Incidentally Detected Liver Metastases during Pancreas Contrast-enhanced Ultrasound
1Department of Ultrasound, Zhongshan Hospital, Fudan University, Shanghai 200032, China
2Shanghai Institute of Medical Imaging, Shanghai 200032, China
3Department of Ultrasound, Shaoxing Yuecheng People’s Hospital, Zhejiang 200032, China
4Department Allgemeine Innere Medizin (DAIM), Kliniken Hirslanden Beau Site, Salem und Permanence, Bern, Switzerland
aThese authors contributed equally to this work.
*Correspondence to Yi Dong, E-mail: dong.yi@zs-hospital.sh.cn
Received: August 5 2021; Revised: September 9 2021; Accepted: November 3 2021; Published Online: December 9 2021
Cite this paper:
Dan Zuo, Ji-Jiang Qian, Yi Dong, Wen-Ping Wang, Xiao-Fan Tian, Yi-Jie Qiu and Christoph Frank Dietrich. Incidentally Detected Liver Metastases during Pancreas Contrast-enhanced Ultrasound. BIO Integration 2021; 2(4): 135–142.
DOI: 10.15212/bioi-2021-0026. Available at: https://bio-integration.org/
Download citation
© 2021 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
Purpose: The purpose of current study was to investigate the value of the late-phase enhancement features of pancreas contrast-enhanced ultrasound (CEUS) in the detection of liver metastases in patients with pancreatic ductal adenocarcinomas (PDAC).
Methods: From October 2020 to March 2021, 86 patients were prospectively enrolled. The gold standard of liver metastatic and PDAC lesions were based on histopathologically diagnoses and multiple imaging modalities results. B-mode ultrasound (BMUS) was performed to detect suspected liver metastases before CEUS. During the late phase of pancreas CEUS, the entire liver was scanned again to detect hypoenhanced liver metastases. Liver metastases were confirmed by biopsy and histopathological results. The number and size of liver metastases detected by BMUS and during CEUS late phase were recorded and compared.
Results: Suspected liver metastases were detected in 14 patients by BMUS (n = 23). During the late phase of CEUS, hypoenhanced liver metastases were detected in 23 patients (n = 87). When compared with BMUS, whole-liver scan during the late phase of CEUS detected more isoechoic, small, or superficially located lesions. Compared with BMUS, the specificity, sensitivity, positive predictive value, and negative predictive value of CEUS in diagnosing of liver metastases in PDAC patients were significantly improved (96.72% vs. 100%, 48% vs. 92%, 85.71% vs. 100%, and 83.10% vs. 96.83%, respectively) (P < 0.05).
Conclusion: The late phase whole liver scan during CEUS of pancreas helps to detect more liver metastases, which is important for further clinical decision-making.
Keywords
Liver metastasis, pancreatic ductal adenocarcinoma (PDAC), contrast-enhanced ultrasound (CEUS), detection, diagnosis.
Background
Pancreatic ductal adenocarcinoma (PDAC) is the most common malignant tumor of the pancreas, and it has poor prognosis and high mortality rate [1]. Surgical resection remains the most effective curative treatment for PDAC, but more than 80% of patients are not amenable for surgery because of extensive local vascular involvement or the presence of distant metastasis [2]. Patients who underwent surgical radical resection has better survival outcomes and quality of life than patients who were not suitable for resection [3]. Liver is the most common organ for pancreatic metastasis [4, 5]. According to current National Comprehensive Cancer Network (NCCN) guidelines, surgery operation is not suitable for patients with pancreatic cancer and multiple liver metastases [6]. Instead, the most accepted therapeutic regimen for pancreatic cancer with hepatic metastases is chemotherapy [7]. Therefore, preoperative noninvasive detection of potential liver metastasis is of vital importance to avoid unnecessary surgery. However, preoperative sensitivity and accurate detection of liver metastasis remain a clinical challenge. Previously, up to 50% of liver metastases, especially small ones, could not be detected before operation [8]. Approximately 70% of small lesions (<1 cm) were undetected due to location close to the diaphragm and under the liver capsule [9]. Besides, approximately 7.5% of liver metastases showed isoechoic on conventional B-mode ultrasound (BMUS) [10].
Preoperative imaging evaluation is used to detect potential metastatic lesions. Currently, computed tomography (CT) and magnetic resonance image (MRI) are most widely used imaging approaches, with sensitivity of 90% [11]. Nevertheless, because of the nephrotoxicity and claustrophobia, clinical applications of CT and MRI are limited in some patients [11, 12]. Contrast-enhanced ultrasound (CEUS) is a real-time and noninvasive imaging method with no risk of exposure to radiation or nephrotoxicity. With injection of ultrasound contrast agent, CEUS has significantly improved the detection rate of focal liver lesions (FLLs) [13]. It was reported that CEUS could correctly characterize FLLs, with 92% sensitivity and 98% specificity [14]. In patients who was incidentally diagnosed with FLLs, the pooled estimates of sensitivity and specificity of CEUS for malignancy were 95.1% and 93.8%, respectively [15]. According to current World Federation for Ultrasound in Medicine and Biology (WFUMB) guidelines, distinctive punched washout during the portal venous and late phases are characteristic features of liver metastatic lesions [16].
The purpose of the current study was to evaluate the value of late-phase whole-liver scan during pancreatic CEUS in detection of liver metastases.
Patients and Methods
This research was approved by the ethical committee of our institute (no. B2021-144). Written informed consent was provided by each patient before CEUS examination.
Patients
From October 2020 to March 2021, patients who underwent preoperative pancreatic CEUS were included. The inclusion criteria were patients with clinically suspected pancreatic malignant lesions, those who planned to undergo surgical resection of pancreatic malignant lesion or biopsy of suspected liver metastatic lesion, those who underwent pancreatic CEUS 1 week before surgery, those who underwent at least two imaging modalities other than CEUS, those whose liver metastasis of PDAC was proven by biopsy and histopathology, or those whose imaging proved no liver metastasis.
Patients with no other imaging scan results except for ultrasound and those who could not tolerate CEUS procedure were excluded.
Ultrasound equipment
The ultrasound equipment was a Siemens ACUSON Sequoia equipment (Siemens Medical Solutions, Chicago, IL, USA) with a 5 C-1 transducer (1–6 MHz). The contrast agent was SonoVue (Bracco Imaging SpA, Milan, Italy), and the injection dose was 1.0–1.5 mL for each patient.
All BMUS and CEUS examinations were performed by an experienced radiologist who was blinded to other imaging diagnoses. The images of CEUS were interpreted according to current WFUMB liver CEUS guidelines [16].
B-mode ultrasound of liver and pancreas
First, BMUS scan of the pancreas was performed. Then, the whole liver was scanned to detect possible metastasis on BMUS. Any hypoechoic or hyperechoic solid lesion was considered a potential liver metastasis. The location, size, echogenicity, and margin of suspected lesions were recorded.
Contrast-enhanced ultrasound examination
After the injection of contrast agent, the enhancement patterns and degree of pancreatic lesion were observed and recorded for 2 minutes. During the late phase of CEUS (2–5 minutes after the initiation of contrast agent injection), the whole liver was scanned carefully under CEUS mode. Liver metastasis was defined as a marked washout lesion on CEUS during late phase. The number and location of those lesions were recorded and compared.
If the diagnosis of a suspicious metastasis was inconclusive, another 1–1.5 mL contrast agent would be injected to confirm enhancement features during the arterial phase.
Ultrasound-guided biopsy and histopathological analysis were performed for the final diagnosis of liver metastasis. In multi-lesion cases, the largest lesion of suspected metastases was chosen for histological confirmation. For patients without liver metastatic lesion, the standard diagnosis was based on at least two imaging modalities except for CEUS.
Statistical analysis
SPSS version 20.0 (IBM Corp., Armonk, NY, USA) was used for the statistical analysis. Descriptive analyses were performed using mean ± standard deviation for quantitative variables. The comparison of diagnostic accuracy between BMUS and CEUS was analyzed with paired chi-square test. A P value <0.05 was considered statistically significant.
Results
Clinical features
From October 2020 to March 2021, a total of 86 patients diagnosed with pancreatic malignant tumors were included. Among them, 25 patients were diagnosed with liver metastases.
B-mode ultrasound
Suspected liver metastases were detected in 14 patients (n = 23) during BMUS scan. Single and multiple lesions were detected in 8 and 6 patients, respectively. All lesions were heterogeneously hypoechoic (21/23) (Figure 1) or hyperechoic (2/23) solid masses with ill-defined margins (Figure 2). The mean size was 19.6 ± 9.4 mm (range, 10.1–44.0 cm) (Table 1).
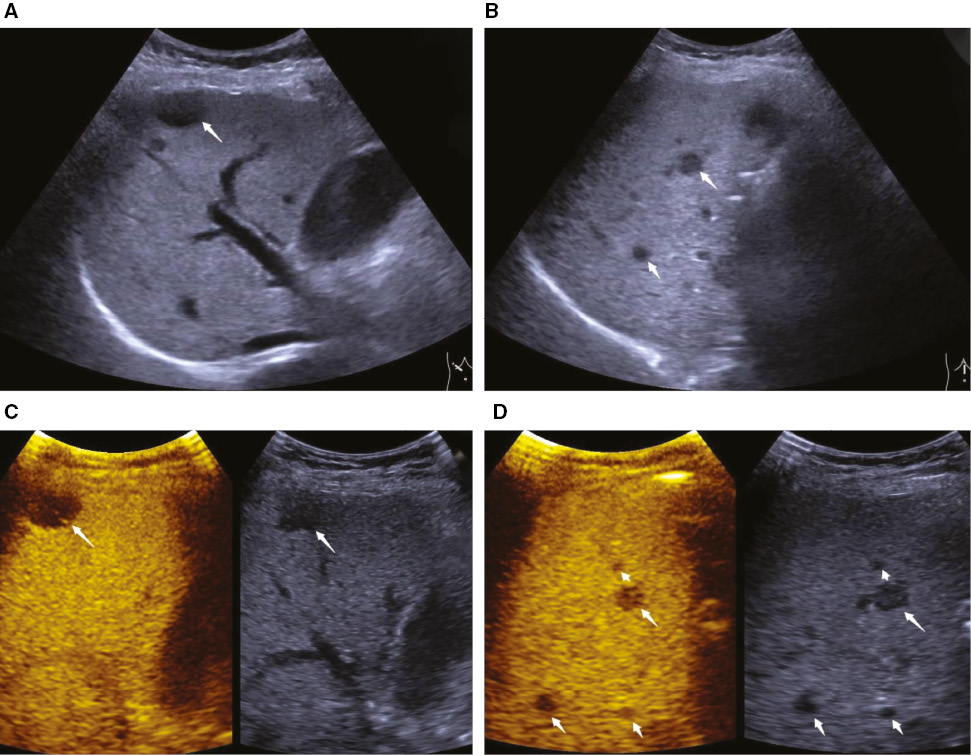
Figure 1 A 63-year-old woman was diagnosed with pancreatic ductal adenocarcinoma with multiple hepatic metastases (arrow). All liver metastases were heterogeneously hypoechoic on B-mode ultrasound (A, B) and hypoenhanced on contrast-enhanced ultrasound during the late phase (C, D).
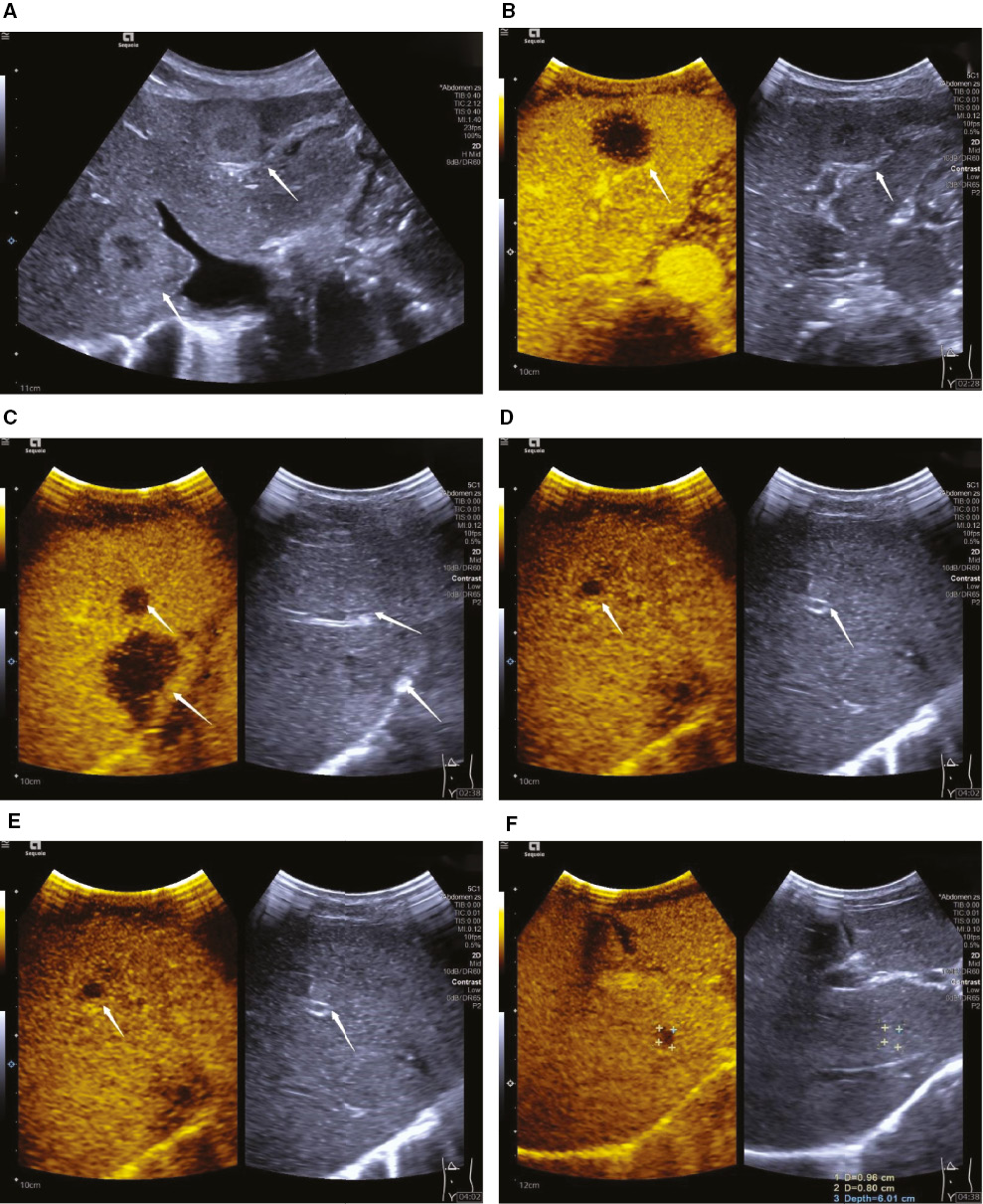
Figure 2 A 67-year-old woman was diagnosed with pancreatic ductal adenocarcinoma and multiple liver metastases (arrow). On B-mode ultrasound, two hypoechoic lesions with hyperechoic rim were detected in the right lobe of the liver (A). During late-phase of pancreatic contrast-enhanced ultrasound, more hypoenhanced and occult lesions were detected (B–F).
Table 1 Comparison of BMUS and CEUS in Detecting Liver Metastases of PDAC
| BMUS | CEUS | |
|---|---|---|
| Number | 20 | 87 |
| Single | 8 | 3 |
| Multiple | 6 | 20 |
| Size (mm) | ||
| Mean size | 19.6 ± 9.4 | 16.4 ± 9.4 |
| Range | 10.1–44.0 | 4.5–49.4 |
| <1 cm | 0 | 22 |
| <2 cm | 15 | 66 |
| Echogenicity of liver lesion | ||
| Hypoechoic | 18 | 18 |
| Isoechoic | 0 | 67 |
| Hyperechoic | 2 | 2 |
| Location | ||
| <2 cm to the ventral liver capsule | 9 | 36 |
| >2 cm to the ventral liver capsule | 11 | 51 |
BMUS, B-mode ultrasound; CEUS, contrast-enhanced ultrasound; PDAC: pancreatic ductal adenocarcinoma.
Contrast-enhanced ultrasound features
After injection of ultrasound contrast agent, liver metastases were detected in 23 patients (n = 87). During the late phase of CEUS scanning of the whole liver, all lesions showed hypoenhancement. Single and multiple lesions were detected in 3 and 20 patients, respectively. The mean size of the 87 lesions was 1.64 ± 0.94 cm (range, 0.45–4.94 cm) (Table 1).
Comparison between BMUS and CEUS
Sixty-seven additional liver metastasis lesions were detected on CEUS in 22 patients (Figure 3). However, all those lesions showed isoechoic or slightly hypoechoic on BMUS. Among the 67 lesions that were not visible on BMUS, 71.64% (48/67) were smaller than 2 cm and 31.34% (21/67) were less than 1 cm. When compared with BMUS, CEUS detected more isoechoic, small, or superficial located lesions (Table 1).
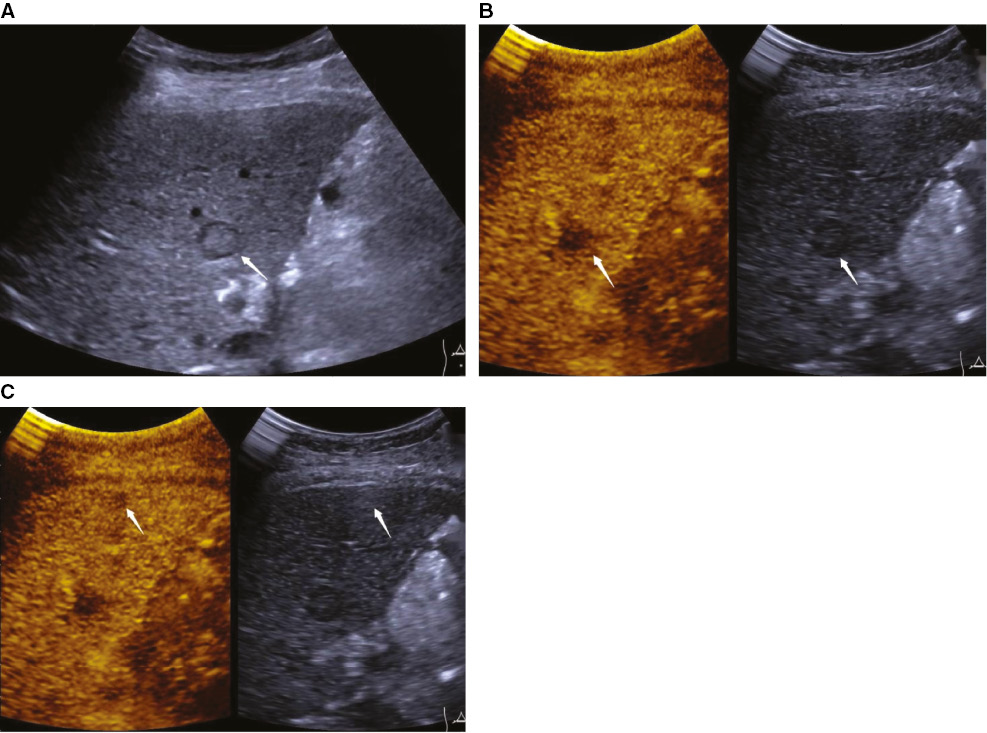
Figure 3 An 80-year-old woman was diagnosed with pancreatic ductal adenocarcinoma with multiple hepatic metastases (arrow). A hepatic metastatic lesion was heterogeneously hypoechoic and ill-defined on B-mode ultrasound (BMUS) (A) with marked washout during the late phase of contrast-enhanced ultrasound (CEUS) (B). During the late phase of CEUS, another smaller, hypoenhanced lesion was detected, which was not visible on BMUS (C).
Among BMUS-detected lesions, one hypoechoic lesion was proved to be liver hemangioma and 2 hypoechoic lesions were proved to be focal fatty infiltration by multiple image modalities. The hemangioma lesion was a hypoechoic mass with clear margin on BMUS. After the injection of contrast agent, this lesion showed peripheral nodular hyperenhancement with centripetal progression during arterial phase and continuous hyperenhancement during portal venous and late phase. While the focal fatty infiltration lesion was hyperechoic on BMUS and iso-enhancement during all phases of CEUS.
Diagnostic efficiency
Among 25 patients with histologically confirmed hepatic metastatic lesions of pancreatic cancers, BMUS correctly diagnosed hepatic metastatic lesions of 13 patients, whereas CEUS correctly diagnosed of 23 patients and the diagnostic accuracy of CEUS was significantly higher than that of BMUS. Taking pathological and multiple imaging modalities results as gold standard, the sensitivity, specificity, positive predictive value, and negative predictive value of BMUS and CEUS for correctly diagnosing hepatic metastases were 48% and 92%, 96.72% and 100%, 85.71% and 100%, and 83.10% and 96.83%, respectively.
Discussion
Accurate detection of potential liver metastases in PDAC patients is important for preoperative staging and further therapeutic decision-making plans of PDCA patients. BMUS is the first-line imaging methods for detection FLLs. However, although the sensitivity and accuracy of BMUS in detecting potential liver metastasis remain unsatisfactory [17, 18], application of CEUS may provide added diagnostic evidence and improve the diagnostic confidence of FLLs [19, 20].
Currently, it is still challenging for BMUS to detect small or isoechoic FLLs [17, 18]. CEUS could improve the diagnose rate of small (<1 cm) and occult lesions. Whole-liver scan during the portal venous and late phases of pancreatic CEUS enables better detection of liver metastases in patients with primary non-hepatic malignant tumors [21]. The washout appearance during the venous and late phases of CEUS may play a confirmatory role when evaluating suspected lesions on BMUS. In our study, 67 additional lesions detected on CEUS were not visible on BMUS. A high proportion of these lesions were small. The smallest lesion detected on CEUS was only 0.45 cm in diameter. Therefore, CEUS might be helpful to make better treatment plans for these patients.
CEUS features may help to differentiate suspected liver metastasis on BMUS. In our study, a hypoechoic hemangioma was detected on BMUS. After injection of contrast agent, this lesion showed typical enhancement pattern on CEUS. In our study, one focal liver infiltration (FFI) lesion was hypoechoic on BMUS. However, on CEUS, the lesion showed no wash-out during the late phase of CEUS. FFI is one of the most common benign liver entities, and it could be misdiagnosed as malignancy on BMUS [22, 23]. FFI shows no difference in vascularity from peripheral parenchyma and contains normal undisturbed vessels [24]. Typically, homogenous iso-enhancement in all phases of CEUS has been regarded as a distinctive feature of FFI [16].
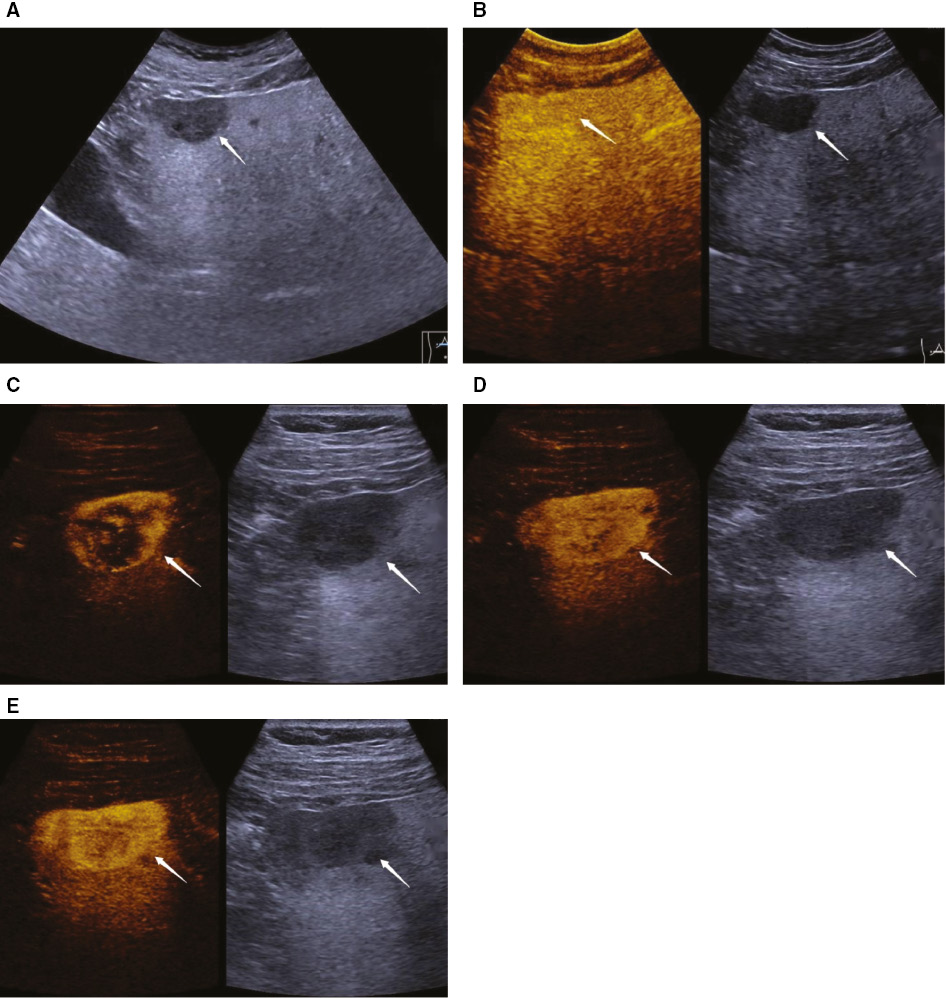
Over the last decade, although CEUS has significantly improves the detection rate of FLLs, it is still to be difficult to detect lesions located close to liver capsule [25, 26]. Compared with conventional low-frequency convex transducer, a high-frequency transducer (7–9 MHz) provides high resolution and better near-field investigations [27, 28]. CEUS performed with high-frequency transducers is an exciting technique for detection of small and superficial lesions. In 2006, Schacherer et al. compared the capability of low- and high-frequency transducers in the detection of liver metastases. They concluded that high-frequency transducer sonography revealed new, potentially malignant hepatic lesions in almost every patient [29]. In 2017, Wang et al. reported that CEUS performed with high-frequency transducer significantly improved the detection rate of FLLs, with overall sensitivity, specificity. and diagnostic accuracy of 88.9%, 92.6%, and 96.2%, respectively [25]. In our study, compared with conventional CEUS, 3 lesions located close to liver capsule showed more imaging details with high-frequency mode (Figure 4).
Figure 4 A 48-year-old man was diagnosed with pancreatic ductal adenocarcinoma. A hypoechoic mass was detected in the left lobe of the liver on B-mode ultrasound (A). The lesion showed iso-enhancement with peripheral liver parenchyma during the late phase of contrast-enhanced ultrasound (B). After contrast agent injection, the lesion showed peripheral nodular hyperenhancement with centripetal progression during the arterial phase (C–E).
Limitation
The limitation of this study is the limited number of patients. Future studies with larger samples size should be performed to further verify the value of CEUS in preoperative detection of liver metastases in PDAC.
Conclusion
The whole-liver scan during the late phase of CEUS is helpful for detecting potential liver metastasis in patients with PDAC.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 82071942), Shanghai Pujiang Program (grant no. 2020PJD008), Clinical Research Plan of SHDC (grant no. SHDC2020CR4060), Shanghai Municipal Science and Technology Medical Guidance Project (grant no. 18411967200), Shanghai Municipal Health and Family Planning Commission Research Project (grant no. 201840215), and Shanghai Municipal Science and Technology Major Project (grant no. 2017SHZDZX01).
Conflicts of interest
There were no conflicts of interest with respect to the authorship or publication of this article.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70(1):7-30. [PMID: 31912902 DOI: 10.3322/caac.21590]
- Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet 2016;388(10039):73-85. [PMID: 26830752 DOI: 10.1016/S0140-6736(16)00141-0]
- Wo JY, Niemierko A, Ryan DP, Blaszkowsky LS, Clark JW, et al. Tolerability and long-term outcomes of dose-painted neoadjuvant chemoradiation to regions of vessel involvement in borderline or locally advanced pancreatic cancer. Am J Clin Oncol 2018;41(7):656-61. [PMID: 28134673 DOI: 10.1097/COC.0000000000000349]
- Raman SP, Reddy S, Weiss MJ, Manos LL, Cameron JL, et al. Impact of the time interval between MDCT imaging and surgery on the accuracy of identifying metastatic disease in patients with pancreatic cancer. AJR Am J Roentgenol 2015;204(1):W37-42. [PMID: 25539271 DOI: 10.2214/AJR.13.12439]
- Allen VB, Gurusamy KS, Takwoingi Y, Kalia A, Davidson BR. Diagnostic accuracy of laparoscopy following computed tomography (CT) scanning for assessing the resectability with curative intent in pancreatic and periampullary cancer. Cochrane Database Syst Rev 2016;7:CD009323. [PMID: 27383694 DOI: 10.1002/14651858.CD009323.pub3]
- Tempero MA, Malafa MP, Chiorean EG, Czito B, Scaife C, et al. Pancreatic Adenocarcinoma, Version 1.2019. J Natl Compr Canc Netw 2019;17(3):202-10. [PMID: 30865919 DOI: 10.6004/jnccn.2019.0014]
- Bellon E, Gebauer F, Tachezy M, Izbicki JR, Bockhorn M. Pancreatic cancer and liver metastases: state of the art. Updates Surg 2016;68(3):247-51. [PMID: 27832445 DOI: 10.1007/s13304-016-0407-7]
- Valls C, Andia E, Sanchez A, Fabregat J, Pozuelo O, et al. Dual-phase helical CT of pancreatic adenocarcinoma: assessment of resectability before surgery. AJR Am J Roentgenol 2002;178(4):821-6. [PMID: 11906855 DOI: 10.2214/ajr.178.4.1780821]
- Stang A, Keles H, Hentschke S, Seydewitz C, Keuchel M, et al. Real-time ultrasonography-computed tomography fusion imaging for staging of hepatic metastatic involvement in patients with colorectal cancer: initial results from comparison to US seeing separate CT images and to multidetector-row CT alone. Invest Radiol 2010;45(8):491-501. [PMID: 20458251 DOI: 10.1097/RLI.0b013e3181ddd3da]
- Nakano S, Tsushima Y, Higuchi T, Taketomi-Takahashi A, Amanuma M. Contrast- and non-contrast-enhanced ultrasonography (US) findings of hepatic metastasis from malignant pheochromocytoma/paraganglioma. Jpn J Radiol 2012;30(4):310-6. [PMID: 22271156 DOI: 10.1007/s11604-012-0051-1]
- Muhi A, Ichikawa T, Motosugi U, Sou H, Nakajima H, et al. Diagnosis of colorectal hepatic metastases: comparison of contrast-enhanced CT, contrast-enhanced US, superparamagnetic iron oxide-enhanced MRI, and gadoxetic acid-enhanced MRI. J Magn Reson Imaging 2011;34(2):326-35. [PMID: 21780227 DOI: 10.1002/jmri.22613]
- Choi YR, Kim JH, Park SJ, Hur BY, Han JK. Therapeutic response assessment using 3D ultrasound for hepatic metastasis from colorectal cancer: Application of a personalized, 3D-printed tumor model using CT images. PLoS One 2017;12(8):e0182596. [PMID: 28797089 DOI: 10.1371/journal.pone.0182596]
- Sidhu PS, Cantisani V, Dietrich CF, Gilja OH, Saftoiu A, et al. The EFSUMB Guidelines and recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in non-hepatic applications: update 2017 (Long Version). Ultraschall Med 2018;39(2):e2-e44. [PMID: 29510439 DOI: 10.1055/a-0586-1107]
- Auer TA, Fischer T, Garcia SRM, Penzkofer T, Jung EM, et al. Value of contrast-enhanced ultrasound (CEUS) in Focal Liver Lesions (FLL) with inconclusive findings on cross-sectional imaging. Clin Hemorheol Microcirc 2020;74(3):327-39. [PMID: 31658052 DOI: 10.3233/CH-190718]
- Westwood M, Joore M, Grutters J, Redekop K, Armstrong N, et al. Contrast-enhanced ultrasound using SonoVue(R) (sulphur hexafluoride microbubbles) compared with contrast-enhanced computed tomography and contrast-enhanced magnetic resonance imaging for the characterisation of focal liver lesions and detection of liver metastases: a systematic review and cost-effectiveness analysis. Health Technol Assess 2013;17(16):1-243. [PMID: 23611316 DOI: 10.3310/hta17160]
- Dietrich CF, Nolsoe CP, Barr RG, Berzigotti A, Burns PN, et al. Guidelines and Good Clinical Practice Recommendations for Contrast Enhanced Ultrasound (CEUS) in the Liver—Update 2020—WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultraschall Med 2020;41(5):562-85. [PMID: 32707595 DOI: 10.1055/a-1177-0530]
- Holzapfel K, Reiser-Erkan C, Fingerle AA, Erkan M, Eiber MJ, et al. Comparison of diffusion-weighted MR imaging and multidetector-row CT in the detection of liver metastases in patients operated for pancreatic cancer. Abdom Imaging 2011;36(2):179-84. [PMID: 20563868 DOI: 10.1007/s00261-010-9633-5]
- Kopljar M, Patrlj L, Busic Z, Kolovrat M, Rakic M, et al. Potential use of Doppler perfusion index in detection of occult liver metastases from colorectal cancer. Hepatobiliary Surg Nutr 2014;3(5):259-67. [PMID: 25392837 DOI: 10.3978/j.issn.2304-3881.2014.09.04]
- Dong Y, Zhang XL, Mao F, Huang BJ, Si Q, Wang WP. Contrast-enhanced ultrasound features of histologically proven small (≤=20 mm) liver metastases. Scand J Gastroenterol 2017;52(1):23-8. [PMID: 27577113 DOI: 10.1080/00365521.2016.1224380]
- Hsiao CY, Chen PD, Huang KW. A prospective assessment of the diagnostic value of contrast-enhanced ultrasound, dynamic computed tomography and magnetic resonance imaging for patients with small liver tumors. J Clin Med 2019;8(9):1353. [PMID: 31480576 DOI: 10.3390/jcm8091353]
- D’Onofrio M, Crosara S, De Robertis R, Canestrini S, Mucelli RP. Contrast-enhanced ultrasound of focal liver lesions. AJR Am J Roentgenol 2015;205(1):W56-66. [PMID: 26102419 DOI: 10.2214/AJR.14.14203]
- von Herbay A, Westendorff J, Gregor M. Contrast-enhanced ultrasound with SonoVue: differentiation between benign and malignant focal liver lesions in 317 patients. J Clin Ultrasound 2010;38(1):1-9. [PMID: 19790253 DOI: 10.1002/jcu.20626]
- Fu R, Qiu T, Ling W, Lu Q, Luo Y. Nodular focal fat sparing of liver mimicking hepatocellular carcinoma in contrast-enhanced ultrasound: a case report. Medicine (Baltimore) 2019;98(22):e15431. [PMID: 31145272 DOI: 10.1097/MD.0000000000015431]
- Kim TK, Jang HJ, Wilson SR. Benign liver masses: imaging with microbubble contrast agents. Ultrasound Q 2006;22(1):31-9. [PMID: 16641791]
- Wang WP, Dong Y, Cao J, Mao F, Xu Y, et al. Detection and characterization of small superficially located focal liver lesions by contrast-enhanced ultrasound with high frequency transducers. Med Ultrason 2017;19(4):349-56. [PMID: 29197910 DOI: 10.11152/mu-1276]
- Wege AK, Schardt K, Schaefer S, Kroemer A, Brockhoff G, et al. High resolution ultrasound including elastography and contrast-enhanced ultrasound (CEUS) for early detection and characterization of liver lesions in the humanized tumor mouse model. Clin Hemorheol Microcirc 2012;52(2-4):93-106. [PMID: 22975935 DOI: 10.3233/CH-2012-1587]
- Buadu A, Meyer MA. Small liver nodule detection with a high-frequency transducer in patients with chronic liver disease: report of 3 cases. J Ultrasound Med 2013;32(2):355-9. [PMID: 23341394 DOI: 10.7863/jum.2013.32.2.355]
- Loss M, Schneider J, Uller W, Wiggermann P, Scherer MN, et al. Intraoperative high resolution linear contrast enhanced ultrasound (IOUS) for detection of microvascularization of malignant liver lesions before surgery or radiofrequeny ablation. Clin Hemorheol Microcirc 2012;50(1-2):65-77. [PMID: 22538536 DOI: 10.3233/CH-2011-1444]
- Schacherer D, Wrede C, Obermeier F, Scholmerich J, Schlottmann K, Klebl F. Comparison of low and high frequency transducers in the detection of liver metastases. Dig Liver Dis 2006;38(9):677-82. [PMID: 16787772 DOI: 10.1016/j.dld.2006.05.006]