Ethnomedical Potentials, Phytochemicals, and Medicinal Profile of Alpinia galanga L.: A Comprehensive Review
1Department of Pharmaceutical Science, Faculty of Pharmacy, Universitas Airlangga, Surabaya, Indonesia
2Department of Biology, Faculty of Science and Technology, Universitas Airlangga, Surabaya, Indonesia
3Virtual Research Center for Bioinformatics and Biotechnology, Surabaya, Indonesia
4Division of Research and Development, Jalan Tengah, Surabaya, Indonesia
5Postgraduate School, Universitas Airlangga, Surabaya, Indonesia
6Uttaranchal Institute of Pharmaceutical Sciences, Uttaranchal University, Dehradun, India
7Department of Food Safety and Quality, Almaty Technological University, Almaty, Republic of Kazakhstan
8Department of Pharmaceutical Technology, S. D. Asfendiyarov Kazakh National Medical University, Almaty, Republic of Kazakhstan
9Department of Scientific Research, V. M. Gorbatov Federal Research Center for Food Systems, Moscow, Russia
10Faculty of Biotechnology and Food Engineering, Ural State Agrarian University, Yekaterinburg, Russia
11Faculty of Medicine, Universitas Wijaya Kusuma Surabaya, Surabaya, Indonesia
12Faculty of Medicine, Universitas Pembangunan Nasional “Veteran” Jawa Timur, Surabaya, Indonesia
*Correspondence to: Arif Nur Muhammad Ansori, E-mail: ansori.anm@gmail.com; Hery Purnobasuki, E-mail: hery-p@fst.unair.ac.id
Received: June 7 2024; Revised: June 22 2024; Accepted: July 4 2024; Published Online: July 24 2024
Cite this paper:
Priyono QAP, Yusniasari PA, Alifiansyah MRT et al. Ethnomedical Potentials, Phytochemicals, and Medicinal Profile of Alpinia galanga L.: A Comprehensive Review. BIO Integration 2024; 5: 1–9.
DOI: 10.15212/bioi-2024-0032. Available at: https://bio-integration.org/
Download citation
© 2024 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
Exploration of the utilization of plant-based natural materials as raw materials for medicines is still being carried out today. Various secondary metabolite compounds from plants have been found in recent decades, one of which is Alpinia galanga L. (greater galangal). The world community has long recognized the Alpinia galanga L. plant as a raw material for traditional medicinal herbs that can help cure several diseases, such as ulcers, headaches, rheumatism, migraines, and diabetes mellitus. Knowledge of the potential of medicinal ingredients from derivatives of metabolite compounds in Alpinia galanga L. has continued in this modern era by researchers through the science of herbal medicine. Researchers have found that all parts of this plant, including leaves, roots, stems, flowers, and rhizomes, have secondary metabolite compounds that have the potential to be developed for medicine. One part that contains an abundance of secondary metabolites, such as 1,8-cineole, α-fenchyl acetate, β-farnesene, β-bisabolene, α-bergamotene, β-pinene, 1′-acetoxychavicol acetate (ACE), galangin, phenylpropanoid, and β-sitosterol diglucoside (AG-7), is the rhizome. Research related to the bioactivity test of these secondary metabolite compounds is still being conducted by researchers to reveal other amazing potentials of Alpinia galanga L. Therefore, this review article provides information related to the ethnomedicinal profile, phytochemicals, and various medical potentials of Alpinia galanga L.
Keywords
Alpinia galanga L., botany, ethnomedicine, medicine, pharmacology.
Introduction
The rapid development of pharmaceutical science has accelerated the discovery of various bioactive compounds. In addition, the high biodiversity of flora and fauna throughout Indonesia supports the development of natural ingredient-based medicine. Indonesia has approximately 40,000 endemic plants spread from the west-to-east of the country. Of these endemic plants, 6000 have been identified as medicinal plants that have been shown to have medical properties for healing degenerative and non-degenerative diseases. Secondary metabolite compounds present in the leaves, roots, stems, and flowers of medicinal plants make the compounds useful for curing various diseases [1]. Research on the sustainable utilization of medicinal materials from medicinal plants is needed to reveal the potential bioactivity to support the development of science.
One of the medicinal plants that has been widely known for treatment is Alpinia galanga L., which comes from the Zingiberaceae family. Alpinia galanga L. is a perennial monocotyledonous plant that is also known as “greater galangal” [2]. Morphologically, the Alpinia galanga L. plant has a rhizoma part that has a distinctive aroma and has many proven properties for medicinal raw materials. The leaves, stems, and roots of this plant have various bioactive compounds to treat diseases [3, 4]. Alpinia galanga L. is widely distributed in the Asian region and is widely cultivated in Indonesia, Malaysia, Thailand, India, and China [5]. This plant thrives in open areas with full sunlight and shade, such as forests and home yards [6].
Ethnomedicinally, Asian people know Alpinia galanga L. as a raw material for the treatment of various diseases, including digestive tract diseases, heart problems, lumbago, rheumatic pain, inflammatory conditions, ulcers, and as a neutralizing sour and spicy taste for chest pain [7–9]. In addition, contemporary research has proven that Alpinia galanga extract is able to treat various diseases based on in vitro and in vivo tests. The diseases that have been proven to be treated by Alpinia galanga L. include back pain, sore throat, rheumatic pain, tuberculosis, gland disorders, diabetes, chest pain, kidney disease, bronchitis, cataracts, and infections. In addition, serving Alpinia galanga L. in the form of a brewed beverage can minimize the occurrence of heart attacks, angina, and gallstones [10–14]. These various medicinal effects can occur because this plant is rich in phytochemical compounds (terpene, phenolic, and alkaloid groups). This manuscript focuses on reviewing the richness of phytochemical compounds and the pharmacologic effects for treatment. All parts of Alpinia galanga L. possess the functional phytochemical constituents of value for the drug material. Thus, this is important and essential information about drug discovery.
Plant description
Alpinia galanga L. has a root with rhizome and has a distinctive aromatic odor. The leaves of the Alpinia galanga L. plant are oblong lanceolate with a sharp tip and green in color on the adaxial and abaxial parts. The flowers of the Alpinia galanga L. plant are green-white with dense flowers and have a panicle length of ±30 cm. The bractea is ovate-lanceolate with irregular 3-toothed tube petals. The corolla is oval with a wide ellipse and a red color. The fruit of Alpinia galanga L. is small and orange-red in color [15–17].
Taxonomy
The taxonomic order of Alpinia galanga L. is as follows:
Kingdom: Plantae
Division: Magnoliophyta
Class: Liliopsida
Subclass: Zingiberidae
Order: Zingiberales
Family: Zingiberaceae
Subfamily: Alpinioideae
Tribe: Alpinieae
Genus: Alpinia
Species: Alpinia galanga L. [18].
Ethnomedicine uses
People around the world, especially Asia, have long used Alpinia galanga L. as a useful herbal concoction to treat various diseases. The use of medicinal plants as traditional medicines is one of the many world heritages in Indonesia [19, 20]. Ethnomedicine research conducted by Rahmadini et al. (2022) on herbal plants in the Pandeglang-Banten region revealed that the herbal rhizome, Alpinia galanga L. (local name, “Laja Goah”) has the potential for development into an anti-ulcer drug [21]. In addition, ancient Indians have long cultivated Alpinia galanga L. for anti-diabetic, anti-fungal, and anti-inflammatory ingredients [22]. Traditional healers in South India often use Alpinia galanga L. rhizomes as a mixture of herbs to treat diabetes mellitus [23]. The leaves, stems, and roots are also widely used by civilizations in China, India, Thailand, Java, and various other regions in Asia to treat non-degenerative diseases [24–26]. The harvest time tends to be faster with abundant crops. In addition, great properties make Alpinia galanga L. a favorite plant for making traditional potions [27]. The flowers and young stems of this plant are also used to add flavor and aroma to food [28].
Ecologic distribution
The habitat of Alpinia galanga L. is in forest areas, thickets, and sunny open places exposed to regular sunlight [22]. The plant is widespread in Asia and is widely used for food and herbal ingredients. Alpinia galanga L. is found growing wild in the Western ghats, Mysore, Goa, Malabar, and Gujarat. However, Alpinia galanga L. is cultivated in abundance to fulfil the demand for medicinal raw materials and consumption in China, Thailand, Malaysia, and Indonesia [29]. Geographically, Alpinia galanga L. is also found in the Arab peninsula, Egypt, and Sri Lanka [30].
Phytochemical compound
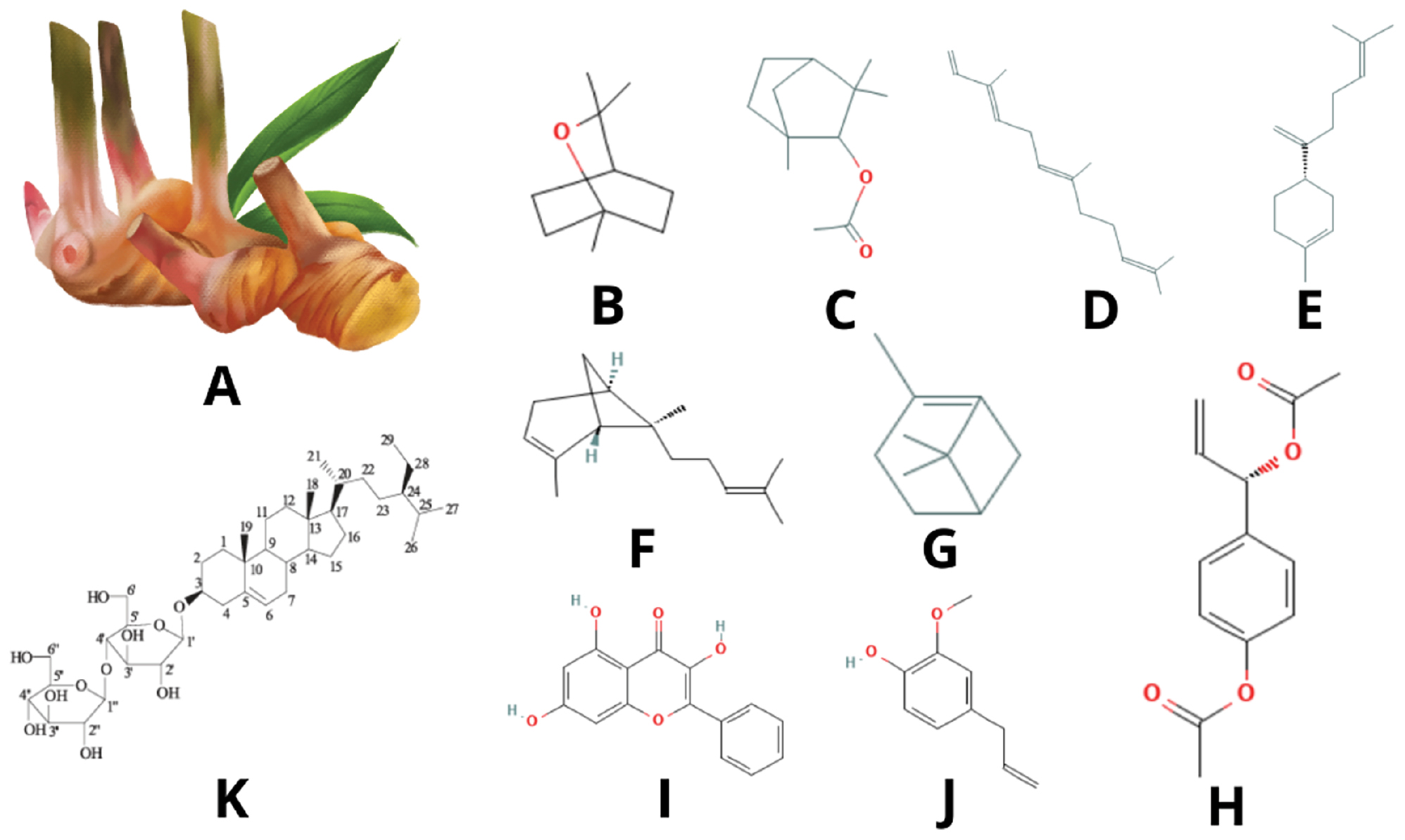
Alpinia galanga L. has many phytochemical constituent compounds that can potentially be utilized for treatment. Abdullah et al. (2015) reported that the dominant bioactive compounds in Alpinia galanga L. plants include 1,8-cineole (44.2–61.7%), β-farnesene (7.0–14.6%), β-bisabolene (0.1–0.8%), trans-α-bergamotene (0.1–0.3%), and β-pinene (0.3–0.9%) (Figure 1) [31, 32]. The compound, 1,8-cineole, is often reported to be a biomarker component of Alpinia galanga L., which is very abundant in the rhizome. In addition, investigation of phytochemical profiles in Alpinia galanga L. also revealed that there are other compounds that make up the rhizome of this plant, including galangin, phenylpropanoids, and β-sitosterol diglucoside (AG-7; Table 1) [31, 33–35].
Figure 1 Phytochemical structure of Alpinia galanga L.: (A) Alpinia galanga plant; (B) 1,8-cineole; (C) α-fenchyl acetate; (D) β-farnesene; (E) β-bisabolene; (F) α-bergamotene; (G) β-pinene; (H) 1′-acetoxychavicol acetate (ACE); (I) galangin; (J) phenylpropanoid; (K) β-sitosterol diglucoside (AG-7).
Table 1 Antioxidant Activity from Flos of Alpinia galanga Essential Oil Compared with BHT and Ascorbic Acid
| Samples | Antioxidant Activity (IC50 μg/mL) | |
|---|---|---|
| DPPH | ABTS | |
| Essential oil | 138.62 ± 3.07 | 40.48 ± 0.49 |
| BHT [2] | 14.16 ± 0.30 | 1.99 ± 0.05 |
| Ascorbic acid [2] | 0.52 ± 0.01 | 1.05 ± 0.02 |
Anti-microbial activity
The essential oil in Alpinia galanga rhizomes is known to have anti-microbial activity against several bacteria and fungi [36]. A previous study succeeded in identifying 26 chemical compounds representing 91.42% of the total components obtained from Alpinia galanga rhizomes [37]. Several chemical compounds in Alpinia galanga rhizomes, such as 1,8 cineole and methyl eugenol, have been reported to have anti-bacterial and anti-fungal activity. Essential oils have anti-microbial activity by damaging the cytoplasmic membrane of microorganisms, which causes disruption of the cell membrane structure and increases cell membrane permeability [38].
Alpinia galanga extract is known to inhibit the growth of Staphylococcus aureus. Research conducted by Oonmetta-aree et al. (2006) confirmed that the inhibitory zone of Alpinia galanga extract against S. aureus bacteria is greater than turmeric and ginger extracts. In addition, it was shown that Alpinia galanga extract inhibits the growth of S. epidermidis and S. cerevisiae. In the same study, analysis of the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) was also performed and the results showed that the MIC of Alpinia galanga extract is at a concentration of 0.325 mg/mL, while the MBC is at a concentration of 1.3 mg/mL [39].
Antioxidant activity
Alpinia galanga rhizomes contain several phenolic components, including galangin, 1,8-cineole, galangal acetate, and kaempferol, which act as antioxidants. These components have been shown to benefit human health by preventing oxidative stress via the removal of free radicals [15]. Phenolic compounds are the key antioxidants because of the compound structure, which includes −OH groups, making phenolic compounds reactive and able to bind to free radical molecules. These compounds function as antioxidants through three mechanisms: donating hydrogen; transferring electrons; and forming chelates [40]. Eugenol, as a class of phenolic compounds found in Alpinia galanga, is known to have strong antioxidant activity based on in vivo and in vitro studies. Moreover, the eugenol derivative, aceteugenol, is known to have an IC50 value 0.12 ± 0.03 μmol/L so that this compound can neutralize free radicals.
DPPH and ABTS tests were used to assess the antioxidant activity of Alpinia galanga flower essential oils, which revealed moderate scavenging effects compared to standard antioxidants. Aceteugenol, isoeugenol, and methyleugenol are derivatives of eugenol and contribute to the oil antioxidant properties. Other compounds in the oil exhibit moderate-to-weak antioxidant effects (Table 1) [41]. There are severl factors that impact the antioxidant effect in the body, one of which is chemical exposure to cadmium and lead acetate [42, 43]. To maintain the antioxidant effect, the body must be healthy and avoid stress level.
Anti-cancer activity
Ahlina et al. (2020) reported that Alpinia galanga L. rhizome extract dissolved in 96% ethanol solvent stimulates the cell cycle associated with aging induction due to increased levels of reactive oxygen species (ROS) in 4T1 breast cancer cells as a response related to increased doxorubicin cells. The IC50 value in this study from Alpinia galanga L. was 135 μg/mL [44]. Another anti-cancer study conducted by Muangnoi et al. (2007) showed that Alpinia galanga L. rhizome extract dissolved in distilled water produced an IC50 value of 100 μg/mL with a target that weakened A549 lung cancer cells, CRL2522 fibroblasts, and MCF-12A breast cancer cells. In addition, the rhizome extract strengthened and maintain normal cells (CRL2321 and CRL2335) so as not to mutate into malignant cancer cells. The mechanism of action involved maintaining the normal threshold of toxicity in the cells [45].
Manse et al. (2016) conducted a study involving B16 skin cancer cells with acetone solvent and showed that Alpinia galanga L. inhibits the mechanism of melanogenesis in tyrosinase with an IC50 value of 7.3 μg/mL [46]. The combination of Alpinia galanga L. and P. emblica rhizome extracts dissolved in ethanol solvent has a hypolipidemic effect with increased gene expression of LDLR, ApoA1, and SR-B1. This combination of rhizome extracts can indirectly inhibit the development of HepG2 liver tumor cells from further developing into liver cancer cells [47].
Comparative testing in vitro with Alpinia galanga L. rhizome extracts in dichloromethane, methanol, and distilled water solvents on HeLa cervical cancer cells conducted by Herrmann et al. (2011) generated a variety of IC50 value results. Various tests with different solvents gave the following results: distilled water (IC50 = 2357.3 μg/mL); dichloromethane (IC50 = 55.7 μg/mL); and methanol (IC50 = 111.7 μg/mL). Saponin and monoterpene bioactive compounds are thought to strongly interact with biomembranes, while polyphenols interact with proteins. Alkaloid compounds from Alpinia galanga L. interact directly with proteins and DNA. This test shows that bioactive compounds from Alpinia galanga L. have strong potential in inhibiting cervical cancer cell proliferation and can be used to prevent cervical cancer [48]. The discovery of low-risk novel therapeutics from natural materials is crucial. Docking experiments are needed before conducting anti-cancer research on Alpinia galanga L. as a predictive tool to prevent potential financial, time, and resource losses in further research stages [49–51].
Anti-diabetic activity
According to studies by Verma et al. (2015), which observed the in vitro anti-diabetic activity of Alpinia galanga Linn. rhizome at a dose of 60 mg/kg body weight in streptozotocin (STZ)-induced diabetic rats, the administration of Alpinia galanga Linn. methanol extract at doses of 200 and 400 mg/kg body weight in diabetic rats significantly lowered blood glucose levels (P < 0.01) compared to the diabetic control group and demonstrated similar efficacy when compared to the diabetic group treated with glibenclamide (10 mg/kg body weight). With a dose of 400 mg/kg body weight after the 4th day (43.5–64.8%) and 200 mg/kg body weight after the 15th day (30%), the greatest drop in blood glucose was recorded. In addition, animals receiving Alpinia galanga methanol extract had significantly lower serum triglycerides, total, and LDL-cholesterol levels, as well as higher HDL-cholesterol levels (P < 0.01) compared to rats with diabetes. Acute toxicity testing also revealed that at different doses of 50, 100, 200, 400, 800, and 1600 mg/kg body weight, Alpinia galanga methanol extract did not result in mortality up to 7 days following treatment. Histopathologic examinations on rats administered Alpinia galanga methanol extract similarly revealed some cell damage at low doses from STZ and alterations in cells toward normal at high doses [52].
The essential oil extracted from rhizome of Alpinia galanga has been shown to have excellent α-glucosidase inhibition ability (IC50 = 0.16 ± 0.03 mg/mL) based on in vitro research by Tian et al. (2022). The inhibitory effect was equal to the positive control, acarbose (IC50 = 0.15 ± 0.01 mg/mL; P < 0.05). Farnesene, the most prevalent component in Alpinia galanga’s essential oil, may have the ability to block α-glucosidase and supports this finding. Moreover, α-glucosidase inhibitory actions are also exhibited by other key components, such as farnesyl acetate, acetyleugenol, eugenol, and E-nerolidol [41].
Hepatoprotective activity
Alpinia galanga is known to have activity as a hepatoprotector. A study conducted by Hemabarathy et al. (2009) showed that administration of Alpinia galanga rhizome extract at a dose of 200–400 mg/kg body weight in the group that was also administered paracetamol (3000 mg/kg body weight) showed a decrease in AST and ALT activity compared to the group that was administered paracetamol alone. This finding showed that there is functional improvement in hepatocyte cells caused by massive parenchymal cell regeneration. In the same study, administration of Alpinia galanga extract to the group that was also administered paracetamol (3000 mg/kg body weight) showed a decrease in malondialdehyde (MDA), where MDA was a marker of oxidative stress in the liver compared to the group that was only administered paracetamol [53].
Anti-malarial and anti-plasmodial activities
Al-Adhroey et al. (2010) reported that the extract from Alpinia galanga roots reduced the number of malaria parasites in the early stage of malaria infection. Different doses were tested, which showed a decrease of 29%, 49%, 63%, and 65% in parasites over 4 d at doses of 50, 100, 200, and 400 mg/kg/day, respectively. This anti-malarial effect was significant compared to the control group. However, increasing the dose from 200 mg/kg to 400 mg/kg only slightly improved the effect. Parasite levels on the 6th day were 5.80, 3.40, 3.40, and 3.20 for doses of 50, 100, 200, and 400 mg/kg/day, respectively. The extract also had preventive effects, reducing parasite levels at doses of 50, 100, 200, and 400 mg/kg/day by 13%, 26%, 39%, and 52%, respectively. While the preventive effects were significant at doses of 200 and 400 mg/kg, the effect was less noticeable at 100 and 50 mg/kg. The extract showed some ability to reduce parasites with doses of 100 and 200 mg/kg being effective. Chloroquine at 20 mg/kg/day also reduced parasites, as in previous studies. The results are shown as percentages; “ns” means no significant difference (P > 0.05), while P values ≤ 0.05 are considered statistically significant. Parasite levels are shown as the mean ± SEM (n = 5) [54].
Alpinia galanga is traditionally used in parts of Peninsular Malaysia. The in vitro anti-plasmodial data from our study (EC50 <10 μg/mL) complements a previous in vitro study by Al-Adhroey et al. (2010). The methanol extract of Alpinia galanga rhizome showed significant suppressive, curative, and prophylactic activities against P. berghei infection in mice. The anti-malarial effects of the methanol extract of Alpinia galanga rhizome could be attributed to its active components like flavonoids and terpenoids [53]. Previous research has demonstrated that compounds related to terpenoids and flavonoids have anti-plasmodial activities against various strains of P. falciparum [55].
Anti-fungal activity
Test studies against Candida albicans using isolates of diterpene compounds from the rhizome of Alpinia galanga proved capable of inhibiting fungal growth significantly [29]. In addition, another study conducted by Khodavandi et al. (2013) mentioned that Alpinia galanga extract proved effective for inhibiting the growth of C. tropicalis and C. glabrata fungi. This can indirectly be used to prevent the occurrence of candidiasis in humans [56]. These various studies are strengthened by the evidence reported by Prastiyanto et al. (2021), which states that Alpinia galanga L. rhizome extract has the effectiveness and potential to be developed into an anti-fungal against C. albicans and Trihophyton rubrum [57].
Anti-allergic activity
Yasuhara et al. (2009) showed that acetoxybenzhydrols are extremely stable and active analogues of 1′S-1′-acetoxychavicol, an effective anti-allergic substance in Alpinia galanga L. Stronger inhibitory activity is shown by 1′S-1′-acetoxychavicol against antigen-induced generation of TNF-α, IL-4, and β-hexosaminidase release. Furthermore, 1′S-1′-acetoxychavicol has been shown to have significant biological properties, including xanthine oxidase inhibitory, anti-cancer, anti-inflammatory, anti-fungal, anti-oxidative, and anti-HIV properties. With respect tp type 1 allergies, 1′S-1′-acetoxychavicol exhibits greater inhibitory activity than synthetic anti-allergic medications (ketotifen fumarate). Nevertheless, 1′S-1′-acetoxychavicol has the disadvantage of being unstable, particularly in acidic environments; 1′S-1′-acetoxychavicol decomposes gradually over several months at ambient temperature, yielding a complex combination in addition to acetic acid [58].
When compared to analogue 1, the acetoxybenzhydrol methylcarboxylate analogue, 16, R2 = COOMe, shows the highest level of active stability. The present investigation looked at the potent inhibitory effects of analogues 15 and 16 on the release of β-hexosaminidase and the synthesis of TNF-α and IL-4 in RBL-2H3 cells 4 h post-treatment. Table 1 illustrates how analogues 15 and 16 had IC50 values between 11 and 28 μM, which was equivalent to the analogue 1 level of inhibition (12–17 μM) over TNF-α and IL-4 production. These results suggest that esters 15 and 16 are useful against type I allergic responses in the early and late phases. The following are the structural prerequisites of acetoxychavicol acetate analogue 1′S-1′ for an inhibitory effect against the release of β-hexosaminidase: (1) To preserve activity, the vinyl group in analogue 1 is swapped out for a phenyl group. (2) This activity requires the presence of the phenyl acetate group. (3) A smaller group is desirable for increased activity in the ester group at position 1′ [58].
Anti-inflammatory activity
According to Subash (2016), the ethanol extract of Alpinia galanga rhizome significantly reduces inflammation in rats with pleuritis generated by carrageenan. The amount of pleural exudate in the control group was 1.25 ± 0.104 mL. The volume of pleural exudate in animals administered Alpinia galanga ethanol extract at oral doses of 100, 200, and 400 mg/kg decreased to 0.96 ± 0.103, 0.62 ± 0.144, and 0.38 ± 0.12 mL, respectively. In inflammatory edema, the ethanol extract of Alpinia galanga significantly inhibits the total leukocyte input and reduction of exudation [59]. Alpinia galanga extract has been shown in another study by Cahyono et al. (2023) to activate growth factor expression on a peripheral blood mononuclear cell acute inflammation model triggered with TNF-α and to create an inflammatory milieu [60].
Anti-larvicidal, anti-molluscidal, and anti-insect activities
Nguyen et al. (2022), using essential oils from the rhizome of Alpinia galanga L., showed effectiveness to kill Aedes aegypti and Culex quinquefasciatus mosquito larvae. In addition, the use of essential oil derivatives from this plant can also be an anti-molluscidal against Gyraulus convexiusculus and Pomacea canaliculata [61]. Exploration of the utilization of trans-sinnamic acid isolate from the rhizome of Alpinia galanga L. against third-instar mortality of Aedes aegypti, C. quinquefasciatus, and Anopheles dirus B larvae showed significant results that are satisfactory for application as a biolarvicide. This finding is due to the high mortality rate of mosquito larvae after exposure to trans-sinnamic acid isolates [62]. Research on the development of repellents from Alpinia galanga L. extracts conducted by Abdullah et al. (2015) showed that rhizome extracts have toxic and anti-feeding activities against Coptotermes gestroi and C. curvignathus termites (Isoptera: Rhinotermitidae) [31].
Antiviral activity
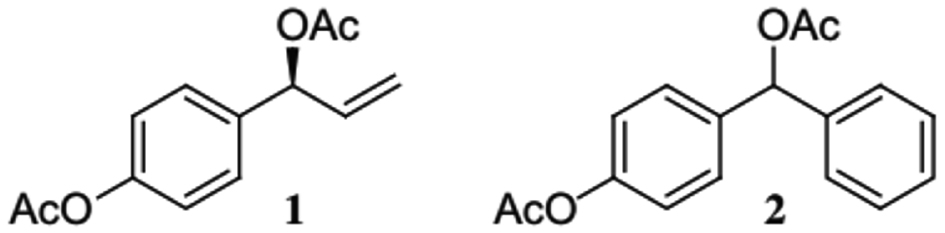
According to studies by Ye et al. (2006), 1′S-1′-acetoxychavicol acetate (ACA) (Figure 2) extracted from Alpinia galanga L. rhizome prevents the transfer of Rev, which in turn prevents the reproduction of human immunodeficiency virus type 1 (HIV-1). The Rev protein facilitates the export of mRNA that is needed to produce viral structural proteins, which is a crucial component of HIV-1 replication. One possible target for the creation of antiviral treatment is this protein. This study showed that at 4 μM, the ACA chemical may fully disrupt Rev transport in an in vitro yeast model and reduce HIV-1 reproduction in peripheral blood mononuclear cells by >80%. By obstructing Rev transport via the chromosomal region maintenance 1 (CRM1) route, ACA inhibits HIV-1 replication. Leptomycin B (LMB) and ACA compete with one another as a Rev transport inhibitor. Full-length HIV-1 RNA can accumulate to a greater extent in the nucleus and accumulate to a lesser extent in the cytoplasm when exposed to ACA, suggesting that ACA is involved in the inhibition of Rev-RNA transport from the nucleus to the cytoplasm [63].
Figure 2 1′S-1′-acetoxychavicol acetate (1) and its phenyl analogue (2).
Anti-ulcer activity
Gastric ulcer is a disease that affects the digestive system where there is a disturbance of the integrity of the gastric or duodenal mucosa. This disease occurs due to an imbalance between gastric ulcer triggering factors, such as pepsin, acid, and H. pylori, with protective factors of the gastric mucosa, such as bicarbonate, prostaglandins, and gastric mucus [64]. A study conducted by Johnley et al. (2020) states that Alpinia galanga rhizome extract reduces the ulceration index and lesions in the gastric wall of Wistar rats that have been induced by indomethacin as a trigger for gastric ulcers [65]. In addition, research conducted by Matsuda et al. (2003) showed that acetone extract of Alpinia galanga rhizome has the ability to reduce the occurrence of lesions on the gastric wall, which is more potent than some synthetic anti-ulcer drugs, such as cimetidine and omeprazole. Some phenylpropanoid group isolates, such as 1′S-1′-acetoxychavicol acetate and 1′S-1′-acetoxyeugenol acetate, are known to have a very significant role in inhibiting the occurrence of gastric lesions [66].
Anti-depressant activity
Certain components of Alpinia galanga L. essential oil, such as eucalyptol, fenchone, and α-terpineol from rhizomes, have been shown to have effects that are comparable to antidepressants. When the effects of inhaling either 1,8 cineole or eucalyptol were further investigated, it was shown that in tests involving mice, doses of 1,8 cineole at 4×10−4 and 4×10−2 mg dramatically decreased immobility time by 44% and 39%, respectively [67]. According to Ganesh and Balaraju (2019), a class of monoterpenoids containing a ketone group (fenchonoid) was also shown to have anti-depressant-like effects in rodents under continuous unpredictable mild stress. When rodents were given oral doses of 400 and 800 mg/kg of fenchone, remission from depression occurred [68].
Anti-mutagenic activity
In vitro anti-mutagenic activity testing of Alpinia galanga L. rhizome methanol extract against erythrocyte cells in the spinal cord of mice showed results in the form of a decrease in micronucleated polychromatic erythrocytes at a dose of 300 mg/kg body weight by 77.27% and at a dose of 600 mg/kg body weight by 63.63% compared to the positive control. This means that Alpinia galanga L. rhizomes have good anti-mutagenic activity [69]. Anti-mutagenic activity testing conducted by Trakoontivakorn et al. (1999) confirmed this finding in a comparative investigation involving Alpinia galanga L. extract with methanol fraction compared to water. The results of the investigation showed that Alpinia galanga L. extract with methanol solvent fraction has higher anti-mutagenic activity compared to water fraction [70].
Conclusion
Alpinia galanga L. has many medical attributes that can potentially be developed as candidate raw materials for medicines. All parts of Alpinia galanga L. have derivatives of secondary metabolite compounds that have different bioactivities. Rhizomes are famously known to have a rich phytochemical constituent that exhibit various bioactivities. Several kinds of bioactivities known to be possessed by Alpinia galanga L. include anti-microbial, antioxidant, anti-cancer activity, anti-diabetic activity, hepatoprotective, anti-malarial, anti-plasmodial, anti-fungal, anti-allergic, anti-inflammatory, anti-larvicidal, anti-molluscidal, anti-insect, antiviral, anti-ulcer, anti-depressant, and anti-mutagenic activities. Therefore, further research and development of the medical attributes of Alpinia galanga L. is warranted to maximize the potential of the secondary metabolites present in this plant.
Authors contribution
All authors have accepted responsibility for the entire content of this manuscript and consented to its submission to the journal, reviewed all the results, and approved the final version of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
This study supported by the Virtual Research Center for Bioinformatics and Biotechnology, Indonesia. Additionally, we thank Jalan Tengah, Indonesia (jalantengah.site) for editing the manuscript.
References
- Nugraha A, Keller P. Revealing indigenous Indonesian traditional medicine: anti-infective agents. Nat Prod Commun 2011;6(12):1953-66. [PMID: 22312747 DOI: 10.1177/1934578X1100601240]
- Rao K, Ch B, Narasu LM, Giri A. Antibacterial activity of Alpinia galanga (L) Willd crude extracts. Appl Biochem Biotechnol 2010;162(3):871-84. [PMID: 20387130 DOI: 10.1007/s12010-009-8900-9]
- Yadav PN, Liu Z, Rafi MM. A diarylheptanoid from lesser galangal (Alpinia officinarum) inhibits proinflammatory mediators via inhibition of mitogen-activated protein kinase, p44/42, and transcription factor nuclear factor-κB. J Pharmacol Exp Therapeut 2003;305(3):925-31. [PMID: 12626645 DOI: 10.1124/jpet.103.049171]
- Zhou YQ, Liu H, He MX, Wang R, Zeng QQ, et al. A review of the botany, phytochemical, and pharmacological properties of galangal. In: Natural and artificial flavoring agents and food dyes (Handbook of food bioengineering). Cambridge, MA: Academic Press; 2018. pp. 351-96.
- Arambewela LSR, Arawwawala M, Owen NL, Jarvis B. Volatile oil of Alpinia galanga Willd. of Sri Lanka. J Essent Oil Res 2007;19(5):455-6. [DOI: 10.1080/10412905.2007.9699950]
- Chouni A, Paul S. A review on phytochemical and pharmacological potential of Alpinia galanga. Pharmacog J 2018;10(1):1-12. [DOI: 10.5530/pj.2018.1.2]
- Abubakar IB, Malami I, Yahaya Y, Sule SM. A review on the ethnomedicine uses, phytochemistry and pharmacology of Alpinia officinarum hance. J Ethnopharmacol 2018;224(1):45-62. [PMID: 29803568 DOI: 10.1016/j.jep.2018.05.027]
- Basri AM, Taha H, Ahmad N. A review on the pharmacological activities and phytochemicals of Alpinia officinarum (galangal) extracts derived from bioassay-guided fractionation and isolation. Pharmacogn Rev 2017;11(21):43-56. [PMID: 28503054 DOI: 10.4103/phrev.phrev_55_16]
- Kaushik D, Yadav J, Kaushik P, Sacher D, Rani R. Current pharmacological and phytochemical studies of the plant Alpinia galanga. Zhong Xi Yi Jie He Xue Bao 2011;9(10):1061-5. [PMID: 22015185 DOI: 10.3736/jcim20111004]
- Nam Hoang N, Kodama T, Nwet Win N, Prema, Do KM, et al. A new monoterpene from the rhizomes of Alpinia galanga and its anti-Vpr activity. Chem Biodivers 2021;18(1):e2100401. [PMID: 34415099 DOI: 10.1002/cbdv.202100401]
- Bian MQ, Kang J, Wang HQ, Zhang QJ, Liu C, et al. Three new norsesquiterpenoids from the seeds of Alpinia galanga. J Asian Nat Prod Res 2014;16(5):459-64. [PMID: 24716441 DOI: 10.1080/10286020.2014.906407]
- Igoli NP, Obanu ZA, Gray AI, Clements C. Bioactive diterpenes and sesquiterpenes from the rhizomes of wild ginger (Siphonochilus aethiopicus (Schweinf) B.L Burtt). Afr J Tradit Complement Altern Med 2011;9(1):88-93. [PMID: 23983325 DOI: 10.4314/ajtcam.v9i1.13]
- Lim HJ, Bak SG, Lim HJ, Lee SW, Lee S, et al. Acyclic triterpenoid isolated from Alpinia katsumadai alleviates formalin-induced chronic mouse paw inflammation by inhibiting the phosphorylation of ERK and NF-kB. Molecules 2020;25(15):3345. [PMID: 32717961 DOI: 10.3390/molecules25153345]
- DeFilipps RA, Krupnick GA. The medicinal plants of Myanmar. PhytoKeys 2018;10(2):1-341. [PMID: 30002597 DOI: 10.3897/phytokeys.102.24380]
- Ghosh S, Rangan L. Alpinia: the gold mine of future therapeutics. Biotech 2013;3(3):173-85. [PMID: 28324376 DOI: 10.1007/s13205-012-0089-x]
- Kolangi F, Shafi H, Memariani Z, Kamalinejad M, Bioos S, et al. Effect of Alpinia officinarum hance rhizome extract on spermatogram factors in men with idiopathic infertility: a prospective double-blinded randomized clinical trial. Andrologia 2017;51(1):e13172. [PMID: 30378695 DOI: 10.1111/and.13172]
- Yang X, Eilerman RG. Pungent principal of Alpinia galanga (L.) swartz and its applications. J Agric Food Chem 1999;47(4):1657-62. [PMID: 10564034 DOI: 10.1021/jf9808224]
- Udjiana S. Food preservation efforts using galangal extract. J Separat Technol 2008;1(2):1-9.
- Oknarida S, Husain F, Wicaksono H. Study of ethnomedicine and the utilization of medicinal plants by local healers in Colo village, Dawe subdistrict, Kudus regency. Solidarity 2018;7(2):480-500.
- Mohsina FP, Aamir Q, Faheem IP, Maheen S, Mukim M, et al. Alpinia galanga – A review of its ethnomedicinal, phytochemical and pharmacological activity. Curr Trends Pharma Clinical Trials 2022;5(1):180042.
- Rahmadini N, Rindita, Prakasa AP, Nugroho A. Ethnomedicinal exploration of medicinal plant in Cihanjuang village, Pandeglang-Banten for curing stomacache. Media Konservasi 2022;27(3):140-6. [DOI: 10.29244/medkon.27.3.140-146]
- Verma RK, Sharma N. Phytochemical and pharmacological activities of Alpinia galangal: a review. Asian J Pharm Pharmacol 2022;8(3):74-85. [DOI: 10.31024/ajpp.2022.8.3.3]
- Wali AF, Jabnoun S, Razmpoor M, Najeeb F, Shalabi H, et al. Account of some important edible medicinal plants and their socio-economic importance. In: Masoodi MH, Rehman MU, editors. Edible plants in health and diseases, cultural, practical and economic value. Singapore: Springer; 2022. pp. 325-76.
- Farnsworth NR, Bunyapraphatsara N. Thai medicinal plants recommended for primary healthcare system. Bangkok: Prachachon; 1992.
- Uhl S. Ingredients: the building blocks for developing new ethnic foods. Food Tech 1996;6:79-84.
- Jain S, Shrivastava S, Nayak S, Sumbhate S. Plant review trends in Curcuma longa Linn. Pharmacog Rev 2007;1(1):119-28.
- Rahman MA, Islam MS. Alpinia calcarata Roscoe: a potential phytopharmacological source of natural medicine. Pharmacogn Rev 2015;9(17):55-62. [PMID: 26009694 DOI: 10.4103/0973-7847.156350]
- Arambewela LSR, Wijesinghe A. Sri Lankan medicinal plant monograph and analysis: Alpinia galangal. 10th ed. Colombo: Industrial Technology Institute and National Science Foundation; 2006.
- Haraguchi H, Kuwata Y, Inada K, Shingu K, Miyahara K, et al. Antifungal activity from Alpinia galanga and the competition for incorporation of unsaturated fatty acids in cell growth. Planta Med 1996;62(4):308-13. [PMID: 8792660 DOI: 10.1055/s-2006-957890]
- Shetty RG, Monisha S. Pharmacology of an endangered medicinal plant Alpinia galanga – a review. Res J Pharm Biol Chem Sci 2015;6(1):499-511.
- Abdullah F, Subramanian P, Ibrahim H, Malek SNA, Lee GS, et al. Chemical composition, antifeedant, repellent, and toxicity activities of the rhizomes of galangal, Alpinia galanga against Asian subterranean termites, Coptotermes gestroi and Coptotermes curvignathus (Isoptera: Rhinotermitidae). J Insect Sci 2015;15(1):1-7. [PMID: 25688085 DOI: 10.1093/jisesa/ieu175]
- Raina AP, Abraham Z. Essential oil profiling of Alpinia species from southern India. Ind J Exp Biol 2017;55:776-81.
- Morikawa T, Ando S, Matsuda H, Kataoka S, Muraoka O, et al. Inhibitors of NO production from the rhizomes of Alpinia galanga: structures of new 8-9′ linked neoligans and sesquineolignan. Chem Pharm Bull 2005;53(6):625-30. [PMID: 15930771 DOI: 10.1248/cpb.53.625]
- Misawa T, Aoyama H, Furuyama T, Dodo K, Sagawa M, et al. Structural development of benzhydrol-type 1-acetoxy-chavicol acetate (ACA) analogs as human leukaemia cell growth inhibitors based on quantitative SAR (QSAR) analysis. Chem Pharm Bull 2008;56(10):1490-95. [PMID: 18827399 DOI: 10.1248/cpb.56.1490]
- Siddiqui AW, Ali M, Naquvi KJ, Husain SS. New aliphatic ester, β-sitosterol diglucoside and vesicaria biflavones from the seeds of Rumex vesicarius L. Acta Pol Pharm 2015;72(5):965-71. [PMID: 26665404].
- Chudiwal AK, Jain DP, Somani RS. Alpinia galanga Willd. – An overview on phyto-pharmacological properties. Indian J Nat Prod Resour 2010;1:143-49.
- Khumpirapang N, Klayraung S, Tima S, Okonogi S. Development of microemulsion containing Alpinia galanga oil and its major compounds: enhancement of antimicrobial activities. Pharmaceutics 2021;13(2):265. [PMID: 33672041 DOI: 10.3390/pharmaceutics13020265]
- Bajpai VK, Sharma A, Baek KH. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control 2013;32:582-90. [DOI: 10.1016/j.foodcont.2013.01.032]
- Oonmetta-aree J, Suzuki T, Gasaluck P, Eumkeb G. Antimicrobial properties and action of galangal (Alpinia galanga Linn.) on Staphylococcus aureus. LWT Food Sci Technol 2006;39(10):1214-20. [DOI: 10.1016/j.lwt.2005.06.015]
- Das J, Ramani R, Suraju MO. Polyphenol compounds and PKC signaling. Biochim Biophys Acta 2016;1860(10):2107-21. [PMID: 27369735 DOI: 10.1016/j.bbagen.2016.06.022]
- Tian Y, Jia X, Wang Q, Lu T, Deng G, et al. Antioxidant, antibacterial, enzyme inhibitory, and anticancer activities and chemical composition of Alpinia galanga flower essential oil. Pharmaceuticals (Basel) 2022;15(9):1069. [PMID: 36145290 DOI: 10.3390/ph15091069]
- Sugiharto, Winarni D, Wibowo AT, Islamatasya U, Bhaktil N, et al. Gynura procumbens adventitious root extract altered expression of antioxidant genes and exert hepatoprotective effects against cadmium-induced oxidative stress in mice. Hayati J Biosci 2022;29(4):479-86. [DOI: 10.4308/hjb.29.4.479-486]
- Sugiharto, Winarni D, Islamatasya U, Muhasyi AH, Merpati AB, et al. The protective effect of Gynura procumbens adventitious root against lead acetate toxicity in mice. J Trop Biodivers Biotechnol 2022;7(2):1-9. [DOI: 10.22146/jtbb.69453]
- Ahlina FN, Nugraheni N, Salsabila IAS, Haryanti M, Da’I E, et al. Revealing the reversal effect of galangal (Alpinia galanga L.) extract against oxidative stress in metastatic breast cancer cells and normal fibroblast cells intended as a Co-chemotherapeutic and anti-aging agent. Asian Pac J Cancer Prev 2020;21(1):107-17. [PMID: 31983172 DOI: 10.31557/APJCP.2020.21.1.107]
- Muangnoi P, Lu M, Lee J, Thepouyporn A, Mirzayans R, et al. Cytotoxicity, apoptosis and DNA damage induced by Alpinia galanga rhizome extract. Planta Med 2007;73(8):748-54. [PMID: 17611930 DOI: 10.1055/s-2007-981542]
- Manse Y, Ninomiya K, Nishi R, Kamei I, Katsuyama Y, et al. Melanogenesis inhibitory activity of a 7-O-9′-linked neolignan from Alpinia galanga fruit. Bioorg Med Chem 2016;24(23):6215-24. [PMID: 27756508 DOI: 10.1016/j.bmc.2016.10.001]
- Tirawanchai N, Homongkol P, Chansriniyom C, Somkasetrin A, Jantaravinid K, et al. Lipid-lowering effect of Phyllanthus embilica and Alpinia galanga extracts on HepG2 cell line. PharmaNutrition 2019;9(1):1-10. [DOI: 10.1016/j.phanu.2019.100153]
- Herrmann F, Romero MR, Blazquez AG, Kaufmann D, Ashour ML, et al. Diversity of pharmacological properties in Chinese and European medicinal plants: cytotoxicity, antiviral and antitrypanosomal screening of 82 herbal drugs. Diversity 2011;3(4):547-80. [DOI: 10.3390/d3040547]
- Sugiharto, Zubaidah U, Winarni D, Manuhara YSW. Gynura procumbens methanolic extracts suppresses proliferation of hepatocellular carcinoma: in vitro assay. AIP Conf Proc 2023;2554:090007. [DOI: 10.1063/5.0104809]
- Herdiansyah MA, Ansori ANM, Kharisma VD, Alifiansyah MRT, Anggraini D, et al. In silico study of cladosporol and its acyl derivatives as anti-breast cancer against alpha-estrogen receptor. Biosaintifika 2024;16(1):142-54. [DOI: 10.15294/biosaintifika.v15i1.949]
- Zainul R, Kharisma VD, Ciuputri P, Ansori ANM, Herdiansyah MA, et al. Antiretroviral activity from elderberry (Sambucus nigra L.) flowers against HIV-2 infection via reverse transcriptase inhibition: a viroinformatics study. Healthc Low-Resource Settings 2024;1(2024):1-12. [DOI: 10.4081/hls.2024.12047]
- Verma RK, Mishra G, Singh P, Jha KK, Khosa RL. Anti-diabetic activity of methanolic extract of Alpinia galanga Linn. aerial parts in streptozotocin induced diabetic rats. Ayu 2015;36(1):91-5. [PMID: 26730146 DOI: 10.4103/0974-8520.169006]
- Hemabarathy B, Budin S, Feizal V. Paracetamol hepatotoxicity in rats treated with crude extract of Alpinia galangal. J Biol Sci 2009;9(1):57-62. [DOI: 10.3923/jbs.2009.57.62]
- Al-Adhroey AH, Nor ZM, Al-Mekhlafi HM, Mahmud R. Median lethal dose, antimalarial activity, phytochemical screening and radical scavenging of methanolic Languas galanga rhizome extract. Molecules 2010;15(11):8366-376. [PMID: 21081857 DOI: 10.3390/molecules15118366]
- Batista R, de Jesus Silva A Jr, De Oliveira AB. Plant-derived antimalarial agents: new leads and efficient phytomedicines. Part II. Non-alkaloidal natural products. Molecules 2009;14(8):3037-72. [PMID: 19701144 DOI: 10.3390/molecules14083037]
- Khodavandi A, Tahzir NAB, Cheng PW, Chen PYV, Alizadeh F, et al. Antifungal activity of Rhizome coptidis and Alpinia galangal against Candida species. J Pure Appl Microbiol 2013;7(3):1725-30.
- Prastiyanto ME, Rohmah N, Efendi L, Arifin R, Wardoyo FA, et al. Antifungal activities of the rhizome extract of five member Zingiberaceae against Candida albicans and Trichophyton rubrum. Biodiversity 2021;22(3):1509-13. [DOI: 10.13057/biodiv/d220355]
- Yasuhara T, Manse Y, Morimoto T, Qilong W, Matsuda H, et al. Acetoxybenzhydrols as highly active and stable analogues of 1′ S-1′-acetoxychavicol, a potent antiallergic principal from Alpinia galanga. Bioorg Med Chem Lett 2009;19(11):2944-46. [PMID: 19414259 DOI: 10.1016/j.bmcl.2009.04.065]
- Subash KR, Prakash GB, Reddy KVC, Manjunath K, Rao KU. Anti-inflammatory activity of ethanolic extract of Alpinia galanga in carrageenan induced pleurisy rats. Natl J Physiol Pharm and Pharmacol 2016;6(5):468-70. [DOI: 10.5455/njppp.2016.6.0719013072016]
- Cahyono B, Suzery M, Amalina ND. Anti-inflammatory effect of Alpinia galanga extract on acute inflammatory cell model of peripheral blood mononuclear cells stimulated with TNF-α. Med Glas (Zenica) 2023;20(2):207-13. [PMID: 37300465 DOI: 10.17392/1561-23]
- Nguyen BV, Hung NH, Satyai P, Dai DN, Huong LT, et al. Chemical composition and pesticidal activity of Alpinia galanga (L.) Willd. essential oils in Vietnam. Rec Nat Prod 2022;16(2):182-7. [DOI: 10.25135/rnp.263.21.05.2074]
- Poonsri W, Pengsook A, Pluempanupat W, Yooboon T, Bullangpoti V. Evaluation of Alpinia galanga (Zingiberaceae) extracts and isolated trans-cinnamic acid on some mosquitoes larvae. Chem Biol Technol Agric 2019;6(17):1-7. [DOI: 10.1186/s40538-019-0157-0]
- Ye Y, Li B. 1′ S-1′-acetoxychavicol acetate isolated from Alpinia galanga inhibits human immunodeficiency virus type 1 replication by blocking Rev transport. J Gen Virol 2006;87(7):2047-53. [PMID: 16760408 DOI: 10.1099/vir.0.81685-0]
- Longo D, Fauci A, Kasper D, Hauser S, Jameson J, et al. Harrison’s principles of internal medicine. 18th ed. New York, NY: McGraw-Hill Professional; 2012. p. 2438.
- Johnley IIR, Somasundaram G, Salwe KJ, Manimekalai K. Anti ulcerogenic and anti-oxidant activity of Alpinia galanga rhizomes aqueous extract in indomethacin induced gastric mucosal damage in wistar albino rats. Biomed Pharmacol J 2020;13(1):55-60. [DOI: 10.13005/bpj/1860]
- Matsuda H, Pongpiriyadacha Y, Morikawa T, Ochi M, Yoshikawa M. Gastroprotective effects of phenylpropanoids from the rhizomes of Alpinia galanga in rats: structural requirements and mode of action. Eur J Pharmacol 2003;471(1):59-67. [PMID: 12809953 DOI: 10.1016/S0014-2999(03)01785-0]
- Dougnon G, Ito M. Inhalation administration of the bicyclic ethers 1,8-and 1,4-cineole prevent anxiety and depressive-like behaviours in mice. Molecules 2020;25(8):1884. [PMID: 32325759 DOI: 10.3390/molecules25081884]
- Ganesh VS, Balaraju J. In vitro assessment of antidepressant upshot of l-fenchone in chronic unpredictable mild stress (CUMS) induced depression like behaviour in rodents (CUMS). World J Curr Med Pharm Res 2019;1:177-83.
- Chasanah DI, Arianingrum R, Atun S. Antimutagenic activity of methanol extracts of galangal rhizome (Alpinia galanga) on erythrocyte cell in mice bone narrow in vitro. Asian J Trop Biotechnol 2014;11(2):36-43.
- Trakoontivakorn G, Nakahara K, Shinmoto H, Tsushida T. Identification of antimutagenic substances (AMES test) from Boesenbergia pandurate Schl. (fingerroot) and Alpinia galanga (galanga). JIRCAS J 1999;7(1999):105-16.