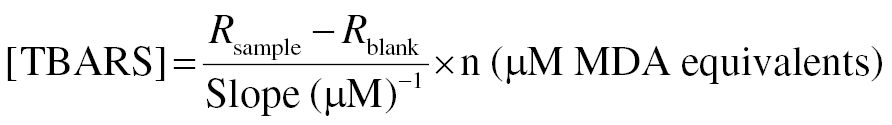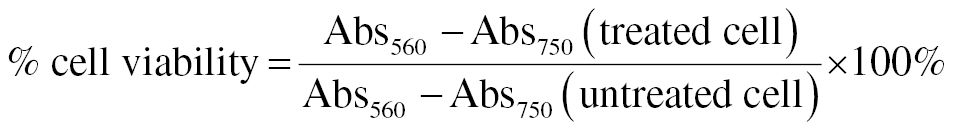Gynura procumbens Adventitious Root Ameliorates Oxidative Stress and has Cytotoxic Activity Against Cancer
1Department of Biology, Faculty of Science and Technology, Universitas Airlangga, Surabaya 60115, Indonesia
2Biotechnology of Tropical Medicinal Plants Research Group, Faculty of Science and Technology, Universitas Airlangga, Surabaya 60115, Indonesia
*Correspondence to: Sugiharto, Department of Biology, Faculty of Science and Technology, Universitas Airlangga, Surabaya 60115, Indonesia, E-mail: sugiharto@fst.unair.ac.id
Received: May 1 2024; Revised: May 26 2024; Accepted: July 4 2024; Published Online: July 24 2024
Cite this paper:
Zubaidah U, Sugiharto, Siregar MIP et al. Gynura procumbens Adventitious Root Ameliorates Oxidative Stress and has Cytotoxic Activity Against Cancer. BIO Integration 2024; 5: 1–9.
DOI: 10.15212/bioi-2024-0020. Available at: https://bio-integration.org/
Download citation
© 2024 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
Background: Lead exposure is a growing concern in recent public health because lead acts as an oxidant, causing oxidative stress. In this context, the administration of exogenous antioxidants is imperative. Our preliminary study revealed that Gynura procumbens leaf and root contain phenolics and flavonoids. The current study aims to determine the potency of G. procumbens adventitious root (ARGp) in preventing oxidative stress caused by lead exposure and the potential as an anti-cancer agent.
Methods: ARGp was induced from leaf explant, propagated inside a bioreactor, and macerated by methanol. Balb/C mice were used in an in vivo study and divided into 5 groups, as follows: P1 (distilled water); P2 (Pb 100 mg/L); P3 (ARGp-100 mg/L + Pb 100 mg/L); P4 (ARGp-200 mg/L + Pb 100 mg/L); and P5 (ARGp-300 mg/L + Pb 100 mg/L). Hematologic parameters, the level of lipid peroxidation, and GPx-4 antioxidant gene expression were subsequently recorded.
Results: Administration of ARGp significantly increased the hematocrit and mean corpuscular volume but did not significantly increase the mean corpuscular hemoglobin compared to lead exposure (P2). In contrast, ARGp significantly lowered the mean corpuscular hemoglobin concentration (MCHC) and white blood count compared to P2. ARGp significantly decreased liver and kidney lipid peroxidation but not in the serum. These findings are consistent with the ability of ARGp to enhance endogenous antioxidant gene expression, especially GPx-4. Furthermore, ARGp exhibited a cytotoxic effect on the hepatoma (Huh7it) cell line with an IC50 44.65 mg/L.
Conclusion: ARGp possesses antioxidants by restoring hematologic damage, lowering lipid peroxidation, and increasing antioxidant gene expression, as well as anti-cancer activity.
Keywords
Gene expression, Gynura procumbens, Huh7it cell line, lipid peroxidation, oxidative stress.
Introduction
Heavy metal pollution has been a major public health concern in recent decades [1]. One of the heavy metals that causes poisoning is lead (Pb). Pb enters the body via the skin, respiratory tract, and digestive system and can then be carried via the bloodstream. The minimum concentration of Pb in the blood that is considered Pb poisoning internationally is 10 μg/dL. Pb binds to erythrocytes, which further accumulates in the brain, liver, and kidney [2]. Once Pb interferes with metabolism, Pb causes cell and even tissue damage. Pb can disturb the oxidant-antioxidant balance and subsequently cause oxidative stress [3]. Other possible mechanisms underlying Pb toxicity involve the interaction with bio-elements, such as DNA, and alterations in gene expression [4].
Oxidative stress begins inside the mitochondria. Our previous study reported that Pb acetate (PbA) exposure lowers superoxide dismutase (SOD) and catalase (CAT) levels in response to excessive amounts of reactive oxygen species [ROS] [5]. In addition, Pb2+ lowers glutathione peroxidase (GPx) and glutathione S-transferase activities and Pb inhibits the thiol group, which results in a lower glutathione (GSH) level. These conditions favor lipid peroxidation, which is characterized by a high level of malondialdehyde [MDA] [6, 7]. Lipid peroxidation alters membrane integrity-induced cell damage. Prolonged PbA administration to mice leads to hepatic cell damage and lowers hematologic parameters. Moreover, heavy metals behave as transcriptional regulators or perform epigenetic regulation that alters gene expression [5]. Under adverse conditions, oxidative stress drives inflammation, and inflammation may cause cell death or carcinogenesis. Carcinogenicity is associated with an inability to repair DNA damage and hinders mutations [8]. In such a case, an antioxidant booster that compromises oxidative stress induced by Pb is needed.
Gynura procumbens, a tropical plant from the Asteraceae family, has well-documented antioxidant activity. The leaves of G. procumbens contain phenolics and have been reported to be hepatoprotective against cadmium (Cd) toxicity, while also suppressing proliferation of hepatomas [9, 10]. However, antioxidant activity depends on the organ. The roots of G. procumbens has the highest antioxidant activity, followed by the leaves and stems [11, 12]. However, the use of roots is destructive to the mother plant. Of note, rapid propagation can be achieved in vitro through plant tissue culture. Adventitious root in vitro cultures have the antioxidant potency of root ex vitro [13]. We concluded that the antioxidant activity of G. procumbens adventitious root could prevent oxidative stress induced by Cd in mice [14].
The current study aimed to unravel the potency of G. procumbens to ameliorate lead toxicity. A hematologic analysis was performed to assess antioxidant intervention in the first part of Pb poisoning. Subsequently, the lipid peroxidation levels in blood, liver, and kidney, as well as expression of the endogenous antioxidant, GPx-4, were evaluated to verify tissue damage amelioration. The toxicity effect on a hepatoma cell line was also observed. This is the first report to demonstrate harnessing the stress oxidative-inflammation-indirect malignancy axis using G. procumbens adventitious root-derived in vitro culture.
Materials and methods
Materials
The following materials were used in the current study: G. procumbens leaf (Surabaya, East Java, Indonesia); Murashige and Skoog (MS) medium (Merck, Darmstadt, Germany); indole butyric acid (IBA) hormone (Merck, Darmstadt, Germany); BALB/c mice (Faculty of Pharmacy Universitas Airlangga, Surabaya, East Java, Indonesia); an MDA TBARS kit (BioAssay Systems, Florida, USA), an SV total RNA isolation system (Promega, Madison, Wisconsin, United States), forward and reverse β-actin and GPx-4 primers (Macrogen, Singapore); 2,5-diphenyl-2H-tetrazolium bromide (MTT) reagent (Thermo Fisher, USA); and Dulbecco’s Modified Eagle Medium (DMEM, Macrogen, Singapore).
Instruments
The following instruments were used in the current study: balloon type bubble bioreactor (BTBB); ABX Micros 60 (Horiba, Japan); ABX Pentra 400 hematology analyzer (Horiba, Japan); Eppendorf micropipette (micropipette (Eppendorf, USA)); centrifuge Eppendorf 5424R (USA); microplate reader (Multiskan Go, Thermo Scientific, USA); qRT-PCR (MyGo Pro, UK); and μDrop (Thermo Scientific, USA).
Production and extraction of G. procumbens adventitious root
Production of G. procumbens adventitious root was performed as described in our previous study [13]. Leaf explant was induced to grow adventitious root under solid MS medium supplemented with 5 mg/L of IBA. The obtained inoculum was further cultured in BTBB in the liquid MS medium with 5 mg/L of IBA. Adventitious root was harvested after a 28-d incubation. The dried adventitious root was macerated by methanol at a 1:10 ratio in triplicate. The yield was concentrated in a rotary evaporator (200 mbar; 45°C) until a thick extract was obtained. G. procumbens adventitious root (ARGp) extract was kept in a refrigerator to prevent desiccation.
In vivo study design
An in vivo study was conducted using 8–10-week-old male mice (Mus musculus, strain BALB/c). The mice were obtained from the Faculty of Pharmacy at the Universitas Airlangga (Surabaya, Indonesia). The use of all mice was under ethical clearance (certificate no. 2. KE. 151.07.2019) issued by the Faculty of Veterinary Ethics Committee (Universitas Airlangga). Mice were randomly grouped into 5 treatment groups as follows: P1, 0.25 mL of distilled water (control); P2, 0.25 mL of Pb (100 mg/L); P3, 0.25 mL of ARGp (100 mg/L) and 0.25 mL of Pb (100 mg/L); P4, 0.25 mL of ARGp (200 mg/L) and 0.25 mL of Pb (100 mg/L); and P5, 0.25 mL of ARGp (300 mg/L) and 0.25 mL of Pb (100 mg/L).
The mice were administered ARGp orally, followed by lead. Oral administration was performed to ensure that the entire treatment solution entered the test animals and was absorbed in the digestive system. The entry of Pb into the digestive system causes competition between Ca, Fe, P, and K ions on the carrier protein binding side of the mucosal digestive tract [4, 15]. All treatments were performed in 1 month. Mice were sacrificed using xylazine. Blood was obtained by intracardiac puncture and prepared for further analysis. The liver and kidneys were also obtained for analysis.
Hematologic analysis
Analysis of hematologic parameters was performed as described by Sugiharto et al. [16]. The hematologic parameters included hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and white blood cell (WBC) count. Measurements were performed using an ABX Micros 60 Hematology analyzer by a spectrophotometric method with ABX Minidil LMG (2.1 mL) and ABX Minilyse LMG (alphalize, 0.52 mL) reagents.
Lipid peroxidation analysis
The MDA level was quantified using a TBARS kit. Serial dilutions of the MDA standard were made into 30, 18, 9, and 0 μM. Samples sources included serum, liver, and kidney homogenates. The serum was added tp 10% trichloroacetic acid (TCA), centrifugated for 5 min at 14,000 rpm [8765 g (radius rotor = 4 cm)], and the supernatant was obtained.
To perform the colorimetric assay, 200 μL of TBA reagent was added to each sample and standard. The samples were homogenized and incubated at 100°C for 1 h to await a color change. The samples and standard were transferred into wells and the absorbance was read at a 535-nm wavelength. TBARS was calculated as follows:
Gene expression analysis
Gene expression analysis was performed on the hepatic GPx-4 gene. Isolation of mRNA was performed according to the spin protocol (Promega). The concentration of mRNA was quantified using μDrop, while agarose gel electrophoresis was performed to assess mRNA quality. mRNA (1 μg) was prepared for cDNA synthesis using reverse transcriptase according to the manufacturer’s instructions (Promega).
qRT-PCR analysis of GPx-4 was performed according to the SYBR green method. Forward and reverse primers were obtained from Macrogen with the following sequences: 5’–TAAGAACGGCTGCGTGGT – 3’; and 5’ – GTAGGGGCACACACTTGTAGG – 3’ β-actin was used as the reference gene. GPx-4 expression was calculated using the comparative ΔΔCt method.
Toxicity analysis in the Huh7it cell line
The toxicity assay was performed as described by Sugiharto et al. [10] using the hepatoma cell line (Huh7it). The cells were purchased from the Laboratory of Natural Product Medicine Research Development (Phytochemistry and Antiviral Assay, Institute of Tropical Disease, Universitas Airlangga). Huh7it cells (2.3 × 104 cells/well) were cultured in a 96-well plate containing DMEM medium and incubated inside an incubator at 37°C in 5% CO2 overnight. Each group was further treated with various concentrations of ARGp (0, 6, 12, 25, 50, 100, 250, and 500 μg/mL) for 48 h. The MTT assay was performed to assess the percentage (%) of cell viability. Absorbance was read at 560 and 750 nm. The relative viability of treated cells compared to control cells (non-treated) was expressed as a % of cell viability. The % of cell viability was calculated according to the following formula:
The IC50 value of extract toxicity was obtained from logarithmic regression of the % of cell viability data. The acquisition of this IC50 value is determined as the best concentration of ARGp to avert malignancy.
Statistical analysis
All data are displayed as the mean ± SD and statistically analyzed by GraphPad Prism. Normality and homogeneity data were first performed. Further analysis of variance and post hoc analysis were performed following the data distribution. Pearson analysis was used to assess the correlation between parameters. The data with a P < 0.05 were considered statistically significant.
Results and discussion
ARGp countered the alteration of hematologic parameters upon Pb poisoning
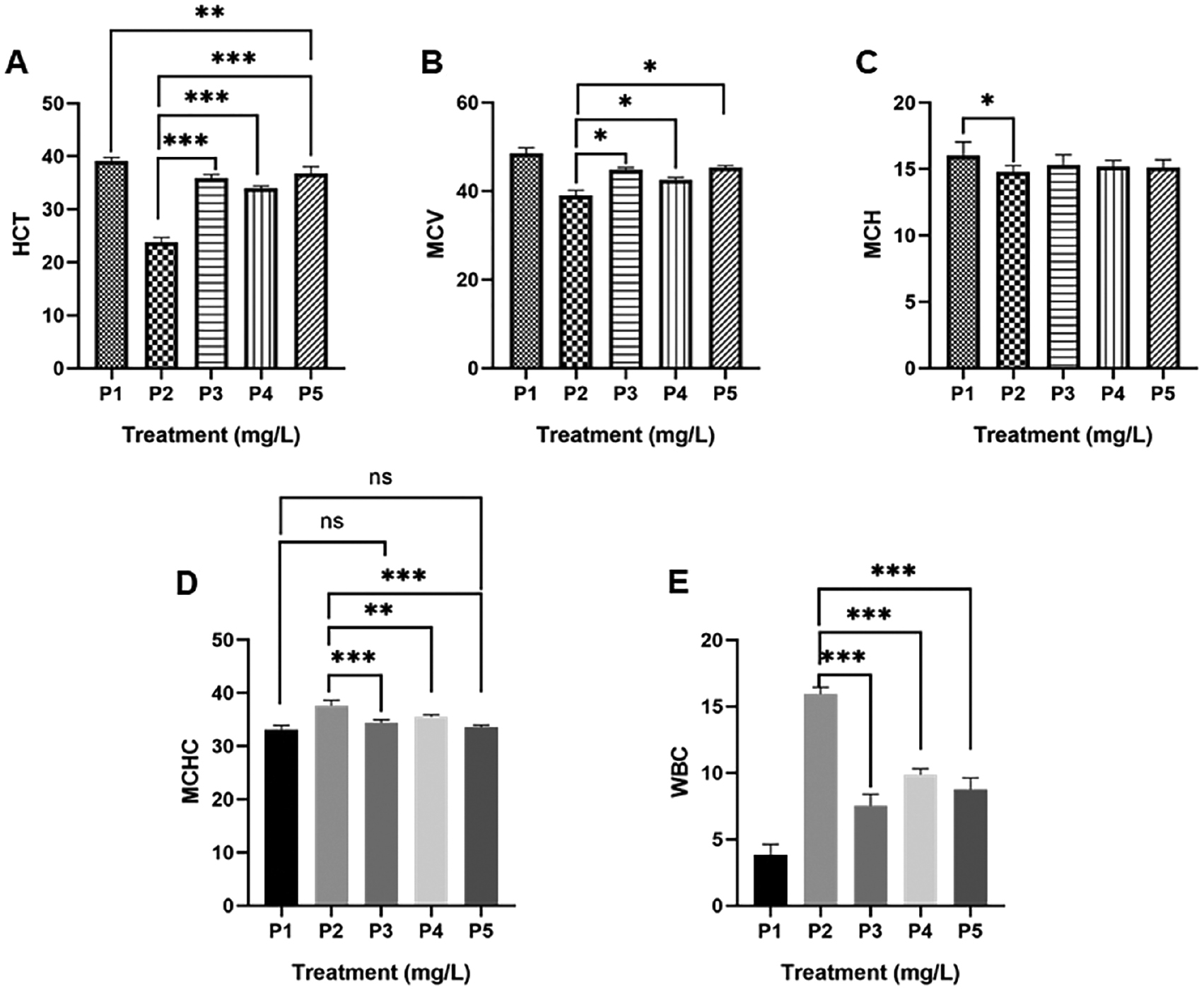
Pb poisoning impairs the hematopoietic system, which can be confirmed by alterations of several hematologic parameters [17, 18]. Administration of Pb (100 mg/L) significantly (P < 0.0001–0.01) reduced the HCT, MCV, and MCH, and increased the MCHC and WBC count compared to control (P1; Figure 1). Pb initially causes membrane fragility and degradation of ribonucleic acid in erythrocytes, which further alters morphology and shortens the life span [17]. The HCT and MCV levels in the P2 treatment group were the lowest. With respect to oxygen transport, Pb disturbs heme synthesis through inhibition of three key enzymes (δ-aminolevulinic acid dehydratase [ALAD], aminolevulinic acid synthetase [ALAS], and ferrochelatase). However, ALAD is more profound because heme synthesis does not decrease until 80%–90% of ALAD is suppressed [17]. Following adverse conditions, the level of MCH was decreased, while MCHC was increased (Figure 1). This finding is intuitive because the majority of Pb are bound in hemoglobin rather than erythrocyte membrane [4]. A recent study was correlated with our previous report in which Pb (50 and 100 mg/L) lowered the erythrocyte count and hemoglobin concentration [16]. The result of the above process is hypochromic microcytic or sideroblastic anemia [19].
Figure 1 Effect G. procumbens adventitious root (ARGp) extract on hematologic parameters. HCT: hematocrit; MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration; WBC: white blood cell; P1 = control; P2 = Pb-100 mg/L; P3 = ARGp-100 mg/L + Pb-100 mg/L; P4 = ARGp-200 mg/L + Pb-100 mg/L; P5 = ARGp-300 mg/L + Pb-100 mg/L. Statistical analysis was performed using one-way ANOVA, the Tukey test, and Duncan test. The asterisk shows significant differences (P < 0.05). P ≥ 0.05 = ns (not significant), P: 0.01–0.05 = * (significant), P: 0.001–0.01 = ** (very significant), P < 0.001 = *** (very significant).
Furthermore, Pb poisoning modulates immune-mediated cell destruction [18]. This was confirmed by an increase in the WBC count (15.93 ± 0.51 103/mm3). A metal-induced type IV delayed-hypersensitivity reaction occurred due to T cell activation. Prolonged sensitization drives inflammation and cellular damage. Dose-dependent Pb exposure improves the number of lymphocytes, monocytes, and granulocytes [16].
Pb is a source of oxidants by which antioxidants scavenge Pb to lower hematologic damage. The administration of all doses of ARGp (P3, P4, and P5) significantly (P < 0.0001) increased the HCT compared to Pb (100 mg/L) treatment. HPLC analysis revealed that G. procumbens adventitious root contains phenolic and flavonoid compounds [13]. Phenolics protect erythrocytes from membrane peroxidation. Alvarez-Suarez et al. [20] reported that phenolics from monofloral honey reduce membrane damage of erythrocytes upon AAPH exposure.
It is known that heavy exposure to Pb impairs heme synthesis. They are taken up by iron machinery and block iron through competitive inhibition [21]. Treatment of natural products may restore heme synthesis. Recently, ARGp was shown to improve the synthesis of heme. As a result, the MCV and MCH levels increased. Even, the MCV of P3 (44.75 ± 0.50 fL) and P5 (45.25 ± 0.50 fL) nearly reached the MCV level of the control cohort (P1 = 48.50 ± 1.29 fL). This ability is presumably correlated with polysaccharides found in G. procumbens [22]. Polysaccharides from Angelica sinensis improve hematopoiesis by increasing the iron serum level, regulating iron homeostasis, and increasing secretion of hematopoietic growth factors [23, 24].
It was previously shown that lead poisoning drives inflammation. Inflammation recruits cell-mediated immune responses, which is destructive rather than beneficial. ARGp also compromises sensitization of metal by reducing the number of leukocytes. The number of WBCs after treatment with 100, 200, and 300 mg/L ARGp was 7.53 ± 0.87 103/mm3, 9.85 ± 0.45 103/mm3, and 8.75 ± 0.88 103/mm3, respectively. Those were significant (P < 0.0001) compared to 100 mg/L of Pb (15.93 ± 0.51 103/mm3). Once again, polyphenols contribute to lower sensitization by reducing proinflammatory mediators and immunosuppression. These compounds also inhibit CCL2, which is responsible for leukocyte recruitment [25].
ARGp slows lipid peroxidation in organs affected by Pb
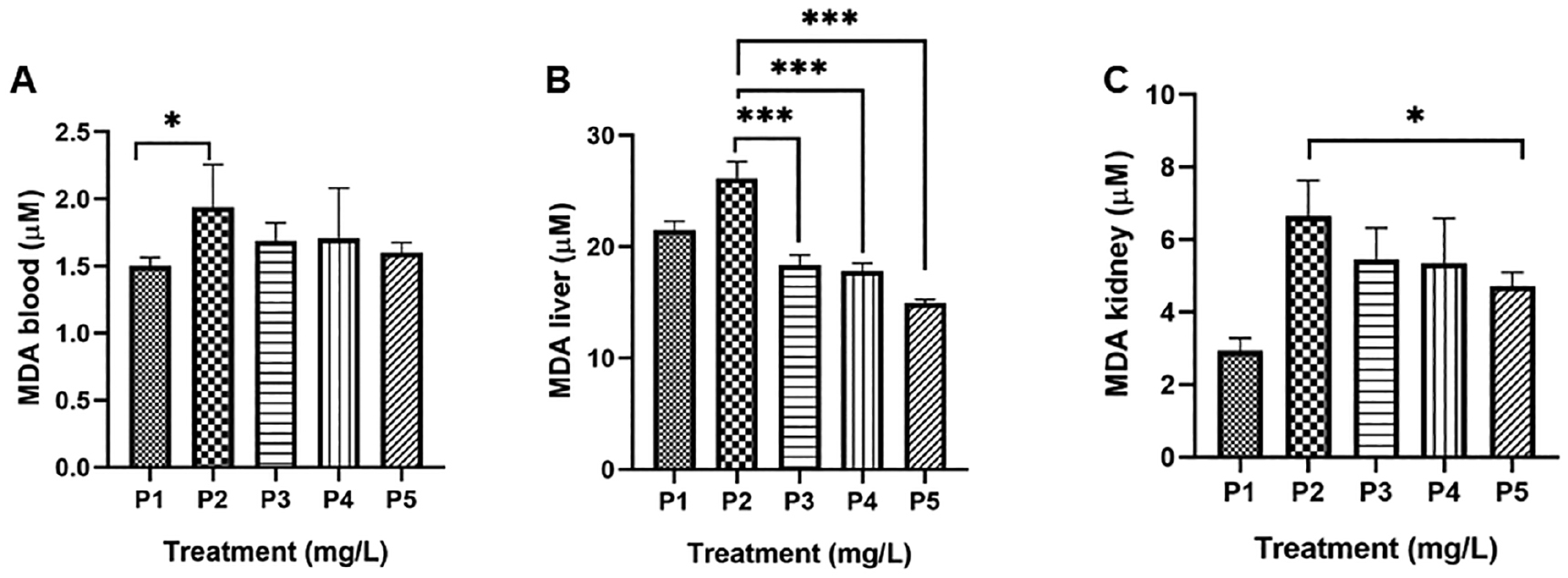
Upon entering the bloodstream, 99% of Pb is bounded by erythrocytes and 1% is retained in the plasma. Pb further interacts with the erythrocyte membrane and causes lipid peroxidation of the membrane [26]. The level of lipid peroxidation is proportional to the increase in the MDA level within cells or tissues because ROS decrease polyunsaturated fatty acids and form reactive MDA, causing stress within the cell and forming covalent bonds with proteins referred to as advanced lipoxidation end-products (ALEs). The production of these aldehydes can be used as biomarkers to measure the level of oxidative stress in an organism [27, 28]. The MDA level of blood serum was significantly (P < 0.05) increased upon exposure to 100 mg/L of Pb (P2) over the control normal (P1). However, treatment of all ARGp doses caused a decrement in the MDA level that was not significant compared to P2 (Figure 2A). A study by Shafiq-ur-Rehman [26] reported that a moderate dose of Pb (0.5–5 μM) degraded both the inner (amino-containing phosphatidyl serine) and outer (amino-containing phosphatidylcholine) cytoplasmic layer of erythrocytes, which makes erythrocytes highly vulnerable to Pb poisoning.
Figure 2 G. procumbens adventitious root (ARGp) decreased MDA in serum (A), liver (B), and kidney (C) homogenates. P1 = control; P2 = Pb-100 mg/L; P3 = ARGp-100 mg/L + Pb-100 mg/L; P4 = ARGp-200 mg/L + Pb-100 mg/L; P5 = ARGp-300 mg/L + Pb-100 mg/L. Statistical analysis was performed using one-way ANOVA, the Tukey test, and Duncan test. The asterisk shows significant differences (P < 0.05). P ≥ 0.05 = ns (not significant), P: 0.01–0.05 = * (significant), P: 0.001–0.01 = ** (very significant), P < 0.001 = *** (very significant).
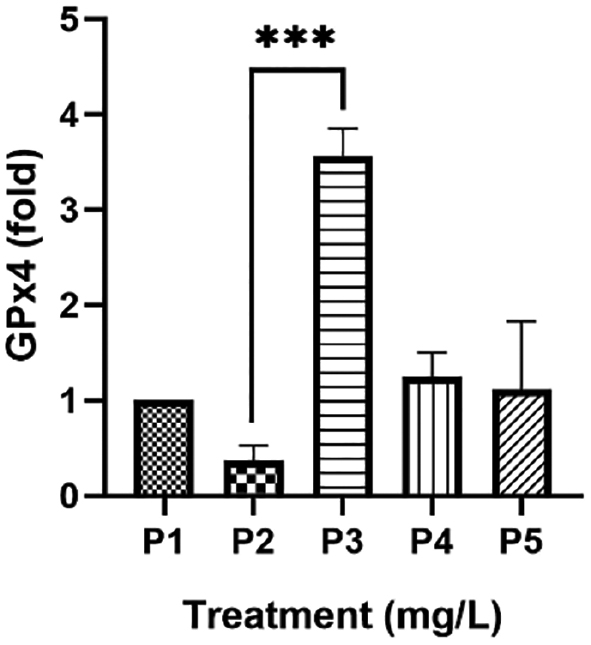
Figure 3 Gynura procumbens adventitious root (ARGp) increased expression of GPx4. P1 = control; P2 = Pb-100 mg/L; P3 = ARGp-100 mg/L + Pb-100 mg/L; P4 = ARGp-200 mg/L + Pb-100 mg/L; P5 = ARGp-300 mg/L + Pb-100 mg/L. Statistical analysis was performed using one-way ANOVA, the Tukey test, and Duncan test. The asterisk shows significant differences (P < 0.05). P ≥ 0.05 = ns (not significant), P: 0.01–0.05 = * (significant), P: 0.001–0.01 = ** (very significant), P < 0.001 = *** (very significant).
In addition to hematopoietic tissue, Pb induces oxidative stress associated with membrane peroxidation in other tissues, including the liver, kidneys, testes, heart, and brain. The liver accumulates more Pb than other tissues (33%), followed by the kidney [29]. The concentration of MDA in the liver was higher (26.15 ± 1.50 μM) than the kidneys (6.63 ± 0.99 μM). The liver and kidneys data were significantly different compared to P1 (P < 0.0001). The profound mechanism underlying Pb poisoning is causing oxidative stress where antioxidants behave as an antidote and a chelator to combat oxidative stress [30]. The high level of MDA was compromised by administration of ARGp at all doses (Figure 2B and 2C). Our previous report demonstrated that G. procumbens leaves lower the MDA level in mice with Cd poisoning. This antioxidant activity was consistent with the total phenolic and flavonoid content. Moreover, a hepatoprotective effect was also shown [9].
An adverse effect of Pb exposure is structural damage to the kidney and alterations in the excretory system [4]. This finding was supported by the increased MDA level in P2 according to a recent study. Treatment with ARGp (300 mg/L) exhibited a significant decrease in the MDA level in the kidneys. The current evidence is associated with quercetin content in G. procumbens. Quercetin increases GPx and decreases MDA in nephrectomy-induced oxidative stress. Moreover, quercetin improves endogenous antioxidant gene expression in rats with chronic kidney disease [31].
ARGp improved GPX-4 expression
Oxidative stress-derived heavy metals are characterized by redox imbalance between oxidant and endogenous antioxidant enzymes. Indeed, the liver is one of the affected organs. Our study showed high marker hepatic cells and aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels upon Cd exposure [14]. Excessive production of ROS initiates oxidative stress. First, metal produces ROS via the Fenton and/or Haber–Weiss reactions. Inside mitochondria, NADPH oxidation results in superoxide (O2•−), which is converted into hydrogen peroxide (H2O2) by SOD. The H2O2 is further transformed into H2O by CAT and GPx. However, all three antioxidant enzymes are suppressed by metal [32]. As indicated in the current findings, exposure to 100 mg/L of Pb caused a decrease in GPx-4 expression (0.36 ± 0.16-fold) compared to control (1.00 ± 0.00-fold). Sugiharto et al. [14] reported an alteration of SOD1 and CAT expression upon exposure to 100 mg/L of Cd. This finding also influenced a decrease in SOD and CAT levels within serum and liver homogenates. The number of antioxidant enzymes is not sufficient to scavenge excessive ROS.
Administration of exogenous antioxidants can help antioxidant enzymes neutralize ROS. The G. procumbens adventitious root antioxidant was confirmed based on total phenolic content (120.24 ± 2.81 mg/g of DW gallic acid), total flavonoid content (289.44 ± 6.94 mg/g of DW quercetin and 1148.15 ± 23.13 mg/g of DW kaempferol), and 2,2-diphenyl-1-picrylhydrazyl or DPPH assay [IC50 = 148.0 μg/mL] [13]. HPLC analysis confirmed that ARGp contains polyphenols from flavonoids subgroup [quercetin, catechin, and kaempferol] [13]. These bioactive compounds are believed to mediate high expression of the GPx-4 liver gene in ARGp [100 mg/L] (P3) compared to Pb treatment [P2] (P < 0.0001). This result is consistent with increased expression of SOD1 and CAT in mice exposed to 100 mg/L of Cd upon treatment with 100 mg/L of ARGp [14]. This effect was one of the hepatoprotective effects of G. procumbens in addition to maintaining normal hepatic cells during PbA poisoning [5]. The current result is indeed interesting because a dose-dependent manner is opposed. There are two possible reasons underlying this result. A drug, as a xenobiotic, can pose harm to cells in excessive amounts, triggering detoxification processes. Xenobiotic metabolism involves three primary stages (absorption, metabolism, and excretion). Among these stages, metabolism is particularly critical. Enzymes, such as cytochrome CYP2C9, CYP2C19, and CYP2D6, have a pivotal role in this phase, catalyzing oxidation reactions that enhance drug solubility and facilitating elimination from the body [31, 33]. Zhou et al. [34] reported that administration of Ginko biloba at a low concentration inhibits CYP isoforms but a high dose induces CTP isoforms by increasing mRNA expression. Furthermore, drugs may share the same ligand structure and undergo competition with the binding site of the respective enzyme [35, 36].
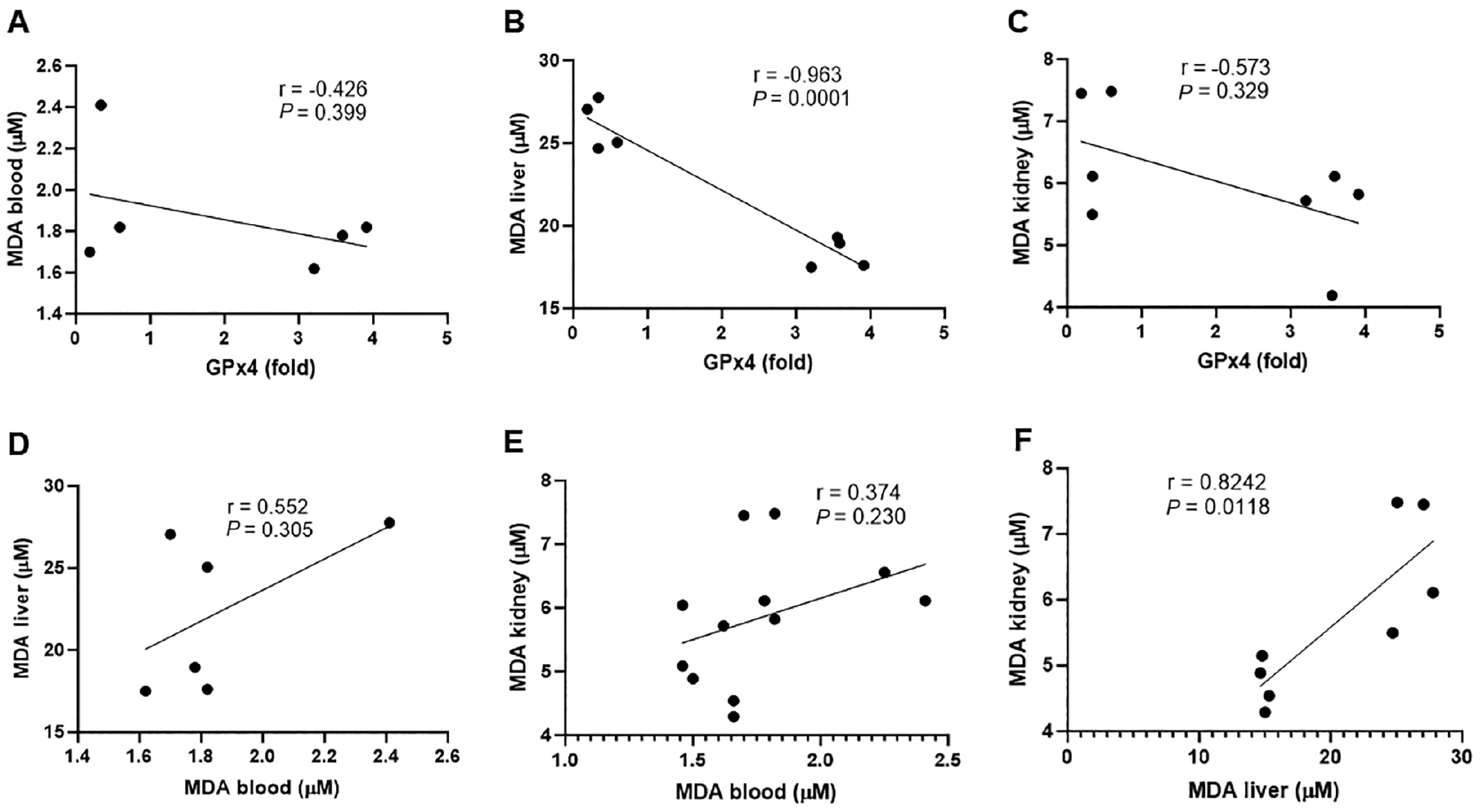
Pearson analysis was performed to assess the correlation among the obtained parameters, such as between the MDA level and GPx4 expression. All correlations of GPx4 expression and the amount of MDA in blood, liver, and kidneys were negative. There was a significant strong negative correlation (r = −0.96; P = 0.0001) between GPx4 and hepatic MDA. GPx4 expression and blood MDA, as well as GPx4 expression and kidney MDA kidney, exhibited a moderate correlation but was not significant (Figure 4A and 4C). Oxidant-antioxidant balance supports body homeostasis. Antioxidant enzymes, such as SOD, CAT, and GSH, scavenge ROS. Excessive ROS can be detrimental to the membrane, resulting in malondialdehyde. The leak of cell membranes increases membrane fluidity and permeability. In this case, the number of endogenous antioxidants expressed by normal gene expression, such as GPx4, cannot compromise ROS and oxidative stress occurs [37]. Prolonged ROS exposure leads to systemic oxidative stress, which affects many organs with different damage severity [38]. There was a high positive correlation but not significant difference between MDA blood and MDA liver (r = 0.552; P = 0.305) but the correlation between MDA blood and kidney had little correlation (Figure 4E). However, a significant strong positive correlation (r = −0.82; P = 0.118) was shown between MDA liver and kidney. This finding indicates that hepatotoxicity caused by oxidants, such as Pb, is perpetuated into the kidney [39].
Figure 4 Pearson correlation displayed a correlation of GPx4 and MDA blood (A), GPx4 and MDA liver (B), GPx4 and MDA kidney (C), MDA blood and MDA liver (D), MDA blood and MDA kidney (E), and MDA liver and MDA kidney (F). MDA: malondialdehyde; P < 0.05 (significant).
ARGp exhibited a cytotoxicity effect against hepatoma cells
It is well-documented that heavy metal toxicity modulates cancer progression, especially in the liver and kidney. There are three major mechanisms contributing to metal carcinogenesis, oxidative stress, DNA repair failure, and alteration of redox-sensitive signal transduction pathways. Oxidative stress becomes the most attractive assumption associated with the mutagenic effect of metals [32].
The aberrant Fenton and/or Haber–Weiss reactions lead to the excessive production of hydroxyl radical (•OH). This type of ROS causes DNA damage. DNA repair is also hampered by metal. The above-mentioned process drives carcinogenesis in the early progression [32]. Interestingly, the amount of ROS is decreased upon the last stage of carcinogenesis, indicating dual roles of ROS-mediated oxidative stress [32].
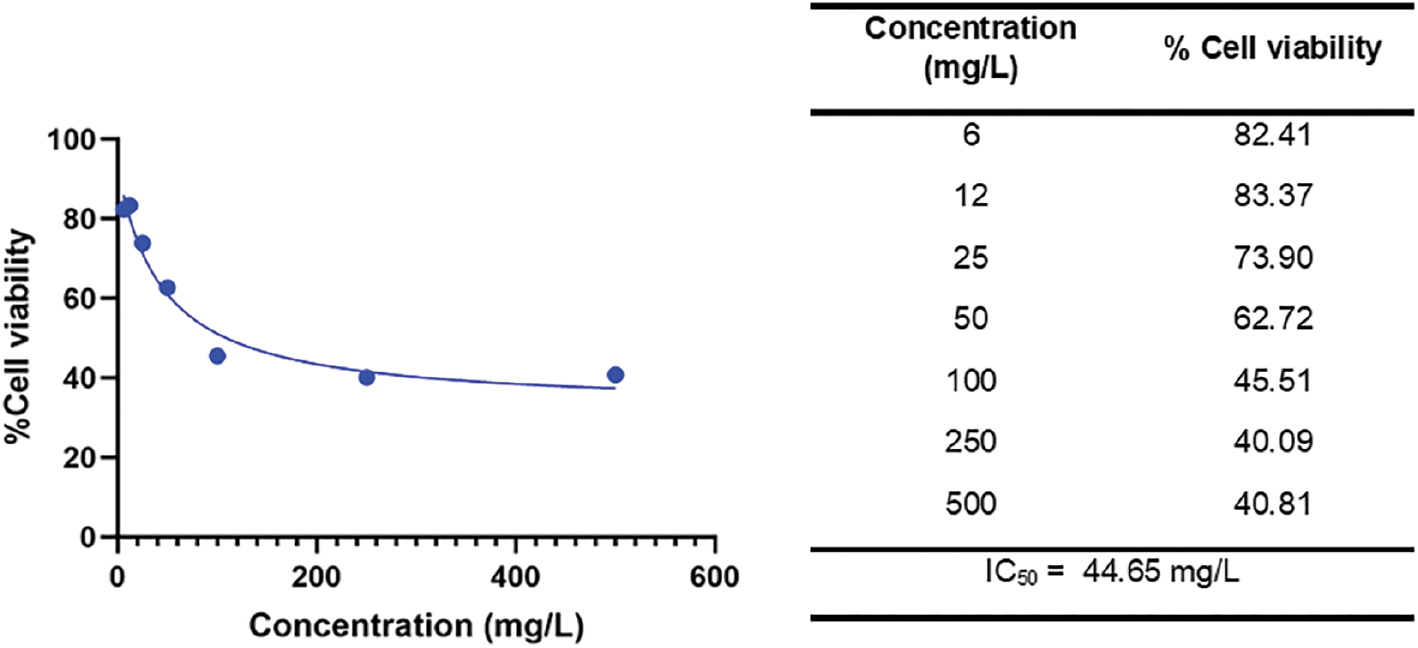
Medicinal plants are believed to alternatively prevent oxidative stress and kill cancer cells. Medicinal plants are rich in polyphenols that behave as antioxidants in the first phase of carcinogenesis but modulate apoptosis in the last phase of carcinogenesis. G. procumbens hosts numerous polyphenols, including quercetin and kaempferol [13]. G. procumbens polyphenols harm liver malignant cells. Treatment of ARGp resulted in low viability of Huh7it (hepatoma) cells in a dose-dependent manner after a 48-h incubation (Figure 5). The IC50 value was 44.65 mg/L, which is a moderate cytotoxic agent according to U.S. National Cancer Institute [40]. The administration of G. procumbens leaf extract is also toxic to Huh7it cells, with an IC50 value of 63.83 μg/mL [10]. The flavonoids present in G. procumbens are assumed to induce intrinsic apoptosis via caspase-3 expression. This assumption is supported by a previous finding in which treatment with nano-ASLE containing flavonoids increased apoptosis in HeLa cells through the intracellular mitochondrial pathway, leading to upregulation of caspase-3 expression [41]. Quercetin, a polyphenol found abundant in G. procumbens, mediated intrinsic apoptosis via modulation of several signaling pathways, such as p53, NF-κB, MAPK, JAK/STAT, PI3K/AKT, and Wnt/β-catenin [42]. Quercetin generates an intrinsic apoptotic mechanism through the activation of caspases 6 and 9 in MCF7, A549, SKOV-3, and A2780 cancer cells [43]. In addition, quercetin also drives extrinsic apoptotic mechanisms in BT474 human breast cancer cells [44].
Figure 5 ARGp reduced viability of the Huh7it cell line.
Conclusion
Pb poisoning causes oxidative stress, as indicated by alteration of hematologic parameters, lipid peroxidation in blood, liver, and kidney, as well as decreased expression of GPx-4. Administration of ARGp with antioxidant activity repaired hematologic damage, reduced the MDA level, and improved GPx-4 expression. These findings indicated prevention of stress oxidation. ARGp further exhibited a moderate anti-cancer effect towards the Huh7it cell line with an IC50 = 44.65 mg/L.
Acknowledgement
This research was funded by the Universitas Airlangga via Hibah Research Group (No. 343/UN3.14/PT/2020) and PUF (No. 2734/UN3.1.8/PT/2021). The authors declare no conflicts of interest.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Mitra S, Chakraborty AJ, Tareq AM, Emran TB, Nainu F, et al. Impact of heavy metals on the environment and human health: novel therapeutic insights to counter the toxicity. J King Saud Univ Sci 2022;34(3):101865. [DOI: 10.1016/j.jksus.2022.101865]
- Virgolini MB, Aschner M. Chapter – Molecular mechanisms of lead neurotoxicity. Adv Neurotoxicol 2021;5:159-213. [PMID: 34263090 DOI: 10.1016/bs.ant.2020.11.002]
- Kianoush S, Balali-Mood M, Mousavi SR, Shakeri MT, Dadpour B, et al. Clinical, toxicological, biochemical, and hematologic parameters in lead exposed workers of a car battery industry. Iran J Med Sci 2013;38(1):30-7. [PMID: 23645955]
- Andjelkovic M, Buha Djordjevic A, Antonijevic E, Antonijevic B, Stanic M, et al. Toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney. Int J Environ Res Public Health 2019;16(2):274. [PMID: 30669347 DOI: 10.3390/ijerph16020274]
- Sugiharto S, Winarni D, Islamatasya U, Muhsyi AH, Merpati AB, et al. The protective effect of Gynura procumbens adventitious root against lead acetate toxicity in mice. J Trop Biodivers Biotechnol 2022;7(2):69453. [DOI: 10.22146/jtbb.69453]
- Caito SW, Aschner M. Mitochondrial redox dysfunction and environmental exposures. Antioxid Redox Signal 2015;23(6):578-95. [PMID: 25826672 DOI: 10.1089/ars.2015.6289]
- Omobowale TO, Oyagbemi AA, Akinrinde AS, Saba AB, Daramola OT, et al. Failure of recovery from lead induced hepatoxicity and disruption of erythrocyte antioxidant defence system in Wistar rats. Environ Toxicol Pharmacol 2014;37(3):1202-11. [PMID: 24814264 DOI: 10.1016/j.etap.2014.03.002]
- Strużyńska L, Dąbrowska-Bouta B, Koza K, Sulkowski G. Inflammation-like glial response in lead-exposed immature rat brain. Toxicol Sci 2007;95(1):156-62. [PMID: 17047031 DOI: 10.1093/toxsci/kfl134]
- Sugiharto S, Tri Wibowo A, Zubaidah U, Dwi Savitri A, Faukib MS, et al. Biological properties of Gynura procumbens leaves extract to MDA levels and antioxidant activities in liver of mice. Res J Pharm Technol 2022;15(12):5829-34. [DOI: 10.52711/0974-360X.2022.00984]
- Sugiharto S, Zubaidah U, Winarni D, Manuhara YSW. Gynura procumbens methanolic extracts suppresses proliferation of hepatocellular carcinoma: In vitro assay. In: AIP Conference Proceedings. AIP Publishing LLC; 2023. pp. 90007.
- Jeon HJ, Kwon HJ. Antioxidant effects and functional evaluation of Gynura procumbens extract as a collaboration material for cosmetics and functional food. Kor J Aesthet Cosmetol 2014;12(4):499-507.
- Krishnan V, Ahmad S, Mahmood M. Antioxidant potential in different parts and callus of Gynura procumbens and different parts of Gynura bicolor. Biomed Res Int 2015;2015:147909. [PMID: 26491654 DOI: 10.1155/2015/147909]
- Sugiharto S, Kusuma DY, Winarni D, Bhakti IN, Muhsyi AH, et al. Comparison of antioxidant potential of Gynura procumbens adventitious root in vitro culture and ex vitro. Ecol Environ Conserv 2021;27(4):1880-4.
- Sugiharto S, Winarni D, Wibowo AT, Islamatasya U, Bhakti IN, et al. Gynura procumbens adventitious root extract altered expression of antioxidant genes and exert hepatoprotective effects against cadmium-induced oxidative stress in mice. Hayati J Biosci 2022;29(4):479-86. [DOI: 10.4308/hjb.29.4.479-486]
- Ros C, Mwanri L. Lead exposure, interactions and toxicity: food for thought. Asia Pac J Clin Nutr 2003;12(4):388-95. [PMID: 14672861]
- Sugiharto S, Kusuma DY, Winarni D, Bhakti IN, Muhsyi AH, et al. The comparison toxicity effects of lead and cadmium exposure on hematological parameters and organs of mice. Ecol Environ Conserv 2020;26(4):1842-6.
- Flora G, Gupta D, Tiwari A. Toxicity of lead: a review with recent updates. Interdiscip Toxicol 2012;5(2):47-58. [PMID: 23118587 DOI: 10.2478/v10102-012-0009-2]
- Capitão C, Martins R, Santos O, Bicho M, Szigeti T, et al. Exposure to heavy metals and red blood cell parameters in children: a systematic review of observational studies. Front Pediatr 2022;10:921239. [PMID: 36275050 DOI: 10.3389/fped.2022.921239]
- Chulilla JAM, Colás MSR, Martín MG. Classification of anemia for gastroenterologists. World J Gastroenterol 2009;15(37):4627. [PMID: 19787825 DOI: 10.3748/wjg.15.4627]
- Alvarez-Suarez JM, Giampieri F, González-Paramás AM, Damiani E, Astolfi P, et al. Phenolics from monofloral honeys protect human erythrocyte membranes against oxidative damage. Food Chem Toxicol 2012;50(5):1508-16. [PMID: 22330201 DOI: 10.1016/j.fct.2012.01.042]
- Hegazy AA, Zaher MM, Abd el-hafez MA, Morsy AA, Saleh RA. Relation between anemia and blood levels of lead, copper, zinc and iron among children. BMC Res Notes 2010;3(1):133. [PMID: 20459857 DOI: 10.1186/1756-0500-3-133]
- Siriamornpun S, Kaewseejan N, Chumroenphat T, Inchuen S. Characterization of polysaccharides from Gynura procumbens with relation to their antioxidant and anti-glycation potentials. Biocatal Agric Biotechnol 2021;32:101957. [DOI: 10.1016/j.bcab.2021.101957]
- Wang PP, Zhang Y, Dai LQ, Wang KP. Effect of Angelica sinensis polysaccharide-iron complex on iron deficiency anemia in rats. Chin J Integr Med 2007;13(4):297-300. [PMID: 18180896 DOI: 10.1007/s11655-007-0297-0]
- Sarker S, Nahar L. Natural medicine: the genus Angelica. Curr Med Chem 2004;11(11):1479-500. [PMID: 15180579 DOI: 10.2174/0929867043365189]
- Fordham JB, Raza Naqvi A, Nares S. Leukocyte production of inflammatory mediators is inhibited by the antioxidants phloretin, silymarin, hesperetin, and resveratrol. Mediators Inflamm 2014;2014:1-11. [PMID: 24707119 DOI: 10.1155/2014/938712]
- Shafiq-ur-Rehman. Effect of lead on lipid peroxidation, phospholipids composition, and methylation in erythrocyte of human. Biol Trace Elem Res 2013;154(3):433-9. [PMID: 23846836 DOI: 10.1007/s12011-013-9745-1]
- Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radic Biol Med 2009;47(5):469-84. [PMID: 19500666 DOI: 10.1016/j.freeradbiomed.2009.05.032]
- Seifried RM, Harrison E, Seifried HE. Antioxidants in health and disease. In: Nutrition in the prevention and treatment of disease. Elsevier; 2017. pp. 321-46.
- Mudipalli A. Lead hepatotoxicity & potential health effects. Indian J Med Res 2007;126(6):518-27. [PMID: 18219078]
- Ilesanmi OB, Adeogun EF, Odewale TT, Chikere B. Lead exposure-induced changes in hematology and biomarkers of hepatic injury: protective role of TrévoTM supplement. Environ Anal Health Toxicol 2022;37(2):e2022007. [PMID: 35878915 DOI: 10.5620/eaht.2022007]
- Layal K, Perdhana IS, Louisa M, Estuningtyas A, Soetikno V. The effects of quercetin on oxidative stress and fibrosis markers in chronic kidney disease rat model. Med J Indonesia 2017;26(3):169-77.
- Xu J, Wise JTF, Wang L, Schumann K, Zhang Z, Shi X. Dual roles of oxidative stress in metal carcinogenesis. J Environ Pathol Toxicol Oncol 2017;36(4):345-76. [PMID: 29431065 DOI: 10.1615/JEnvironPatholToxicolOncol.2017025229]
- Esteves F, Rueff J, Kranendonk M. The central role of cytochrome P450 in xenobiotic metabolism—a brief review on a fascinating enzyme family. J Xenobiot 2021;11(3):94-114. [PMID: 34206277 DOI: 10.3390/jox11030007]
- Zhou XW, Ma Z, Geng T, Wang ZZ, Ding G, et al. Evaluation of in vitro inhibition and induction of cytochrome P450 activities by hydrolyzed ginkgolides. J Ethnopharmacol 2014;158:132-9. [PMID: 25456428 DOI: 10.1016/j.jep.2014.10.023]
- Susa ST, Hussain A, Preuss CV. Drug metabolism. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. [PMID: 28723052]
- Elfaki I, Mir R, Almutairi FM, Duhier FMA. Cytochrome P450: polymorphisms and roles in cancer, diabetes and atherosclerosis. Asian Pac J Cancer Prev 2018;19(8):2057-70. [PMID: 30139042 DOI: 10.22034/APJCP.2018.19.8.2057]
- Sharifi-Rad M, Anil Kumar NV, Zucca P, Varoni EM, Dini L, et al. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Front Physiol 2020;11:694. [PMID: 32714204 DOI: 10.3389/fphys.2020.00694]
- Jomova K, Raptova R, Alomar SY, Alwasel SH, Nepovimova E, et al. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch Toxicol 2023;97(10):2499-574. [PMID: 37597078 DOI: 10.1007/s00204-023-03562-9]
- Nwogueze BC, Ojieh AE, Aloamaka CP, Igweh JC, Onyesom I. Levels of glutathione-related antioxidants in some tissues of stressed Wistar rats. Indian J Physiol Pharmacol 2021;65(3):167-76.
- Widiandani T, Tandian T, Zufar BD, Suryadi A, Purwanto BT, et al. In vitro study of pinostrobin propionate and pinostrobin butyrate: cytotoxic activity against breast cancer cell T47D and its selectivity index. J Public Health Afr 2023;14:2516. [PMID: 37492547 DOI: 10.4081/jphia.2023.2516]
- Fadholly A, Ansori ANM, Proboningrat A, Nugraha AP, Iskandar RPD, et al. Apoptosis of HeLa cells via caspase-3 expression induced by chitosan-based nanoparticles of Annona squamosa leaf extract: in vitro study. Ind J Pharm Educ Res 2020;54(2):416-21. [DOI: 10.5530/ijper.54.2.47]
- Asgharian P, Tazekand AP, Hosseini K, Forouhandeh H, Ghasemnejad T, et al. Potential mechanisms of quercetin in cancer prevention: focus on cellular and molecular targets. Cancer Cell Int 2022;22(1):257. [PMID: 35971151 DOI: 10.1186/s12935-022-02677-w]
- Chou CC, Yang JS, Lu HF, Ip SW, Lo C, et al. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Arch Pharm Res 2010;33(8):1181-91. [PMID: 20803121 DOI: 10.1007/s12272-010-0808-y]
- Seo HS, Ku JM, Choi HS, Choi YK, Woo JK, et al. Quercetin induces caspase-dependent extrinsic apoptosis through inhibition of signal transducer and activator of transcription 3 signaling in HER2-overexpressing BT-474 breast cancer cells. Oncol Rep 2016;36(1):31-42. [PMID: 27175602 DOI: 10.3892/or.2016.4786]