Biophotonics in Photomedicine
Shanxi Eye Hospital 100 Fudong St, Liu Xiang Shang Quan Xinghualing District, Taiyuan 030002, China
*Correspondence to: E-mail: hl486@cornell.edu
Published Online: April 30 2021
Cite this paper:
Hui Liu and Juan Cheng. Biophotonics in Photomedicine. BIO Integration 2021; 2(3): 91–93.
DOI: 10.15212/bioi-2020-0043. Available at: https://bio-integration.org/
Download citation
© 2021 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
The first biophotonic application can be dated back to the 16th century, when the optical microscope was invented to observe tissues. A few hundred years later, the 21st century is called “the age of photonic.” The advancement of photonic technology has enabled it to replace electronics in many areas, with some intrinsic advantages, such as broader bandwidth at higher frequency and faster speed with no mass [1]. Biophotonics, a new field covered extensively in areas where biology and photonics meet, has applications not only in the human body but also in fields such as agriculture [2, 3]. After transforming communication and data storage, photonics has continued its influence in life science. Its benefits penetrates our lives seamlessly before we know it. This short editorial does not cover the whole territory but instead serves as a modest attempt to focus on the medical application of biophotonics.
Photomedicine, the application of biophotonic applications in healthcare, is one of the most productive translational medicine areas. A photon has natural characteristics of noncontact sterile interaction, high-resolution, and the ability to carry rich information [4], making it an ideal candidate for therapeutic and diagnostic applications. Technologies to generate and manipulate photons have provided many new light sources to broaden the applications in both territories. The development of short-pulse high-power laser technology is a good example. For therapeutic applications, it has transformed the surgical application of medical lasers to be much more precise and less damaging; for diagnostics application, it has revolutionized nonlinear optical imaging with broadened spatial–temporal scales when compared with the traditional imaging tools, allowing people to see structures at another level in a noninvasive way [5–8]. Biophotonics impacts almost all medical domains. For example, as one of the most benefitted disciplines, ophthalmology has two of the most successful models used daily: optical coherent tomography for diagnostic purposes and laser-assisted in situ keratomileusis for surgery.
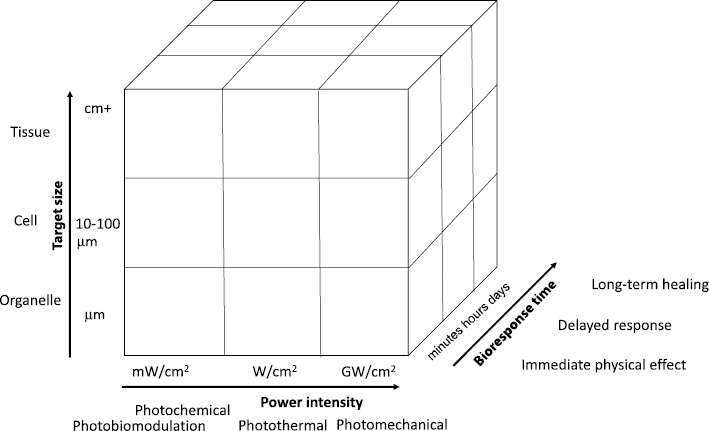
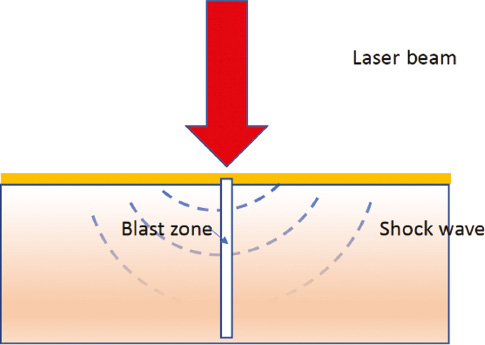
Besides constantly adopting new technologies, researchers have also dig into the light–tissue interactions to determine complications of clinical application over the past 30 years [9–13]. The complications of laser treatment in the 1970s have been reduced significantly with the understanding of this new biological process [9, 10] and the use of the right laser technology. Dr. Anderson paved the road for correct laser parameters for surgical purposes based on a tissue’s properties [10]. Dr. Jacques developed a Monte-Carlo simulation for biological tissues and made it an open-source project that enabled many excellent numeric modeling works [14, 15]. More recently, many complex experiments added to the bio-optical property knowledge base and improved medical procedures [11, 16, 17]. Depending on the response time scale and target size scale, light–tissue interaction has the following characteristic processes (Figure 1): photomechanical, photothermal, photochemical [12], and photobiomodulation [18]. Their work range and biological responses are significantly different. The photomechanical process needs a very high peak power laser to blast off local tissues with minimum heat effect (Figure 2). The higher the peak power, the shorter time required for the laser application; thus, less surrounding tissue damage occurs from the extra heat. It enables precision cuttings as a ultra-sharp knife, which is critical to saving vital functional biological tissues. Some examples of photomechanical processes are picosecond laser for tattoo removal in dermatology [19] and femtosecond lasers in eye surgeries [20]. Meanwhile, photothermal procedures [21–25] maximize the selective heat absorption of specific biological tissue to generate signals for imaging or achieve ablation or coagulation for treatment. The photochemical process works at the molecular level using relatively low-level intensity light (<1 W/cm2) to have an immediate effect on organelles. Photodynamic therapy (PDT) is a photochemical process that has applications in various medical fields [26]. It targets organelles, with delayed cell death in hours and healing response in days [12].
Figure 1 Light–tissue interaction. Adapted from Steven Jacques, “Laser–tissue interaction: photochemical, photothermal and photomechanical,” Laser in General Surgery, 1995.
Figure 2 Demonstration of photomechanical effect. A strong laser beam generates shock waves to blast off the targeted tissue with clear boundaries.
Last but not least, photobiomodulation, previously called low-level light therapy, has recently gained popularity in clinical applications and home therapy, owing to its safety, low cost, and effectiveness [18, 27]. It works at an even lower energy level than PDT. Dr. Endre Mester first discovered it in Hungary in 1967 when he tried to duplicate the laser treatment of tumors by Dr. Paul Edward McGuff in Boston, Massachusetts. However, the laser he used had much lower energy than the one used by Dr McGuff in Boston. Instead of killing cancer tumors, he saw the laser promoted hair growth and wound healing. Thirty years later, Dr. Michael Hamblin, at the Wellman Center of Photomedicine, noticed over 100% viability in the no-drug, light-only control group of PDT experiments. He has been dedicated to studying the mechanism behind the phenomenon ever since and led significant progress in the scientific discovery and medical application of photobiomodulation. In this case, light is not used as a tool but rather as a drug. A specific biomedical optical event may involve more than one of the processes above. It is not the clear differentiation of those processes by name that matters, but rather the understanding of the true nature of different biological responses does in order to improve the precision and enable the innovations of medical applications.
As a cross-disciplinary field, biophotonics is a natural platform for innovation. For example, researchers have taken advantages of the recently developed nanostructures to optimize imaging signals and improve drug delivery efficiency [6, 28]. Active investment in healthcare also contributes to the quick clinical transitions of biophotonic innovations. However, to genuinely and successfully improve people’s lives, many gaps have to be bridged. Horizontally, the gaps are between scientists from different fields to make new fundamental breakthroughs through knowledge merging; vertically, the gaps lie in scientists, engineer medical professionals, and physicists to make innovated, reliable, and practical products. Learning another field’s language is never easy; promoting this learning requires leaders to advocate communication and understanding among experts. Although it is still a relatively young field, with abundant success so far and booming innovations coming along, it is reasonable to expect giant waves of healthcare transformation involving biophotonics to continue for the next few decades.
References
- DeMaria AJ. Photonics vs. electronics technologies. Optics News 1989;15:22-37. [DOI: 10.1364/ON.15.4.000022]
- Tan JY, Ker PJ, Lau KY, Hannan MA, Tang SGH. Applications of photonics in agriculture sector: a review. Molecules 2019;24:2025. [PMID: 31137897 DOI: 10.3390/molecules24102025]
- Photonic N. The next big thing? Nat Photon 2007;1:485-5. [DOI: 10.1038/nphoton.2007.149]
- Goda K. Biophotonics and beyond. APL Photon 2019;4:050401. [DOI: 10.1063/1.5100614]
- Marble CB, Yakovlev VV. Biomedical optics applications of advanced lasers and nonlinear optics. J Biomed Opt 2020;25:1-9. [PMID: 32329266 DOI: 10.1117/1.JBO.25.4.040902]
- Huang B, Babcock H, Zhuang X. Breaking the diffraction barrier: super-resolution imaging of cells. Cell 2010;143:1047-58. [PMID: 21168201 DOI: 10.1016/j.cell.2010.12.002]
- Fujimoto JG, Pitris C, Boppart SA, Brezinski ME. Optical coherence tomography: an emerging technology for biomedical imaging and optical biopsy. Neoplasia 2000;2:9-25. [PMID: 10933065 DOI: 10.1038/sj.neo.7900071]
- Xu C, Wise FW. Recent advances in fiber lasers for nonlinear microscopy. Nat Photon 2013;7:875–82. [PMID: 24416074 DOI: 10.1038/nphoton.2013.284]
- Anderson RR, Parrish JA. The optics of human skin. J Invest Dermatol 1981;77:13-9. [PMID: 7252245 DOI: 10.1111/1523-1747.ep12479191]
- Rox R, Anderson JAP. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science 1983;220:524-7. [PMID: 6836297 DOI: 10.1126/science.6836297]
- Jacques SL. Optical properties of biological tissues: a review. Phys Med Biol 2013;58:18. [PMID: 23666068 DOI: 10.1088/0031-9155/58/11/R37]
- Jacques SL. Laser–tissue interactions photochemical, photothermal, and photomechanical. Lasers Gen Surg 1995;72:28. [PMID: 1589829 DOI: 10.1016/s0039-6109(16)45731-2]
- Jacques SL. Ratio of entropy to enthalpy in thermal transitions in biological tissues. J Biomed Opt 2006;11:041108. [PMID: 16965136 DOI: 10.1117/1.2343437]
- Liu Y, Jacques SL, Azimipour M, Rogers JD, Pashaie R, Eliceiri KW. OptogenSIM: a 3D Monte Carlo simulation platform for light delivery design in optogenetics. Biomed Opt Express 2015;6:4859-70. [PMID: 26713200 DOI: 10.1364/BOE.6.004859]
- Wang L, Jacques SL, Zheng L. MCML-Monte Carlo modeling of light transport in multi-layered tissues. Comput Method Programs Biomed 1995;47:16. [PMID: 7587160 DOI: 10.1016/0169-2607(95)01640-f]
- Bargo PR, Prahl SA, Goodell TT, Sleven RA, Koval G, et al. In vivo determination of optical properties of normal and tumor tissue with white light reflectance and an empirical light transport model during endoscopy. J Biomed Opt 2005;10:034018. [PMID: 16229662 DOI: 10.1117/1.1921907]
- Lee S, Vu DH, Hinds MF, Davis SJ, Liang A, et al. Pulsed diode laser-based singlet oxygen monitor for photodynamic therapy: in vivo studies of tumor-laden rats. J Biomed Opt 2008;13:064035. [PMID: 19123681 DOI: 10.1117/1.3042265]
- Hamblin MR. Photobiomodulation or low-level laser therapy. J Biophoton 2016;9:1122-4. [PMID: 27973730 DOI: 10.1002/jbio.201670113]
- Kasai K. Picosecond laser treatment for tattoos and benign cutaneous pigmented lesions (secondary publication). Laser Ther 2017;26:274-81. [PMID: 29434427 DOI: 10.5978/islsm.17-RE-02]
- Soong HK, Malta JB. Femtosecond lasers in ophthalmology. Am J Ophthalmol 2009;147:189-97.e2. [PMID: 18930447 DOI: 10.1016/j.ajo.2008.08.026].
- Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc 2016;128:2115-20. [PMID: 16464114 DOI: 10.1021/ja057254a]
- Zou L, Wang H, He B, Zeng L, Tan T, et al. Current approaches of photothermal therapy in treating cancer metastasis with nanotherapeutics. Theranostics 2016;6:762-72. [PMID: 27162548 DOI: 10.7150/thno.14988]
- Totachawattana A, Liu H, Mertiri A, Hong MK, Erramilli S, et al. Vibrational mid-infrared photothermal spectroscopy using a fiber laser probe: asymptotic limit in signal-to-baseline contrast. Opt Lett 2016;41:179-82.
- Rajakarunanayake YN, Wickramasinghe HK. Nonlinear photothermal imaging. Appl Phys Lett 1986;48:218. [DOI: 10.1063/1.96800]
- Wangzhong S, Sha H, William JS, Adah A. Review of the progress toward achieving heat confinement—the holy grail of photothermal therapy. J Biomed Opt 2017;22:1-16. [PMID: 28776627 DOI: 10.1117/1.JBO.22.8.080901]
- Celli JP, Spring BQ, Rizvi I, Evans CL, Samkoe KS, et al. Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem Rev 2010;110:2795-838. [PMID: 20353192 DOI: 10.1021/cr900300p]
- Hamblin MR, Waynant RW, Demidova TN, Anders J. Mechanisms of low level light therapy. Proc SPIE 2006;6140:614001. [DOI: 10.1117/12.646294]
- Chen H, He J, Lanzafame R, Stadler I, Hamidi HE, et al. Quantum dot light emitting devices for photomedical applications. J Soc Inform Display 2017;25:177-84. [PMID: 28867926 DOI: 10.1002/jsid.543]