insulin action at a molecular level-100 years of progress. Mol Metab 2021;52:101304. [PMID: 34274528 DOI: 10.1016/j.molmet.2021.101304]
- Shorning BY, Dass MS, Smalley MJ, Pearson HB. The PI3K-AKT-mTOR pathway and prostate cancer: at the crossroads of AR, MAPK, and WNT signaling. Int J Mol Sci 2020;21(12):4507. [DOI: 10.3390/ijms21124507]
- Wen X, Zhang B, Wu B, Xiao H, Li Z, et al. Signaling pathways in obesity: mechanisms and therapeutic interventions. Signal Transduct Target Ther 2022;7(1):298. [PMID: 36031641 DOI: 10.1038/s41392-022-01149-x]
- Patwekar M, Quazi A, Faheem IP, Kamal MA, Mukim M, et al. In vitro inhibitory effect on alpha amylase enzyme by polyherbal dip tea in diabetes. Indo Glob J Pharm Sci 2022;12(1):156-65. [DOI: 10.35652/IGJPS.2022.12018]
- Helmstädter J, Keppeler K, Küster L, Münzel T, Daiber A, et al. Glucagon-like peptide-1 (GLP-1) receptor agonists and their cardiovascular benefits-the role of the GLP-1 receptor. Br J Pharmacol 2022;179(4):659-76. [PMID: 33764504 DOI: 10.1111/bph.15462]
- Kopp KO, Glotfelty EJ, Li Y, Greig NH. Glucagon-like peptide-1 (GLP-1) receptor agonists and neuroinflammation: implications for neurodegenerative disease treatment. Pharmacol Res 2022;186:106550. [PMID: 36372278 DOI: 10.1016/j.phrs.2022.106550]
- Ikonomidis I, Pavlidis G, Thymis J, Birba D, Kalogeris A, et al. Effects of glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter-2 inhibitors, and their combination on endothelial glycocalyx, arterial function, and myocardial work index in patients with type 2 diabetes mellitus after 12-month treatment. J Am Heart Assoc 2020;9(9):e015716. [PMID: 32326806 DOI: 10.1161/JAHA.119.015716]
- Lv X, Dong Y, Hu L, Lu F, Zhou C, et al. Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) for the management of nonalcoholic fatty liver disease (NAFLD): a systematic review. Endocrinol Diabetes Metab 2020;3(3):e00163. [PMID: 32704576 DOI: 10.1002/edm2.163]
- Xu D, Nair A, Sigston C, Ho C, Li J, et al. Potential roles of glucagon-like peptide 1 receptor agonists (GLP-1 RAs) in nondiabetic populations. Cardiovasc Ther 2022;2022:6820377. [PMID: 36474714 DOI: 10.1155/2022/6820377]
- Urva S, Coskun T, Loghin C, Cui X, Beebe E, et al. The novel dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 (GLP-1) receptor agonist tirzepatide transiently delays gastric emptying similarly to selective long-acting GLP-1 receptor agonists. Diabetes Obes Metab 2020;22(10):1886-91. [PMID: 32519795 DOI: 10.1111/dom.14110]
- Kumar S, Khatik GL, Mittal A. In silico molecular docking study to search new SGLT2 inhibitor based on dioxabicyclo[3.2.1] octane scaffold. Curr Comput Aided Drug Des 2020;16(2):145-54. [PMID: 30345926 DOI: 10.2174/1573409914666181019165821]
- Palapra H, Viswam SK, Kalaiselvan V, Undela K. SGLT2 inhibitors associated pancreatitis: signal identification through disproportionality analysis of spontaneous reports and review of case reports. Int J Clin Pharm 2022;44(6):1425-33. [PMID: 36224513 DOI: 10.1007/s11096-022-01476-7]
- Sinha B, Ghosal S. Meta-analyses of the effects of DPP-4 inhibitors, SGLT2 inhibitors and GLP1 receptor analogues on cardiovascular death, myocardial infarction, stroke and hospitalization for heart failure. Diabetes Res Clin Pract 2019;150:8-16. [PMID: 30794833 DOI: 10.1016/j.diabres.2019.02.014]
- Moinul M, Amin SA, Kumar P, Patil UK, Gajbhiye A, et al. Exploring sodium glucose cotransporter (SGLT2) inhibitors with machine learning approach: a novel hope in anti-diabetes drug discovery. J Mol Graph Model 2022;111:108106. [PMID: 34923429 DOI: 10.1016/j.jmgm.2021.108106]
- Suijk DL, van Baar MJ, van Bommel EJ, Iqbal Z, Krebber MM, et al. SGLT2 inhibition and uric acid excretion in patients with type 2 diabetes and normal kidney function. Clin J Am Soc Nephrol 2022;17(5):663-71. [PMID: 35322793 DOI: 10.2215/CJN.11480821]
- Singh M, Sharma R, Kumar A. Safety of SGLT2 inhibitors in patients with diabetes mellitus. Curr Drug Saf 2019;14(2):87-93.
- Tian Q, Guo K, Deng J, Zhong Y, Yang L. Effects of SGLT2 inhibitors on haematocrit and haemoglobin levels and the associated cardiorenal benefits in T2DM patients: a meta-analysis. J Cell Mol Med 2022;26(2):540-7. [PMID: 34878225 DOI: 10.1111/jcmm.17115]
- Moeckli B, Pham TV, Slits F, Latrille S, Peloso A, et al. FGF21 negatively affects long-term female fertility in mice. Heliyon 2022;8(11):e11490. [PMID: 36406708 DOI: 10.1016/j.heliyon.2022.e11490]
- Chikamatsu M, Watanabe H, Shintani Y, Murata R, Miyahisa M, et al. Albumin-fused long-acting FGF21 analogue for the treatment of non-alcoholic fatty liver disease. J Control Release 2023;355:42-53. [PMID: 36690035 DOI: 10.1016/j.jconrel.2023.01.039]
- Tan H, Yue T, Chen Z, Wu W, Xu S, et al. Targeting FGF21 in cardiovascular and metabolic diseases: from mechanism to medicine. Int J Biol Sci 2023;19(1):66-88. [PMID: 36594101 DOI: 10.7150/ijbs.73936]
- Claflin KE, Sullivan AI, Naber MC, Flippo KH, Morgan DA, et al. Pharmacological FGF21 signals to glutamatergic neurons to enhance leptin action and lower body weight during obesity. Mol Metab 2022;64:101564. [PMID: 35944896 DOI: 10.1016/j.molmet.2022.101564]
- Watanabe H, Miyahisa M, Chikamatsu M, Nishida K, Minayoshi Y, et al. Development of a long acting FGF21 analogue-albumin fusion protein and its anti-diabetic effects. J Control Release 2020;324:522-31. [PMID: 32450094 DOI: 10.1016/j.jconrel.2020.05.036]
- Guo C, Zhao L, Li Y, Deng X, Yuan G. Relationship between FGF21 and drug or nondrug therapy of type 2 diabetes mellitus. J Cell Physiol 2021;236(1):55-67. [PMID: 32583417 DOI: 10.1002/jcp.29879]
- Lam CS, Xia YX, Chen BS, Du YX, Liu KL, et al. Dihydro-resveratrol attenuates oxidative stress, adipogenesis and insulin resistance in in vitro models and high-fat diet-induced mouse model via AMPK activation. Nutrients 2023;15(13):3006. [PMID: 37447331 DOI: 10.3390/nu15133006]
- Yoon KJ, Zhang D, Kim SJ, Lee MC, Moon HY. Exercise-induced AMPK activation is involved in delay of skeletal muscle senescence. Biochem Biophys Res Commun 2019;512(3):604-10. [PMID: 30910357 DOI: 10.1016/j.bbrc.2019.03.086]
- Steele HE, Guo Y, Li BY, Na S. Mechanotransduction of mitochondrial AMPK and its distinct role in flow-induced breast cancer cell migration. Biochem Biophys Res Commun 2019;514(2):524-9. [PMID: 31060777 DOI: 10.1016/j.bbrc.2019.04.191]
- Chen H, Nie T, Zhang P, Ma J, Shan A. Hesperidin attenuates hepatic lipid accumulation in mice fed high-fat diet and oleic acid induced HepG2 via AMPK activation. Life Sci 2022;296:120428. [PMID: 35218767 DOI: 10.1016/j.lfs.2022.120428]
- Feng TY, Lv DL, Zhang X, Du YQ, Yuan YT, et al. Rosmarinic acid improves boar sperm quality, antioxidant capacity and energy metabolism at 17°C via AMPK activation. Reprod Domest Anim 2020;55(12):1714-24. [PMID: 32969084 DOI: 10.1111/rda.13828]
- Watkins LR, Orlandi C. In vitro profiling of orphan G protein coupled receptor (GPCR) constitutive activity. Br J Pharmacol 2021;178(15):2963-75. [PMID: 33784795 DOI: 10.1111/bph.15468]
- Grisshammer R. The quest for high-resolution G protein-coupled receptor-G protein structures. Proc Natl Acad Sci U S A 2020;117(13):6971-3. [PMID: 32179692 DOI: 10.1073/pnas.2002665117]
- Ren H, Li J, Zhang N, Hu LA, Ma Y, et al. Function-based high-throughput screening for antibody antagonists and agonists against G protein-coupled receptors. Commun Biol 2020;3(1):146. [PMID: 32218528 DOI: 10.1038/s42003-020-0867-7]
- Shi Y, Chen Y, Deng L, Du K, Lu S, et al. Structural understanding of peptide-bound G protein-coupled receptors: peptide-target interactions. J Med Chem 2023;66(2):1083-111. [PMID: 36625741 DOI: 10.1021/acs.jmedchem.2c01309]
- van Gastel J, Leysen H, Boddaert J, Luttrell LM, Martin B, et al. Aging-related modifications to G protein-coupled receptor signaling diversity. Pharmacol Ther 2021;223:107793. [PMID: 33316288 DOI: 10.1016/j.pharmthera.2020.107793]
- Kimura T, Pydi SP, Pham J, Tanaka N. Metabolic functions of G protein-coupled receptors in hepatocytes-potential applications for diabetes and NAFLD. Biomolecules 2020;10(10):1445. [PMID: 33076386 DOI: 10.3390/biom10101445]
- Smits MM, Fluitman KS, Herrema H, Davids M, Kramer MH, et al. Liraglutide and sitagliptin have no effect on intestinal microbiota composition: a 12-week randomized placebo-controlled trial in adults with type 2 diabetes. Diabetes Metab 2021;47(5):101223. [PMID: 33429063 DOI: 10.1016/j.diabet.2021.101223]
- Lee JS, Han P, Chaudhury R, Khan S, Bickerton S, et al. Metabolic and immunomodulatory control of type 1 diabetes via orally delivered bile-acid-polymer nanocarriers of insulin or rapamycin. Nat Biomed Eng 2021;5(9):983-97. [PMID: 34616050 DOI: 10.1038/s41551-021-00791-0]
- Song Y, Sun L, Ma P, Xu L, Xiao P. Dihydromyricetin prevents obesity via regulating bile acid metabolism associated with the farnesoid X receptor in ob/ob mice. Food Functi 2022;13(5):2491-503. [PMID: 35147634 DOI: 10.1039/d1fo03971g]
- Ito K, Okumura A, Takeuchi JS, Watashi K, Inoue R, et al. Dual agonist of farnesoid X receptor and takeda G protein-coupled receptor 5 inhibits hepatitis B virus infection in vitro and in vivo. Hepatology 2021;74(1):83-98. [PMID: 33434356 DOI: 10.1002/hep.31712]
- Shi M, Tang J, Zhang T, Han H. Swertiamarin, an active iridoid glycoside from Swertia pseudochinensis H. Hara, protects against alpha-naphthylisothiocyanate-induced cholestasis by activating the farnesoid X receptor and bile acid excretion pathway. J Ethnopharmacol 2022;291:115164. [PMID: 35278607 DOI: 10.1016/j.jep.2022.115164]
- Kårhus ML, Sonne DP, Thomasen M, Ellegaard AM, Holst JJ, et al. Enterohepatic, gluco-metabolic, and gut microbial characterization of individuals with bile acid malabsorption. Gastro Hep Adv 2022;1(3):299-312. [PMID: 39131668 DOI: 10.1016/j.gastha.2021.12.007]
- Wagle SR, Kovacevic B, Walker D, Ionescu CM, Jones M, et al. Pharmacological and advanced cell respiration effects, enhanced by toxic human-bile nano-pharmaceuticals of probucol cell-targeting formulations. Pharmaceutics 2020;12(8):708. [PMID: 32751051 DOI: 10.3390/pharmaceutics12080708]
- Okamoto T, Shimada T, Matsumura C, Minoshima H, Ban T, et al. New approach to drug discovery of a safe mitochondrial uncoupler: OPC-163493. ACS Omega 2021;6(26):16980-8. [PMID: 34250356 DOI: 10.1021/acsomega.1c01993]
- Ramesh T. Oxidative stress and hepatocellular mitochondrial dysfunction attenuated by Asiatic acid in streptozotocin-induced diabetic rats. J King Saud Univ Sci 2021;33(3):101369. [DOI: 10.1016/j.jksus.2021.101369]
- Xiong Y, He YL, Li XM, Nie F, Zhou XK. Endogenous asymmetric dimethylarginine accumulation precipitates the cardiac and mitochondrial dysfunctions in type 1 diabetic rats. Eur J Pharmacol 2021;902:174081. [PMID: 33901463 DOI: 10.1016/j.ejphar.2021.174081]
- Dorighello GG, Rovani JC, Paim BA, Rentz T, Assis LH, et al. Mild mitochondrial uncoupling decreases experimental atherosclerosis, a proof of concept. J Atheroscler Thromb 2022;29(6):825-38. [PMID: 34092712 DOI: 10.5551/jat.62796]
- Lu H, Buchan RJ, Cook SA. MicroRNA-223 regulates Glut4 expression and cardiomyocyte glucose metabolism. Cardiovasc Res 2010;86(3):410-20. [PMID: 20080987 DOI: 10.1093/cvr/cvq010]
- Nikzamir A, Palangi A, Kheirollaha A, Tabar H, Malakaskar A, et al. Expression of glucose transporter 4 (GLUT4) is increased by cinnamaldehyde in C2C12 mouse muscle cells. Iran Red Crescent Med J 2014;16(2):e13426. [PMID: 24719730 DOI: 10.5812/ircmj.13426]
- Atkinson BJ, Griesel BA, King CD, Josey MA, Olson AL. Moderate GLUT4 overexpression improves insulin sensitivity and fasting triglyceridemia in high-fat diet-fed transgenic mice. Diabetes 2013;62(7):2249-58. [PMID: 23474483 DOI: 10.2337/db12-1146]
- Dehghan F, Hajiaghaalipour F, Yusof A, Muniandy S, Hosseini SA, et al. Saffron with resistance exercise improves diabetic parameters through the GLUT4/AMPK pathway in-vitro and in-vivo. Sci Rep 2016;6(1):25139. [PMID: 27122001 DOI: 10.1038/srep25139]
- Zheng S, Rollet M, Pan YX. Protein restriction during gestation alters histone modifications at the glucose transporter 4 (GLUT4) promoter region and induces GLUT4 expression in skeletal muscle of female rat offspring. J Nutr Biochem 2012;23(9):1064-71. [PMID: 22079207 DOI: 10.1016/j.jnutbio.2011.05.013]
- McCoull W, Addie MS, Birch AM, Birtles S, Buckett LK, et al. Identification, optimisation and in vivo evaluation of oxadiazole DGAT-1 inhibitors for the treatment of obesity and diabetes. Bioorg Med Chem Lett 2012;22(12):3873-8. [PMID: 22608962 DOI: 10.1016/j.bmcl.2012.04.117]
- Meyers CD, Tremblay K, Amer A, Chen J, Jiang L, et al. Effect of the DGAT1 inhibitor pradigastat on triglyceride and apoB48 levels in patients with familial chylomicronemia syndrome. Lipids Health Dis 2015;14(1):8. [PMID: 25889044 DOI: 10.1186/s12944-015-0006-5]
- Shih CC, Ciou JL, Lin CH, Wu JB, Ho HY. Cell suspension culture of Eriobotrya japonica regulates the diabetic and hyperlipidemic signs of high-fat-fed mice. Molecules 2013;18(3):2726-53. [PMID: 23455665 DOI: 10.3390/molecules18032726]
- Xiao C, Dash S, Morgantini C, Hegele RA, Lewis GF. Pharmacological targeting of the atherogenic dyslipidemia complex: the next frontier in CVD prevention beyond lowering LDL cholesterol. Diabetes 2016;65(7):1767-78. [PMID: 27329952 DOI: 10.2337/db16-0046]
- Banerji MA, Dunn JD. Impact of glycemic control on healthcare resource utilization and costs of type 2 diabetes: current and future pharmacologic approaches to improving outcomes. Am Health Drug Benefits 2013;6(7):382. [PMID: 24991370]
- NamKoong C, Kim MS, Jang BT, Lee YH, Cho YM, et al. Central administration of GLP-1 and GIP decreases feeding in mice. Biochem Biophys Res Commun 2017;490(2):247-52. [PMID: 28610922 DOI: 10.1016/j.bbrc.2017.06.031]
- Jiang N, Jing L, Li Q, Su S, Yang Q, et al. Design of novel Xenopus GLP-1-based dual glucagon-like peptide 1 (GLP-1)/glucagon receptor agonists. Eur J Med Chem 2021;212:113118. [PMID: 33422984 DOI: 10.1016/j.ejmech.2020.113118]
- Willard FS, Bueno AB, Sloop KW. Small molecule drug discovery at the glucagon-like peptide-1 receptor. Exp Diabetes Res 2012;2012:709893. [PMID: 22611375 DOI: 10.1155/2012/709893]
- Cao Y, Hölscher C, Hu MM, Wang T, Zhao F, et al. DA5-CH, a novel GLP-1/GIP dual agonist, effectively ameliorates the cognitive impairments and pathology in the APP/PS1 mouse model of Alzheimer’s disease. Eur J Pharmacol 2018;827:215-26. [PMID: 29551659 DOI: 10.1016/j.ejphar.2018.03.024]
- Mirzaei F, Khodadadi I, Majdoub N, Vafaei SA, Tayebinia H, et al. Role of glucagon-like peptide-1 (GLP-1) agonists in the management of diabetic patients with or without COVID-19. Open Med Chem J 2022;16(1):e187410452212130. [DOI: 10.2174/18741045-v16-e2212130]
- Jones AB. Peroxisome proliferator-activated receptor (PPAR) modulators: diabetes and beyond. Med Res Rev 2001;21(6):540-52. [PMID: 11607934 DOI: 10.1002/med.1025]
- Ram VJ. Therapeutic significance of peroxisome proliferator-activated receptor modulators in diabetes. Drugs Today (Barc) 2003;39(8):609-32. [PMID: 14566384 DOI: 10.1358/dot.2003.39.8.799408]
- Patsouris D, Müller M, Kersten S. Peroxisome proliferator activated receptor ligands for the treatment of insulin resistance. Curr Opin Investig Drugs 2004;5(10):1045-50. [PMID: 15535425]
- Moore-Carrasco R, Poblete Bustamante M, Gonzalez Guerra O, Leiva Madariaga E, Mujica Escudero V, et al. Peroxisome proliferator-activated receptors: targets for the treatment of metabolic illnesses (Review). Mol Med Rep 2008;1(3):317-24. [PMID: 21479412]
- Wagner KD, Wagner N. Peroxisome proliferator-activated receptor beta/delta (PPARβ/δ) acts as regulator of metabolism linked to multiple cellular functions. Pharmacol Ther 2010;125(3):423-35. [PMID: 20026355 DOI: 10.1016/j.pharmthera.2009.12.001]
- Kim HW, Lee JE, Cha JJ, Hyun YY, Kim JE, et al. Fibroblast growth factor 21 improves insulin resistance and ameliorates renal injury in db/db mice. Endocrinology 2013;154(9):3366-76. [PMID: 23825123 DOI: 10.1210/en.2012-2276]
- Schwenk BM, Hartmann H, Serdaroglu A, Schludi MH, Hornburg D, et al. TDP-43 loss of function inhibits endosomal trafficking and alters trophic signaling in neurons. EMBO J 2016;35(21):2350-70. [PMID: 27621269 DOI: 10.15252/embj.201694221]
- Kim HW, Lee JE, Cha JJ, Hyun YY, Kim JE, et al. Fibroblast growth factor 21 improves insulin resistance and ameliorates renal injury in db/db mice. Endocrinology 2013;154(9):3366-76. [PMID: 23825123 DOI: 10.1210/en.2012-2276]
- Arifa I, Aditsania A, Kurniawan I. The implementation of genetic algorithm-ensemble learning on QSAR study of diacylglycerol acyltransferase-1 (DGAT1) inhibitors as anti-diabetes. In: The International Conference on Data Science and Emerging Technologies. Singapore: Springer Nature Singapore; 2022. pp. 282-92.
- Kellerer M, Kaltoft MS, Lawson J, Nielsen LL, Strojek K, et al. Effect of once-weekly semaglutide versus thrice-daily insulin aspart, both as add-on to metformin and optimized insulin glargine treatment in participants with type 2 diabetes (SUSTAIN 11): a randomized, open-label, multinational, phase 3b trial. Diabetes Obes Metab 2022;24(9):1788-99. [PMID: 35546450 DOI: 10.1111/dom.14765]
- Heller SR, DeVries JH, Wysham C, Hansen CT, Hansen MV, et al. Lower rates of hypoglycaemia in older individuals with type 2 diabetes using insulin degludec versus insulin glargine U100: results from SWITCH 2. Diabetes Obes Metab 2019;21(7):1634-41. [PMID: 30891886 DOI: 10.1111/dom.13708]
- Yuan X, Guo X, Zhang J, Dong X, Lu Y, et al. Improved glycaemic control and weight benefit with iGlarLixi versus insulin glargine 100 U/mL in Chinese people with type 2 diabetes advancing their therapy from basal insulin plus oral antihyperglycaemic drugs: results from the LixiLan-L-CN randomized controlled trial. Diabetes Obes Metab 2022;24(11):2182-91. [PMID: 35762489 DOI: 10.1111/dom.14803]
- Battelino T, Danne T, Edelman SV, Choudhary P, Renard E, et al. C ontinuous glucose monitoring-based time-in-range using insulin glargine 300 units/ml versus insulin degludec 100 units/ml in type 1 diabetes: the head-to-head randomized controlled InRange trial. Diabetes Obes Metab 2023;25(2):545-55. [PMID: 36263928 DOI: 10.1111/dom.14898]
- Chou CA, Chuang SF. Evaluation of the efficacy of low-dose liraglutide in weight control among Taiwanese non-diabetes patients. J Diabetes Investig 2020;11(6):1524-31. [PMID: 32506681 DOI: 10.1111/jdi.13314]
- Ramadan NM, Malek HA, Abd-El Rahman K, El-Kholy E, Shaalan D, et al. Liraglutide effect on ventricular transient outward K+ channel and Connexin-43 protein expression. Exp Clin Endocrinol Diabetes 2021;129(12):899-907. [PMID: 32559789 DOI: 10.1055/a-1162-8196]
- Kahal H, Kilpatrick E, Rigby A, Coady A, Atkin S. The effects of treatment with liraglutide on quality of life and depression in young obese women with PCOS and controls. Gynecol Endocrinol 2019;35(2):142-5. [PMID: 30599799 DOI: 10.1080/09513590.2018.1505848]
- Yu DN, Wang LJ, Cheng B, Li M, Pan Q, et al. [The effects of liraglutide on body composition and muscle strength in adult obese patients with type 2 diabetes mellitus]. Zhonghua Nei Ke Za Zhi 2021;60(11):982-6. [PMID: 34689519 DOI: 10.3760/cma.j.cn112138-20210205-00105]
- Kochar IS, Sethi A. Efficacy and safety of liraglutide in Indian adolescents with obesity. Obes Sci Pract 2019;5(3):251-7. [PMID: 31275599 DOI: 10.1002/osp4.328]
- Sedky AA. Improvement of cognitive function, glucose and lipid homeostasis and serum osteocalcin levels by liraglutide in diabetic rats. Fundam Clin Pharmacol 2021;35(6):989-1003. [PMID: 33683755 DOI: 10.1111/fcp.12664]
- Omar M, Jensen J, Ali M, Frederiksen PH, Kistorp C, et al. Associations of empagliflozin with left ventricular volumes, mass, and function in patients with heart failure and reduced ejection fraction: a substudy of the empire HF randomized clinical trial. JAMA Cardiol 2021;6(7):836-40. [PMID: 33404637 DOI: 10.1001/jamacardio.2020.6827]
- Ku EJ, Lee DH, Jeon HJ, Oh TK. Long-term effectiveness and safety of quadruple combination therapy with empagliflozin versus dapagliflozin in patients with type 2 diabetes: 3-year prospective observational study. Diabetes Res Clin Pract 2021;182:109123. [PMID: 34740742 DOI: 10.1016/j.diabres.2021.109123]
- Pasqua MR, Jafar A, Kobayati A, Tsoukas MA, Haidar A. Low-dose empagliflozin as adjunct to hybrid closed-loop insulin therapy in adults with suboptimally controlled type 1 diabetes: a randomized crossover controlled trial. Diabetes Care 2023;46(1):165-72. [PMID: 36331522 DOI: 10.2337/dc22-0490]
- Mason T, Coelho-Filho OR, Verma S, Chowdhury B, Zuo F, et al. Empagliflozin reduces myocardial extracellular volume in patients with type 2 diabetes and coronary artery disease. JACC Cardiovasc Imaging 2021;14(6):1164-73. [PMID: 33454272 DOI: 10.1016/j.jcmg.2020.10.017]
- Verzijl CR, Van De Peppel IP, Struik D, Jonker JW. Pegbelfermin (BMS-986036): an investigational PEGylated fibroblast growth factor 21 analogue for the treatment of nonalcoholic steatohepatitis. Expert Opin Investig Drugs 2020;29(2):125-33. [PMID: 31899984 DOI: 10.1080/13543784.2020.1708898]
- Brown EA, Minnich A, Sanyal AJ, Loomba R, Du S, et al. Effect of pegbelfermin on NASH and fibrosis-related biomarkers and correlation with histological response in the FALCON 1 trial. JHEP Rep 2023;5(4):100661. [PMID: 36866389 DOI: 10.1016/j.jhepr.2022.100661]
- Charles ED, Neuschwander-Tetri BA, Pablo Frias J, Kundu S, Luo Y, et al. Pegbelfermin (BMS-986036), PEGylated FGF21, in patients with obesity and type 2 diabetes: results from a randomized phase 2 study. Obesity (Silver Spring) 2019;27(1):41-9. [PMID: 30520566 DOI: 10.1002/oby.22344]
- Thompson KE, Guillot M, Graziano MJ, Mangipudy RS, Chadwick KD. Pegbelfermin, a PEGylated FGF21 analogue, has pharmacology without bone toxicity after 1-year dosing in skeletally-mature monkeys. Toxicol Appl Pharmacol 2021;428:115673. [PMID: 34364948 DOI: 10.1016/j.taap.2021.115673]
- Patwekar M, Patwekar F, Mezni A, Sanaullah S, Fatema SR, et al. Assessment of antioxidative and alpha-amylase potential of polyherbal extract. Evid Based Complement Alternat Med 2022;2022:7153526. [PMID: 35685725 DOI: 10.1155/2022/7153526]
- Ma T, Tian X, Zhang B, Li M, Wang Y, et al. Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature 2022;603(7899):159-65. [PMID: 35197629 DOI: 10.1038/s41586-022-04431-8]
- Quazi A, Mohsina FP, Faheem IP, Priya S. In silico ADMET analysis, molecular docking and in vivo anti diabetic activity of polyherbal tea bag formulation in Streptozotocin-nicotinamide induced diabetic rats. Int J Health Sci 2022;6:343-72. [DOI: 10.53730/ijhs.v6nS3.5189]
- Szymczak-Pajor I, Wenclewska S, Śliwińska A. Metabolic action of metformin. Pharmaceuticals (Basel) 2022;15(7):810. [PMID: 35890109 DOI: 10.3390/ph15070810]
- Mohsina FP, Faheem IP, Priya S, Husain SM. Evaluation OF anti diabetic activity OF ichnocarpus frutescens L. Int J Adv Pharm Biotechnol 2018;4:1-2. [DOI: 10.38111/ijapb.20180402001]
- Bramante CT, Huling JD, Tignanelli CJ, Buse JB, Liebovitz DM, et al. Randomized trial of metformin, ivermectin, and fluvoxamine for Covid-19. N Engl J Med 2022;387(7):599-610. [PMID: 36070710 DOI: 10.1056/NEJMoa2201662]
- Faheem IP, Gopalakrishna B, Mohsina FP, Priya S. Antidiabetic potential of ethanolic leaf extract of Crataeva magna in streptozotocin-induced diabetic model. Innov Pharmaceutical Pharm 2021;9:1-7.
- Ameen O, Samaka RM, Abo-Elsoud RA. Metformin alleviates neurocognitive impairment in aging via activation of AMPK/BDNF/PI3K pathway. Sci Rep 2022;12(1):17084. [PMID: 36224264 DOI: 10.1038/s41598-022-20945-7]
- Faheem IP, Gopalakrishana B, Mohsina FP, Ahmad N. Antidiabetic activity OF FICUS dalhousiae miq leaves ethanolic extract ON streptozotocin induced diabtes IN wistar albino rats. World J Pharm Res 2020;10(1):1258.
- Goodwin PJ, Chen BE, Gelmon KA, Whelan TJ, Ennis M, et al. Effect of metformin vs placebo on invasive disease-free survival in patients with breast cancer: the MA.32 randomized clinical trial. JAMA 2022;327(20):1963-73. [PMID: 35608580 DOI: 10.1001/jama.2022.6147]
- Quazi A, Patwekar M, Patwekar F, Mezni A, Ahmad I, et al. Evaluation of wound healing activity (excision wound model) of ointment prepared from infusion extract of polyherbal tea bag formulation in diabetes-induced rats. Evid Based Complement Alternat Med 2022;2022:1372199. [PMID: 35707477 DOI: 10.1155/2022/1372199]
- Hasanvand A. The role of AMPK-dependent pathways in cellular and molecular mechanisms of metformin: a new perspective for treatment and prevention of diseases. Inflammopharmacology 2022;30(3):775-88. [PMID: 35419709 DOI: 10.1007/s10787-022-00980-6]
- Goel S, Singh R, Singh V, Singh H, Kumari P, et al. Metformin: activation of 5′ AMP-activated protein kinase and its emerging potential beyond anti-hyperglycemic action. Front Genet 2022;13:1022739. [PMID: 36386794 DOI: 10.3389/fgene.2022.1022739]
- Di Folco U, Vallecorsa N, Nardone MR, Pantano AL, Tubili C. Effects of semaglutide on cardiovascular risk factors and eating behaviors in type 2 diabetes. Acta Diabetol 2022;59(10):1287-94. [PMID: 35842847 DOI: 10.1007/s00592-022-01936-6]
- Stretton B, Kovoor J, Bacchi S, Chang S, Ngoi B, et al. Weight loss with subcutaneous semaglutide versus other glucagon-like peptide 1 receptor agonists in type 2 diabetes: a systematic review. Intern Med J 2023;53(8):1311-20. [PMID: 37189293 DOI: 10.1111/imj.16126]
- Ahmed NR, Kulkarni VV, Pokhrel S, Akram H, Abdelgadir A, et al. Comparing the efficacy and safety of obeticholic acid and semaglutide in patients with non-alcoholic fatty liver disease: a systematic review. Cureus 2022;14(5):e24829. [PMID: 35693370 DOI: 10.7759/cureus.24829]
- Liu SY, Huang CC, Yang YY, Huang SF, Lee TY, et al. Obeticholic acid treatment ameliorates the cardiac dysfunction in NASH mice. PLoS One 2022;17(12):e0276717. [PMID: 36490253 DOI: 10.1371/journal.pone.0276717]
- Inoue Y, Wada Y, Sato M, Sato S, Okamoto T, et al. Carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone-induced toxicities in rats: comparative study with other mitochondrial uncouplers (2,4-dinitrophenol, OPC-163493 and tolcapone). Toxicol Res 2023;39(4):611-23. [PMID: 37779591 DOI: 10.1007/s43188-023-00189-x]
- Tingle SJ, Thompson ER, Bates L, Ibrahim IK, Govaere O, et al. Pharmacological testing of therapeutics using normothermic machine perfusion: a pilot study of 2,4-dinitrophenol delivery to steatotic human livers. Artif Organs 2022;46(11):2201-14. [PMID: 35546070 DOI: 10.1111/aor.14309]
- Müller TD, Blüher M, Tschöp MH, DiMarchi RD. Anti-obesity drug discovery: advances and challenges. Nat Rev Drug Discov 2022;21(3):201-23. [PMID: 34815532 DOI: 10.1038/s41573-021-00337-8]
- Zhang Z, Zhou D, Luan X, Wang X, Zhu Z, et al. Biodegradable hollow nanoscavengers restore liver functions to reverse insulin resistance in type 2 diabetes. ACS Nano 2023;17(10):9313-25. [PMID: 37155357 DOI: 10.1021/acsnano.3c00875]
- Halim SA, Lodhi HW, Waqas M, Khalid A, Abdalla AN, et al. Targeting α-amylase enzyme through multi-fold structure-based virtual screening and molecular dynamic simulation. J Biomol Struct Dyn 2024;42(11):5617-30. [PMID: 37378513 DOI: 10.1080/07391102.2023.2227721]
- Jones-Tabah J. Targeting G protein-coupled receptors in the treatment of Parkinson’s disease. J Mol Biol 2023;435(12):167927. [PMID: 36563742 DOI: 10.1016/j.jmb.2022.167927]
- Zhang D, Zhang Y, Sun B. The molecular mechanisms of liver fibrosis and its potential therapy in application. Int J Mol Sci 2022;23(20):12572. [PMID: 36293428 DOI: 10.3390/ijms232012572]
- Ikegami H, Hiromine Y, Noso S. Insulin-dependent diabetes mellitus in older adults: current status and future prospects. Geriatr Gerontol Int 2022;22(8):549-53. [PMID: 35711119 DOI: 10.1111/ggi.14414]
- Yin W, Zhang Z, Xiao Z, Li X, Luo S, et al. Circular RNAs in diabetes and its complications: current knowledge and future prospects. Front Genet 2022;13:1006307. [PMID: 36386812 DOI: 10.3389/fgene.2022.1006307]