Nanocarriers in the Enhancement of Therapeutic Efficacy of Natural Drugs
1Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Medical Research Center, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou 510120, China
2The First Clinical Medical School of Guangzhou University of Chinese Medicine, Guangzhou University of Chinese Medicine, Guangzhou 510004, China
3Center for Nanomedicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA
*Correspondence to: Wei Tao, E-mail: taiwei.thu@gmail.com; Phei Er Saw, E-mail: caipeie@mail.sysu.edu.cn
Received: November 5 2020; Revised: December 15 2020; Accepted: January 15 2021; Published Online: February 6 2021
Cite this paper:
Xiuling Li, Shunung Liang, Chee Hwee Tan, Shuwen Cao, Xiaoding Xu, Phei Er Saw and Wei Tao. Nanocarriers in the Enhancement of Therapeutic Efficacy of Natural Drugs. BIO Integration 2021; 2(2): 40–49.
DOI: 10.15212/bioi-2020-0040. Available at: https://bio-integration.org/
Download citation
© 2021 The Authors. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See https://bio-integration.org/copyright-and-permissions/
Abstract
Since time immemorial, plant derived natural products have been used for the treatment of various human diseases before the intervention of modern medicine. The basis of modern medicine is still being inspired from traditional medicine and therapies. However, despite their tremendous therapeutic potential, these natural drugs often have poor bioavailability, metabolic instability, and aqueous insolubility. These factors greatly impede a natural drug’s commercialization potential as a mainstream medicine. Therefore, the development of nanocarrier drug delivery systems is indispensable in overcoming the various constraints of the bottlenecks which occur with natural drugs. Of particular interest in this review are four plant materials endogenous to China with the common names of barrenwort or horny goat weed (Epimedium), Shu Di Huang (Rehmannia glutinosa, RG), ginseng (Panax ginseng), and Dong Quai or female ginseng (Angelica sinensis, AS), each having been scientifically investigated for a wide range of therapeutic uses as has been originally discovered from the long history of traditional usage and anecdotal information by local population groups in Asia. The integration of natural drugs from the East and nanocarrier drug delivery systems developed from the West is paving the way towards further accurate and efficient medicine therapy. We further discuss the potential benefits of these plants and the enhancement of their therapeutic efficacy by nanotechnology intervention.
Keywords
Nanocarriers Intervention, Natural Drugs, Therapeutic Efficacy.
Introduction
Natural products have served as indispensable sources of medicines since the beginning of time. Almost a quarter to a half of all commonly used drugs are originated from the natural products [1]. Because of their better therapeutic activity and because they have less side effects compared with homeopathic chemical medicine, phytomedicines are attracting increasing interest in clinical trials than was previously the case. Natural products usually contain various drug constituents as crude extracts, they have surprising potential in in-vitro experiments while they have a mediocre performance in-vivo due to their poor solubility and inappropriate size, resulting in low availability and absorption.
A nanocarrier drug delivery system can serve as a novel efficient method to conquer the limits of natural products. Versatile drug delivery systems like polymeric nanoparticles (NPs), magnetic NPs, liposomes, solid lipid NPs, micelles, dendrimers, and carbon nanotubes have been proven to have ability to improve the therapy effect of herbal medicines [2]. Judah Folkman at Harvard first put forward the concept of drug delivery systems in the mid-1960s. The Germen physicist Gred Bing and the Swiss physicist Heinrich Rohrer invented the scanning tunneling microscope (STM) in a Swiss laboratory in 1981. STM enabled human beings to observe the array status of individual atoms on the surface of a substance and to observe, for the first time, the physical chemical properties related to surface electronic behavior in real time. A few years later, the first nanometer size materials were discovered by Herbert Gleiter in Australia, which initiated research in the field of nanomaterials and contributed to the advances in instrumentation. Thereafter, the NP field begun to rapidly develop over the next 30 years in Western countries [3–5]. The novel nanocarrier delivery system have attracted worldwide research. Nanotechnology enhances the bioavailability and bioactivity of phytomedicine by decreasing the size into NPs, modifying surface characteristics, and promoting aqueous solubility and permeability across biological membrane [6]. The combined application of nanotechnology and natural products is a rapidly evolving field. Nanotechnology brings multiple benefits from natural compounds to many chronic human diseases.
Traditional natural tonics consist of four types including: aphrodisiacs, Yin medicine, Qi boosters, and blood tonics. Epimedium, Rehmannia glutinosa (RG), Panax ginseng, and Angelica sinensis (AS) are representative drugs in these four categories of tonics. Epimedium can nourish the kidneys and works as an aphrodisiac, strengthening muscles and bones; Rehmannia can nourish yin, enrich fluid production, ease pyrexia, and stem bleeding [7]; Ginseng is popular as a strong tonic in strengthening vitality, activating circulation, as well as calming the mind [8–11]; Angelica nourishes the blood, promoting good blood circulation, lubricating the intestine, and regulating bowel movement [12–16]. They are commonly used in traditional Chinese medicine in a variety of diseases and are mainly given by oral administration and are sometimes made into drops or given as injections. Natural products have their shortcomings but have been proved to have strong therapeutic effects, similar to nanocarrier drug delivery systems used to treat the same condition. Integrating Eastern and Western medicine may complement their advantages to accomplish targeted efficient treatment as well as decrease side effects, gradually pushing medical discovery [17]. The purpose of this review is to generalize the therapeutic effects of these plants and discuss their further usages using nanotechnology.
Epimedium
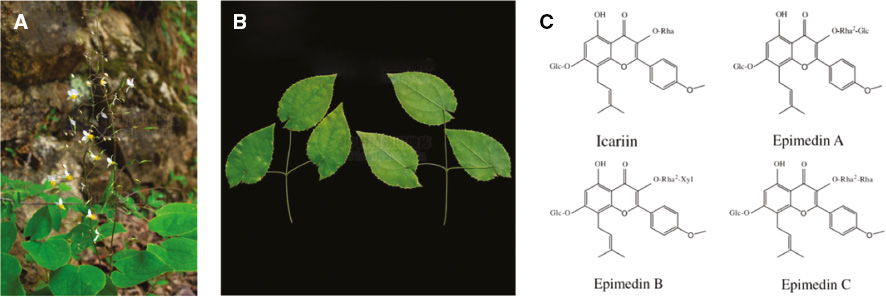
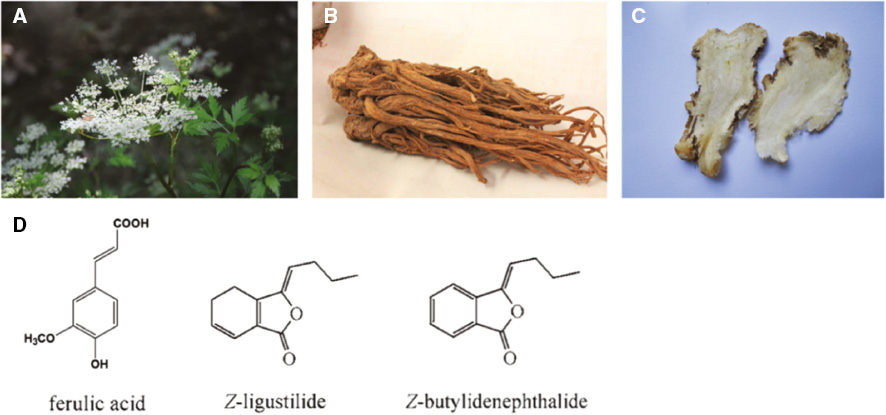
Epimedium is a herb in the Berberidaceae family and describes the dried leaves of the Epimedium plants: E. brevicornum Maxim., E. sagittatum Maxim., E. pubescens Maxim., or E. koreanum Nakai., also known as herba Epimdii, Yinyanghuo, fairy wings, rowdy lamb herb, barrenwort, bishop’s hat, or horny goat weed (Figure 1A, B). It has been used as a medicinal herb in East Asia to increase libido and improve sexual health, cognitive function and brain health, for cardiovascular diseases, to boost testosterone boost, for osteoporosis and neurasthenia [18]. The main constituents of Epimedium are flavonoids, alkaloids, polysaccharides, terpenoids, and lignan compounds [19], which are usually abstracted by conventional Soxhlet extraction, boiling extraction, or using an ultrasonic technique [20]. Flavonoids, the major constituents in Epimedium, including icariin and epimedin A–C (Figure 1C), have been shown to implement various clinical efficacies such as promoting cardiovascular health, stimulating sexual function, and as an anti-inflammation, anti-cancer, anti-osteoporosis, and anti-oxidation agents [21, 22]. Alkaloid has also been found to play an important role as antioxidants and anti-glycemic agents [23, 24].
Figure 1 (A) The whole plant of Epimedium; (B) the medicinal part of Epimedium (from the Plant Photo Bank of China); (C) the chemical structures and names of the major compounds isolated from Epimedium.
Recent studies have demonstrated that icariin, as the major pharmacologically active flavonoid component of Epimedium, induced G2/M arrest of the cell cycle and cell death through the activation of the mitogen-activated protein kinase (MAPK)/extracellular-signal-regulated kinase (ERK) pathway to inhibit the growth of breast cancer [25]. Thus it might be a potent growth inhibitor and trigger apoptosis of cancer cell via nuclear factor kappa B (NF-κB)-mediated cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein (c-FLIP) expression [26] and the mitochondria- and Fas-mediated caspase-dependent pathways [27], or its regulatory mechanism of miR-21 which targets phosphatase and tensin homolog (PTEN), reversion inducing cysteine rich protein with Kazal motifs (RECK) and BCL2 apoptosis regulator (BCL2) [28]. The Epimedium herb was further investigated to show that it has anti-angiogenic effects by acting on ERK signaling pathway in human umbilical vein endothelial cells (HUVECs) [29] and overcomes T790M mutation-mediated drug resistance in nonsmall-cell lung cancer (NSCLC) via the combination with gefitinib [30].
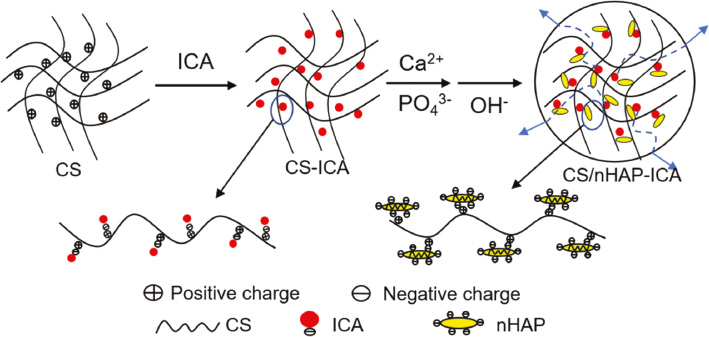
In previous studies, the clinical application of icariin has been hindered because of its poor absorption and low bioavailability after intravenous or intra-gastric administration, implying that new efficient carriers should be developed for icariin injection. Yang et al. described that icariin propylene glycol-liposome suspension (ICA-PG-liposomes) had different pharmacokinetic behavior and better tissue distribution compared with ICA-PG-solution [31]. They prepared the ICA-PG-liposome suspension by mixing trehalose solution and ICA-PG solution and collected the tissues and plasma after intraperitoneal administration from mice. In animal experiments, the ICA-PG-liposome with its nanometer size and high encapsulation, could significantly prolong the mean retention time, strengthen icariin absorption, raise the maximum concentration and the area under curve in plasma, while it led to abundant icariin being enriched to the spleen, liver, and other tissues. Thus, liposome-based carriers fulfill the drug therapy effects when combined with icariin than icariin therapy alone. The sudden release and the release rate of drugs are the problems that commonly hamper their application in clinical applications. Chen et al. designed an organic–inorganic hybrid composite microsphere encapsulating icariin with chitosan/nanohydroxyapatite (CS/nHAP) as a drug delivery carrier [32]. Due to the electrostatic interaction, icariin carrying reactive negative hydroxyl (–OH) could combine to the positive amine groups (–NH2) on the net of CS, and the nHAP inside the CS retained a homogeneously dispersed condition (Figure 2). These two points showed that icariin-loaded microspheres could keep a continuous release in in-vitro studies. As a promising suitable drug delivery system, icariin-loaded CS/nHAP had been proven to do well on up-regulating the bioactivity of osteoblasts. Another study fabricated a CS/gelatin multilayer film coated with icariin-loaded titanium dioxide (TiO2) nanotubes that successfully enhance the spreading and adhesion of osteoblasts as well as stimulated the activity of bone-related genes. The composite structure can conquer the shortages (like bio-inertia, passive bone integration, and deficient osteo-induction) of a Ti-implant while controlling the release profile of icariin to achieve better bone repair compared with pure Ti or multilayer coated nanotubes, resulting in the development of implants in the field of orthopedic and dental research [33].
Figure 2 The scheme of the icariin-loaded CS/nHAP microspheres.
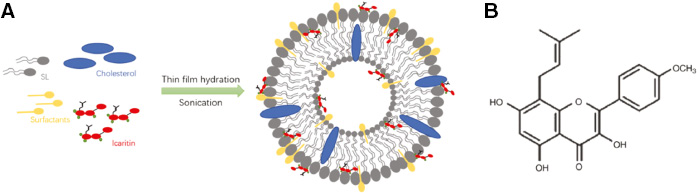
Kedong Tai et al. monitored the characteristic of icaritin-loaded liposomes under different surfactants and cholesterol-to-soybean-lecithin (Chol/SL) mass ratios [34] (Figure 3A). Lipophilic icaritin (Figure 3B) is the derivate of icariin as a prenylated flavonoid, usED in various fields like cancer intervention, immunosuppressive therapy, and bone repair with the limitation of being sensitive to light, heat, oxidants, and oxygen which makes it to store and limits its application. The study found that the average diameter and encapsulation efficiency exhibited growth when added with cholesterol and icaritin into bilayers, also surfactants such as sucrose esters and Tween-80 can benefit liposomal systems with the highest release levels (75.68 ± 0.25% and 77.23 ± 0.58%) in in vitro digestion experiments and show the best physicochemical stability. Liposomes could be a candidate vesicle, which can be used to treat the dispersion of icaritin and other flavonoid compounds in aqueous solutions because of their better biological activity.
Figure 3 (A) The scheme of icaritin-loaded liposomes with surfactants; (B) the chemical structure of icaritin [34].
Rehmannia glutinosa
RG, the radix of R. glutinosa Libosch. belongs to the family Scrophulariaceae, is usually harvested in the autumn and processed into ‘Shu-Dihuang’ by stewing with liquor or steaming (Figure 4A–C). The main compounds which are responsible for the pharmacological activity of Rehmannia contain iridoid glycosides, catalpol (Figure 4D) and RG polysaccharides [35]. It is considered that iridoid glycosides have a potential anti-osteoporotic effect, RG polysaccharides can regulate immune response [36] and glucolipid metabolism [37], as well as catalpol which is used in in-vitro experiments in various disease like cancer, diabetes, atherosclerosis, neurovascular diseases, and so on [38–42]. Pharmacological research has shown that RG may act as a potential candidate drug in diabetes, osteoporosis, gynecological, and hematological diseases.
Figure 4 (A) Aerial part of R. glutinosa Libosch.; (B) fresh radix R. glutinosa; (C) Shu Dihuang, processed product of radix R. glutinosa (from the Plant Photo Bank of China); (D) chemical structure of catalpol.
RG might become a prospective medicine to prevent the Alzheimer’s disease via the protein kinase C (PKC) and ERK1/2 pathways by upregulating the expression of the glial cell line-derived neurotrophic factor gene, which can revive the midbrain embryonic dopaminergic neurons [43]. RG extract could (1) downregulate the expression of transcription factors (NF-κB, c-Fos, NFATc1) and activation of MAPK to defeat against ovariectomy (OVX)-induced bone loss, osteoclast differentiation, and maturation and release of inflammatory cytokine [44], (2) stimulate the IGF-1/PI3K/mTOR pathways to enhance bone formation, resulting in being a hopeful candidate agent to prevent diabetic osteoporosis [45]. Catalpol was found to be able to alleviate atherosclerosis by modulating the PGC-1α/TERT pathway [46].
Rehmannia glutinosa polysaccharide (RGP) has been found that struggling in short action time, poor targeting effect and being easily cleared in the body while need to use a large clinical dosage in the clinical use. Huang et al. used the thin film hydration method and ultra-sonication technique to self-assemble the PEGylation nano-RGP (pRL), and they optimized the preparation to fit the drug targeting effect and enhance the immunological function [47]. RGP solution was hydrated with the dry film which was made with lipid and cholesterol. After being roto-evaporated, homogenized, and filtered, the pRL became a homogeneous solution with the size of 31.98 ± 2.6 nm and encapsulation efficiency of 95.81 ± 1.58%. Polymer polyethylene glycol (PEG) is widely used in nanotechnology and drug delivery for its biocompatibility and because it is non-toxic. Huang et al.’s report found that PEGylation of NPs could effectively improve its pharmacokinetic profile, reduce dosing concentration, and promote the targeting effect in the use of RGP. In the in-vitro experiments, pRL promoted the release of pro-inflammatory cytokines, encouraged macrophage proliferation, and enhanced cellular uptake which was better than nano-RGP.
Qin et al. combined the RGP-liposome (RGPL) with antigen to show the role of NP-based vaccine adjuvant [48]. RGP was known to have the desired immunomodulation enhancement in a low dosage after encapsulating by liposomes. This study further researched how RGPL worked in the immunological environment. When carrying the antigen [OVA-specific immunoglobulin (IgG) or porcine circovirus type 2 (PCV-2)-specific IgG18] on the surface, RGPL could stimulate a higher level of antibodies than RGP. In addition, (1) larger production of interferon-gamma (IFN-γ) and interferon (IL)-4, and a strong IgG response were found in in vivo experiments which were regarded as the signal of the activation of Th1 and Th2 subsets, and (2) RGPL did a good job in generating immune memory and activating dendritic cell (DC) maturation compared with antigen-carried RGP. Polysaccharide-based liposome adjuvants might be a promising candidate in developing a novel vaccine adjuvant system.
Panax ginseng
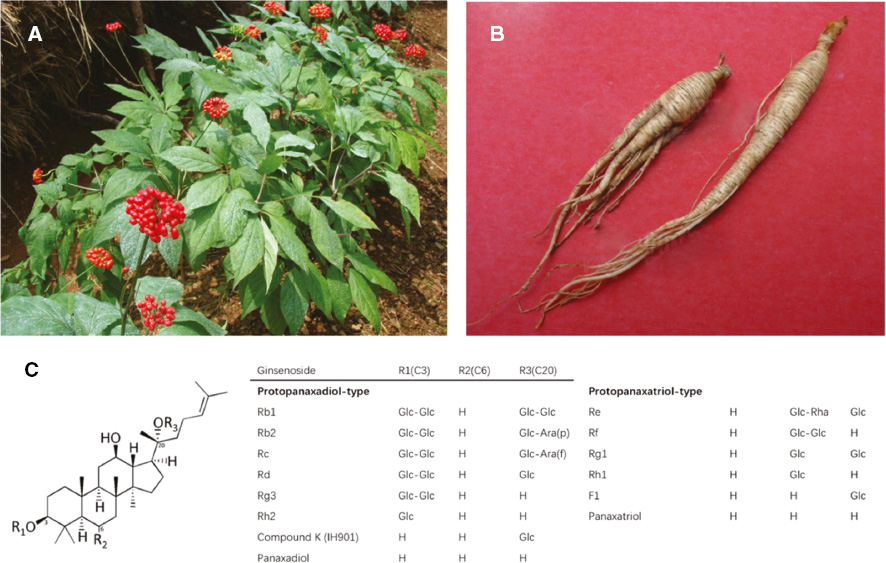
Panax ginseng, the dry radix and rhizome of P. ginseng C. A. Mey. in the family Araliaceae (Figure 5A, B), means ‘all-healing’ due to its wide clinical therapeutic effects [49]. Depending on its different planting environments and processing methods, ginseng has different product names like ‘garden ginseng’ (artificial cultivation), ‘Zi-Hai’ (grown naturally in the wild in mountain forests), ‘Shengshai ginseng’ (washed and dried) and ‘red ginseng’ (steamed and dried), etc. [50]. Several clinical reports on ginseng have proved its therapeutic properties such as promoting glucose metabolism, moderating the immune response, regulating hypertension, and strengthening the body [51]. Saponins, polysaccharides, and phenolics are the key compounds of ginseng exact [52–54] (Figure 5C). Saponins in ginseng are usually known as ginsenosides, which have been reported to have anti-cancer and immune-modulatory capacity with polysaccharides [55, 56]. Phenolics are known to have antioxidant activity.
Figure 5 (A) Aerial portion of P. ginseng; (B) the dry radix and rhizome of P.x ginseng (from the Plant Photo Bank of China); (C) the chemical structure of ginsenoside. R1, R2, R3 are the sites of sugar branched chain on the steroidal saponins skeleton of ginsenoside.
As one of the active metabolites of ginseng saponin, compound K can induce tumor cell apoptosis by reviving epigenetically-silenced genes and suppressing DNA methyltransferase 1 protein expression to have antitumor effects [57]. Polysaccharides were reported to modulate Twist and target its downstream gene expression to accomplish cell apoptosis and cell cycle arrest [58], while ginsenoside Rp1, through regulating CD29-mediated cell adhesion, suppresses the production of IL-1β by the NF-κB pathway [59, 60]. And Rp1 can reduce the proliferation of cancer cells via the insulin-like growth factor 1 receptor/Akt pathway [61].
Crude and major active compounds of ginseng are degraded by intestinal microbiota after oral administration, resulting in low oral bioavailability, limited solubility, and untargeted cytotoxicity. As a result, the development of a drug delivery system and biomolecular conjugation techniques may inspire the clinical application of ginseng extract.
Kim et al. explored the new ingredient of gold NPs (AuNPs) synthesized from ginseng berry (GB) extract in the field of cosmetics [62]. They found that the components of GB extract could make the metal salts stabilize and turn into biocompatible functional NPs. GB-AuNPs were synthesized by adding gold (III) chloride trihydrate, used as precursor salt, into aqueous GB extract. After co-cultivating with the human dermal fibroblast (HDF) and murine melanoma B16BL6 (B16) cells, cell viability was still in a stable rate while GB-AuNPs could decrease the damage caused by hydrogen peroxide (H2O2), generally enhance the moisture retention function, and download the expression of cellular tyrosinase and melanin. GB-medicated AuNPs, being multifunctional and biocompatible, could be a promising cosmetic ingredient.
In another report, Ahn et al. used the leaf extract of P. ginseng Meyer (PG) to complex with AuNPs and discovered that PG-AuNPs could exert an anti-inflammatory effect via the p38 MAPK pathway to block lipopolysaccharide (LPS)-medicated NF-κB activation in macrophages, downregulate the expression of nitric oxide (NO), prostaglandin E2 (PEG2), IL-6, and tumor necrosis factor-alpha (TNF-α) [63]. The research on PG-AuNPs may provide a novel therapy method to medicate inflammation. The silver NP (AgNP) synthesized from PG could also stimulate the MAPK pathway to control cell apoptosis, and reduce the viability and migration of cancer cells in anti-cancer therapy [64].
Singh et al. first found that fresh root extract of P. ginseng was suitable to composite with gold and silver NPs and manufactured a rapid and convenient method for the biosynthesis [65]. In brief, ginseng root stock filtrate was mixed with sterile water, silver nitrate was then added and the reaction mixture was kept at 80°C for 2 h. Using the same methodology for AuNPs, gold (III) chloride trihydrate was added instead at 80°C for 5 min. The change in color of the mixture indicated the end of reaction, the synthesis of AgNPs and AuNPs was done when the color turned from being white to being brown or pink. Ginsenosides and polysaccharides, the phytochemicals of P. ginseng, can influence the production of NPs due to its efficient reduction action. The saponin glycosides can stabilize the NPs by providing a coating on the surface of the NPs.
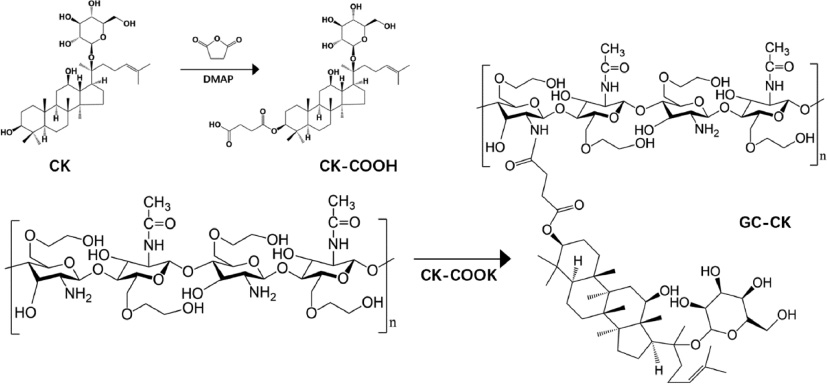
Ramya et al. designed a self-assembled conjugated spherical NP, using hydrophobic ginsenoside compound K (CK) and hydrophilic glycol chitosan (GC), to enhance water solubility and the targeted delivery of ginsenosides [66]. Through an acid-labile linkage, CK was covalently conjugated to the frame of GC and had two particles sizes (255 nm and 296 nm) according to the degree of CK substitution (Figure 6). CK-GC NPs gradually degrades in acidic conditions (pH 5.0) and remains stable in a physiological buffer (pH 7.4), this made it possible for CK targeted intracellular or tumor tissue delivery for suitable pH conditions. Compared with the free drug, utilizing nanosized drug carriers to encapsulate the drugs could improve its pharmacological efficacy. Due to its targeted delivery and the higher cytotoxicity of cancer cells, CK-GC conjugate could promote the anti-tumor activity of CK as a tumor-specific delivery carrier. In addition, using polyglutamic acid (PGA) or fucoidan (Fu), which are antithrombotic materials, to encapsulate the red ginseng, extract loaded CS NPs could enhance ginsenoside solubility and support its release in an acidic environment [67].
Figure 6 Synthetic route for preparation of GC-CK conjugates.
Angelica sinensis
AS, the radix of the A. sinensis (Oliv.) Diels in the Umbelliferae family (Figure 7A–C), is a traditional herbal medicine known as ‘Danggui’ in Chinese for its ‘blood nourishing’ quality [68]. It is commonly used to animate and replenish blood, alleviate pain, and lubricate the intestines as a medicinal and edible plant. Recent pharmacological studies showed that AS extract has wide bioactive effects including: anti-inflammatory, anti-cancer, anti-cardiovascular, memory amelioration, neuroprotective, immunomodulatory, anti-oxidative, radioprotective, and anti-hepatotoxic, etc. [69]. AS has been used therapeutically in nephrotic syndrome, gynecologic diseases, nervous system diseases, and cardiovascular disease. Phthalides, ferulic acid, and polysaccharides are considered as the active components of AS [70] (Figure 7D). Phthalides are reported in diastolic vascular smooth muscle and fight against oxidative stress [71]; ferulic acid exhibits estrogenic activity, and polysaccharides contribute to anti-cancer theraoy and immunomodulation [72].
Figure 7 (A) Aerial portion of A. sinensis (Oliv.) Diels; (B) radix A. sinensis; (C) processed product of radix A. sinensis (from the Plant Photo Bank of China); (D) the chemical structures and names of A. sinensis.
Cancer studies have reported that two polysaccharides from AS can promote the proliferation of the splenocytes, advance the mRNA expression of IL-2/6, IFN-γ in splenocytes, and impel the release of TNF-α and NO in peritoneal macrophages to display anti-tumor activities [72]. Other studies suggested that AS can activate the Nrf2 pathway, downregulate IL-2β and cause TNF-α secretion of LPS induced to protect against oxidative stress [73]; as well as demethylate the Nrf2 promoter CpGs, re-express Nrf2 and its target genes to prevent the development of prostate cancer [74].
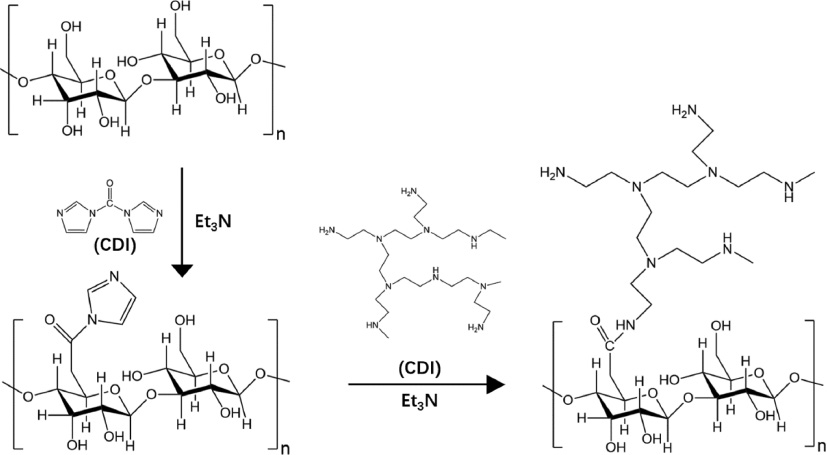
Deng et al. provided the first report showing that A. sinensis polysaccharide (ASP) had been tested extensively as an efficient gene delivery material for its advantages including biodegradability, biocompatibility, and reduced cytotoxicity. They designed a gene vector cationizated ASP (cASP) which was modified with a branched low-molecular-weight poly-ethylenimine (LMW PEI) (1200 Da) through the linkage of the –NHCO– group to deliver the plasmid DNA encoding transforming growth factor β1 (pTGF-β1) into mesenchymal stem cells [75] (Figure 8). cASP has a positive charge on the surface so that it can combine and reverse the charge of plasmid DNA from negative to positive while ASP without modification could not really retain DNA. Polysaccharides could be transported into cells through the known biological process, resulting in the better transfection. More importantly, the combination with low molecular weight polyethylenimine (LMW PEI), which has low cytotoxicity, and biodegradable natural polysaccharide cASP might help shape cASP/pTGF-β1 NPs to have reduced toxicity and efficient transfection. The cationized ASP (cASP) was combined with the plasmid encoding transforming growth factor-beta 1 (pTGF-β1) to form a spherical nano-scaled particle (cASP/pTGF-β1 nanoparticle). It suggested that cASP/pTGF-β1 NPs could advance the delivery of genes into cells faster and more efficiently than the traditional transfection method Lipofectamine 2000 group which was treated with pTGF-β1 plus Lipofectamine 2000 (200 ng pTGF-β1 complexed with 0.5 μL Lipofectamine 2000 for each well).
Figure 8 Schematic drawing for the preparation of A. sinensis polysaccharide (cASP).
In another example, Gu et al. used a water/oil/water (w1/o/w2) double emulsion technique to encapsulate ASP into the poly (lactic-co-glycolic acid) (PLGA), resulting in constituting the ASP-loaded PLGA NP system which is able to impel the proliferation of lymphocytes and T cells compared with blank PLGA and free ASP [76]. Due to some characteristics like fast metabolism, low biocompatibility, and non-concentrated action scope, the application of ASP is limited in clinical use. As a widely used drug delivery system, PLGA can control the secretion of the encapsulated drugs to reach the drug target and have better bioavailability. An ASP-PLGA conjugate may combine the immunological activity of ASP with PLGA targeted delivery, with an emerging synergistic effect compared with ASP alone.
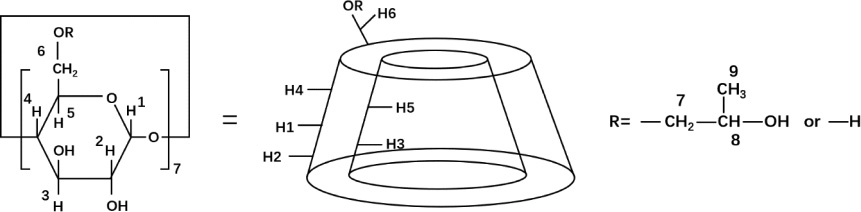
Hsu et al. fabricated a complex mixing AS extract with 2-hydroxypropyl-β-cyclodextrin (HP-β-CD) (Figure 9) to overcome the insolubility of AS extract [77]. Using the freeze-drying method, AS-HP-β-CD complex was prepared and gradually tested to have better aqueous solubility and homogeneous dispersibility, improving the uptake and cytotoxicity of hepatocellular carcinoma compared with the AS extract.
Figure 9 Chemical structures of HP-β-CD.
Conclusion and future outlook
Traditional herbal medicines have potential effects which can inspire the development of modern medicines. Mean active ingredients in botanical herbs are gradually being extracted for clinical use as pharmaceutical formulas. Natural products have great biological activity in vitro, however, they suffer the challenges of poor solubility, large molecular size that would cause them to be trapped by the membrane barrier, degradation in the gastrointestinal environment, and the need of taking larger doses to reach effective concentrations. Nanocarrier drug delivery systems can break free from the limits of natural drugs. By changing the NP sizes and characteristics of its surface, using different polymers of nanomaterials to encapsulate active pharmaceutical compounds or to attach to the surface of the drugs to modify its surface properties, nanotechnology can enhance targeted delivery, sustain and control the release of drugs, and change pharmacokinetics to accomplish better clinical outcomes [78]. Epimedium, RG, Panax ginseng, and AS are commonly used in tonics, and their effects have been amplified by the intervention of nanocarrier delivery systems. Integrating natural products and nanocarriers is a major leap in clinical trials, and it gives several inspiring avenues to pharmaceutical development and other areas. The plant-derived drugs combined with nanocarriers will become a potential candidates in clinical therapy.
References
- Kingston DG. Modern natural products drug discovery and its relevance to biodiversity conservation. J Nat Prod 2011;74:496-511. [PMID: 21138324 DOI: 10.1021/np100550t]
- Khan T, Gurav P. PhytoNanotechnology: enhancing delivery of plant based anti-cancer drugs. Front Pharmacol 2017;8:1002. [PMID: 29479316 DOI: 10.3389/fphar.2017.01002]
- Hoffman AS. The origins and evolution of “controlled” drug delivery systems. J Control Release 2008;132:153-63. [PMID: 18817820 DOI: 10.1016/j.jconrel.2008.08.012]
- Müller RH, Shegokar R, Keck CM. 20 years of lipid nanoparticles (SLN and NLC): present state of development and industrial applications. Curr Drug Discov Technol 2011;8:207-27. [PMID: 21291409 DOI: 10.2174/157016311796799062]
- Faucher S, Le Coustumer P, Lespes G. Nanoanalytics: history, concepts, and specificities. Environ Sci Pollut Res Int 2019;26:5267-81. [PMID: 29549615 DOI: 10.1007/s11356-018-1646-6]
- Hans ML, Lowman AM. Biodegradable nanoparticles for drug delivery and targeting. Curr Opin Solid State Mater Sci 2002;6:319-27. [DOI: 10.1016/S1359-0286(02)00117-1]
- Cheng YY, Hsieh CH, Tsai TH. Concurrent administration of anticancer chemotherapy drug and herbal medicine on the perspective of pharmacokinetics. J Food Drug Anal 2018;26:S88-95. [PMID: 29703390 DOI: 10.1016/j.jfda.2018.01.003]
- Nocerino E, Amato M, Izzo AA. The aphrodisiac and adaptogenic properties of ginseng. Fitoterapia 2000;71:S1-5. [PMID: 10930706 DOI: 10.1016/s0367-326x(00)00170-2]
- Radad K, Gille G, Liu L, Rausch WD. Use of ginseng in medicine with emphasis on neurodegenerative disorders. J Pharmacol Sci 2006;100:175-86. [PMID: 16518078 DOI: 10.1254/jphs.crj05010x]
- Park HJ, Kim DH, Park SJ, Kim JM, Ryu JH. Ginseng in traditional herbal prescriptions. J Ginseng Res 2012;36:225-41. [PMID: 23717123 DOI: 10.5142/jgr.2012.36.3.225]
- Choi SH, Jung SW, Lee BH, Kim HJ, Hwang SH, et al. Ginseng pharmacology: a new paradigm based on gintonin-lysophosphatidic acid receptor interactions. Front Pharmacol 2015;6:245. [PMID: 26578955 DOI: 10.3389/fphar.2015.00245]
- Cho CH, Mei QB, Shang P, Lee SS, So HL, et al. Study of the gastrointestinal protective effects of polysaccharides from Angelica sinensis in rats. Planta Medica 2000;66:348-51. [PMID: 10865452 DOI: 10.1055/s-2000-8552]
- Zhou S, Zhang B, Liu X, Teng Z, Huan M, et al. A new natural angelica polysaccharide based colon-specific drug delivery system. J Pharm Sci 2009;98:4756-68. [PMID: 19408300 DOI: 10.1002/jps.21790]
- Wei WL, Zeng R, Gu CM, Qu Y, Huang LF. Angelica sinensis in China – a review of botanical profile, ethnopharmacology, phytochemistry and chemical analysis. J Ethnopharmacol 2016;190:116-41. [PMID: 27211015 DOI: 10.1016/j.jep.2016.05.023]
- Hua Y, Yao W, Ji P, Wei Y. Integrated metabonomic-proteomic studies on blood enrichment effects of Angelica sinensis on a blood deficiency mice model. Pharm Biol 2017;55:853-63. [PMID: 28140733 DOI: 10.1080/13880209.2017.1281969]
- Ji P, Wei Y, Hua Y, Zhang X, Yao W, et al. A novel approach using metabolomics coupled with hematological and biochemical parameters to explain the enriching-blood effect and mechanism of unprocessed Angelica sinensis and its 4 kinds of processed products. J Ethnopharmacol 2018;211:101-16. [PMID: 28958590 DOI: 10.1016/j.jep.2017.09.028]
- Er Saw P, Jiang S. The significance of interdisciplinary integration in academic research and application. BIO Integration 2020;1:2-5. [DOI: 10.15212/bioi-2020-0005]
- Ulbricht CE, Natural Standard Research Collaboration. An evidence-based systematic review of yin yang huo (Epimedium spp.) by the Natural Standard Research Collaboration. J Diet Suppl 2016;13:136-64. [PMID: 26268839 DOI: 10.3109/19390211.2015.1008817]
- Ren L, Guo MY, Pang XH. Identification and classification of medicinal plants in Epimedium. Chinese Herbal Med 2018;10:249-54. [DOI: 10.1016/j.chmed.2018.05.004]
- Zhang HF, Yang TS, Li ZZ, Wang Y. Simultaneous extraction of epimedin A, B, C and icariin from Herba Epimedii by ultrasonic technique. Ultrason Sonochem 2008;15:376-85. [PMID: 17951093 DOI: 10.1016/j.ultsonch.2007.09.002]
- Meng FH, Li YB, Xiong ZL, Jiang ZM, Li FM. Osteoblastic proliferative activity of Epimedium brevicornum Maxim. Phytomedicine 2005;12:189-93. [PMID: 15830840 DOI: 10.1016/j.phymed.2004.03.007]
- Song J, Shu L, Zhang Z, Tan X, Sun E, et al. Reactive oxygen species-mediated mitochondrial pathway is involved in baohuoside I-induced apoptosis in human non-small cell lung cancer. Chem Biol Interact 2012;199:9-17. [PMID: 22687635 DOI: 10.1016/j.cbi.2012.05.005]
- Račková L, Májeková M, Košt’álová D, Štefek M. Antiradical and antioxidant activities of alkaloids isolated from Mahonia aquifolium. Structural aspects. Bioorg Med Chem 2004;12:4709-15. [PMID: 15358297 DOI: 10.1016/j.bmc.2004.06.035]
- Patel MB, Mishra SM. Magnoflorine from Tinospora cordifolia stem inhibits α-glucosidase and is antiglycemic in rats. J Funct Foods 2012;4:79-86. [DOI: 10.1016/j.jff.2011.08.002]
- Guo Y, Zhang X, Meng J, Wang Z-Y. An anticancer agent icaritin induces sustained activation of the extracellular signal-regulated kinase (ERK) pathway and inhibits growth of breast cancer cells. Eur J Pharmacol 2011;658:114-22. [PMID: 21376032 DOI: 10.1016/j.ejphar.2011.02.005]
- Han H, Xu B, Hou P, Jiang C, Liu L, et al. Icaritin sensitizes human glioblastoma cells to TRAIL-induced apoptosis. Cell Biochem Biophys 2015;72:533-42. [PMID: 25577511 DOI: 10.1007/s12013-014-0499-y]
- Sun L, Peng Q, Qu L, Gong L, Si J. Anticancer agent icaritin induces apoptosis through caspase-dependent pathways in human hepatocellular carcinoma cells. Mol Med Rep 2015;11:3094-100. [PMID: 25434584 DOI: 10.3892/mmr.2014.3007]
- Li J, Jiang K, Zhao F. Icariin regulates the proliferation and apoptosis of human ovarian cancer cells through microRNA-21 by targeting PTEN, RECK and Bcl-2. Oncol Rep 2015;33:2829-36. [PMID: 25845681 DOI: 10.3892/or.2015.3891]
- Yu X, Tong Y, Han XQ, Kwok HF, Yue GG, et al. Anti-angiogenic activity of Herba Epimedii on zebrafish embryos in vivo and HUVECs in vitro. Phytother Res 2013;27:1368-75. [PMID: 23147754 DOI: 10.1002/ptr.4881]
- Song J, Zhong R, Huang H, Zhang Z, Ding D, et al. Combined treatment with Epimedium koreanum Nakai extract and gefitinib overcomes drug resistance caused by T790M mutation in non-small cell lung cancer cells. Nutr Cancer 2014;66:682-9. [PMID: 24738693 DOI: 10.1080/01635581.2014.895392]
- Yang W, Yu XC, Chen XY, Zhang L, Lu CT, et al. Pharmacokinetics and tissue distribution profile of icariin propylene glycol-liposome intraperitoneal injection in mice. J Pharm Pharmacol 2012;64:190-8. [PMID: 22221094 DOI: 10.1111/j.2042-7158.2011.01388.x]
- Chen J, Pan P, Zhang Y, Zhong S, Zhang Q. Preparation of chitosan/nano hydroxyapatite organic–inorganic hybrid microspheres for bone repair. Colloids Surf B Biointerfaces 2015;134:401-7. [PMID: 26218713 DOI: 10.1016/j.colsurfb.2015.06.072]
- Zhang Y, Chen L, Liu C, Feng X, Wei L, et al. Self-assembly chitosan/gelatin composite coating on icariin-modified TiO2 nanotubes for the regulation of osteoblast bioactivity. Mater Des 2016;92:471-9. [DOI: 10.1016/j.matdes.2015.12.023]
- Tai K, He X, Yuan X, Meng K, Gao Y, et al. A comparison of physicochemical and functional properties of icaritin-loaded liposomes based on different surfactants. Colloids Surf A Physicochem Eng Asp 2017;518:218-31. [DOI: 10.1016/J.COLSURFA.2017.01.019]
- Liu C, Ma R, Wang L, Zhu R, Liu H, et al. Rehmanniae radix in osteoporosis: a review of traditional Chinese medicinal uses, phytochemistry, pharmacokinetics and pharmacology. J Ethnopharmacol 2017;198:351-62. [PMID: 28111216 DOI: 10.1016/j.jep.2017.01.02]
- Zhang Z, Meng Y, Guo Y, He X, Liu Q, et al. Rehmannia glutinosa polysaccharide induces maturation of murine bone marrow derived dendritic cells (BMDCs). Int J Biol Macromol 2013;54:136-43. [PMID: 23246902 DOI: 10.1016/j.ijbiomac.2012.12.005]
- Zhou J, Xu G, Ma S, Li F, Yuan M, et al. Catalpol ameliorates high-fat diet-induced insulin resistance and adipose tissue inflammation by suppressing the JNK and NF-kappaB pathways. Biochem Biophys Res Commun 2015;467:853-8. [PMID: 26474703 DOI: 10.1016/j.bbrc.2015.10.054]
- Gao N, Tian JX, Shang YH, Zhao DY, Wu T. Catalpol suppresses proliferation and facilitates apoptosis of OVCAR-3 ovarian cancer cells through upregulating microRNA-200 and downregulating MMP-2 expression. Int J Mol Sci 2014;15:19394-405. [PMID: 25347277 DOI: 10.3390/ijms151119394]
- Liu C, Wu F, Liu Y, Meng C. Catalpol suppresses proliferation and facilitates apoptosis of MCF-7 breast cancer cells through upregulating microRNA-146a and downregulating matrix metalloproteinase-16 expression. Mol Med Rep 2015;12:7609-14. [PMID: 26458573 DOI: 10.3892/mmr.2015.4361]
- Liu JY, Zhang DJ. Amelioration by catalpol of atherosclerotic lesions in hypercholesterolemic rabbits. Planta Med 2015;81:175-84. [PMID: 25671384 DOI: 10.1055/s-0034-1396240]
- Zhou J, Xu G, Yan J, Li K, Bai Z, et al. Rehmannia glutinosa (Gaertn.) DC. polysaccharide ameliorates hyperglycemia, hyperlipemia and vascular inflammation in streptozotocin-induced diabetic mice. J Ethnopharmacol 2015;164:229-38. [PMID: 25698243 DOI: 10.1016/j.jep.2015.02.026]
- Huang JZ, Wu J, Xiang S, Sheng S, Jiang Y, et al. Catalpol preserves neural function and attenuates the pathology of Alzheimer’s disease in mice. Mol Med Rep 2016;13:491-6. [PMID: 26531891 DOI: 10.3892/mmr.2015.4496]
- Yu H, Oh-Hashi K, Tanaka T, Sai A, Inoue M, et al. Rehmannia glutinosa induces glial cell line-derived neurotrophic factor gene expression in astroglial cells via cPKC and ERK1/2 pathways independently. Pharmacol Res 2006;54:39-45. [PMID: 16600621 DOI: 10.1016/j.phrs.2006.01.014]
- Lee SY, Lee KS, Yi SH, Kook SH, Lee JC. Acteoside suppresses RANKL-mediated osteoclastogenesis by inhibiting c-Fos induction and NF-kappaB pathway and attenuating ROS production. PLoS One 2013;8:e80873. [PMID: 24324641 DOI: 10.1371/journal.pone.0080873]
- Gong W, Zhang N, Cheng G, Zhang Q, He Y, et al. Rehmannia glutinosa Libosch extracts prevent bone loss and architectural deterioration and enhance osteoblastic bone formation by regulating the IGF-1/PI3K/mTOR Pathway in streptozotocin-induced diabetic rats. Int J Mol Sci 2019;20:3964. [PMID: 31443143 DOI: 10.3390/ijms20163964]
- Zhang Y, Wang C, Jin Y, Yang Q, Meng Q, et al. Activating the PGC-1alpha/TERT pathway by catalpol ameliorates atherosclerosis via modulating ROS production, DNA damage, and telomere function: implications on mitochondria and telomere link. Oxid Med Cell Longev 2018;2018:2876350. [PMID: 30046372 DOI: 10.1155/2018/2876350]
- Huang Y, Nan L, Xiao C, Ji Q, Li K, et al. Optimum preparation method for self-assembled pegylation nano-adjuvant based on Rehmannia glutinosa polysaccharide and its immunological effect on macrophages. Int J Nanomed 2019;14:9361-75. [PMID: 31819437 DOI: 10.2147/IJN.S221398]
- Huang Y, Qin T, Huang Y, Liu Z, Bo R, et al. Rehmannia glutinosa polysaccharide liposome as a novel strategy for stimulating an efficient immune response and their effects on dendritic cells. Int J Nanomed 2016;11:6795-808. [PMID: 28008254 DOI: 10.2147/IJN.S119108]
- Wong AS, Che CM, Leung KW. Recent advances in ginseng as cancer therapeutics: a functional and mechanistic overview. Nat Prod Rep 2015;32:256-72. [PMID: 25347695 DOI: 10.1039/c4np00080c]
- Choi J, Kim TH, Choi TY, Lee MS. Ginseng for health care: a systematic review of randomized controlled trials in Korean literature. PLoS One 2013;8:e59978. [PMID: 23560064 DOI: 10.1371/journal.pone.0059978]
- Rhee MY, Kim YS, Bae JH, Nah DY, Kim YK, et al. Effect of Korean red ginseng on arterial stiffness in subjects with hypertension. J Altern Complement Med 2011;17:45-9. [PMID: 21235416 DOI: 10.1089/acm.2010.0065]
- Choi KT. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin 2008;29:1109-18. [PMID: 18718180 DOI: 10.1111/j.1745-7254.2008.00869.x]
- Xiao D, Xiu Y, Yue H, Sun X, Zhao H, et al. A comparative study on chemical composition of total saponins extracted from fermented and white ginseng under the effect of macrophage phagocytotic function. J Ginseng Res 2017;41:379-85. [PMID: 28701881 DOI: 10.1016/j.jgr.2017.03.009]
- Guo N, Zhu L, Song J, Dou D. A new simple and fast approach to analyze chemical composition on white, red, and black ginseng. Ind Crops Prod 2019;134:185-94. [DOI: 10.1016/j.indcrop.2019.03.057]
- Sun Y. Structure and biological activities of the polysaccharides from the leaves, roots and fruits of Panax ginseng C.A. Meyer: an overview. Carbohydr Polym 2011;85:490-9. [DOI: 10.1016/j.carbpol.2011.03.033]
- Oh J, Jeon SB, Lee Y, Lee H, Kim J, et al. Fermented red ginseng extract inhibits cancer cell proliferation and viability. J Med Food 2015;18:421-8. [PMID: 25658580 DOI: 10.1089/jmf.2014.3248]
- Kang KA, Kim HS, Kim DH, Hyun JW. The role of a ginseng saponin metabolite as a DNA methyltransferase inhibitor in colorectal cancer cells. Int J Oncol 2013;43:228-36. [PMID: 23652987 DOI: 10.3892/ijo.2013.1931]
- Li C, Tian ZN, Cai JP, Chen KX, Zhang B, et al. Panax ginseng polysaccharide induces apoptosis by targeting Twist/AKR1C2/NF-1 pathway in human gastric cancer. Carbohydr Polym 2014;102:103-9. [PMID: 24507261 DOI: 10.1016/j.carbpol.2013.11.016]
- Kim BH, Cho JY. Regulatory role of ginsenoside Rp1, a novel ginsenoside derivative, on CD29-mediated cell adhesion. Planta Med 2009;75:316-20. [PMID: 19165715 DOI: 10.1055/s-0028-1112213]
- Kim BH, Lee YG, Park TY, Kim HB, Rhee MH, et al. Ginsenoside Rp1, a ginsenoside derivative, blocks lipopolysaccharide-induced interleukin-1beta production via suppression of the NF-kappaB pathway. Planta Med 2009;75:321-6. [PMID: 19145554 DOI: 10.1055/s-0028-1112218]
- Kang JH, Song KH, Woo JK, Park MH, Rhee MH, et al. Ginsenoside Rp1 from Panax ginseng exhibits anti-cancer activity by down-regulation of the IGF-1R/Akt pathway in breast cancer cells. Plant Foods Hum Nutr 2011;66:298-305. [PMID: 21748437 DOI: 10.1007/s11130-011-0242-4]
- Jiménez Z, Kim YJ, Mathiyalagan R, Seo KH, Mohanan P, et al. Assessment of radical scavenging, whitening and moisture retention activities of Panax ginseng berry mediated gold nanoparticles as safe and efficient novel cosmetic material. Artif Cells Nanomed Biotechnol 2018;46:333-40. [PMID: 28393568 DOI: 10.1080/21691401.2017.1307216]
- Ahn S, Singh P, Castro-Aceituno V, Yesmin Simu S, Kim YJ, et al. Gold nanoparticles synthesized using Panax ginseng leaves suppress inflammatory – mediators production via blockade of NF-kappaB activation in macrophages. Artif Cells Nanomed Biotechnol 2017;45:270-6. [PMID: 27611566 DOI: 10.1080/21691401.2016.1228661]
- Castro-Aceituno V, Ahn S, Simu SY, Singh P, Mathiyalagan R, et al. Anticancer activity of silver nanoparticles from Panax ginseng fresh leaves in human cancer cells. Biomed Pharmacother 2016;84:158-65. [PMID: 27643558 DOI: 10.1016/j.biopha.2016.09.016]
- Singh P, Kim YJ, Wang C, Mathiyalagan R, Yang DC. The development of a green approach for the biosynthesis of silver and gold nanoparticles by using Panax ginseng root extract, and their biological applications. Artif Cells Nanomed Biotechnol 2016;44:1150-7. [PMID: 25771716 DOI: 10.3109/21691401.2015.1011809]
- Mathiyalagan R, Subramaniyam S, Kim YJ, Kim YC, Yang DC. Ginsenoside compound K-bearing glycol chitosan conjugates: synthesis, physicochemical characterization, and in vitro biological studies. Carbohydr Polym 2014;112:359-66. [PMID: 25129755 DOI: 10.1016/j.carbpol.2014.05.098]
- Kim ES, Lee J-S, Lee HG. Nanoencapsulation of red ginseng extracts using chitosan with polyglutamic acid or fucoidan for improving antithrombotic activities. J Agric Food Chem 2016;64:4765-71. [DOI: 10.1021/acs.jafc.6b00911]
- Fung FY, Linn YC. Steroids in traditional Chinese medicine: what is the evidence? Singapore Med J 2017;58:115-20. [PMID: 28361161 DOI: 10.11622/smedj.2017016]
- Chao W-W, Lin B-F. Bioactivities of major constituents isolated from Angelica sinensis (Danggui). Chin Med 2011;6:1-7. [PMID: 21851645 DOI: 10.1186/1749-8546-6-29]
- Chen XP, Li W, Xiao XF, Zhang LL, Liu CX. Phytochemical and pharmacological studies on radix Angelica sinensis. Chin J Nat Med 2013;11:577-87. [PMID: 24345498 DOI: 10.1016/S1875-5364(13)60067-9]
- Kan WL, Cho CH, Rudd JA, Lin G. Study of the anti-proliferative effects and synergy of phthalides from Angelica sinensis on colon cancer cells. J Ethnopharmacol 2008;120:36-43. [PMID: 18718517 DOI: 10.1016/j.jep.2008.07.027]
- Cao W, Li XQ, Wang X, Li T, Chen X, et al. Characterizations and anti-tumor activities of three acidic polysaccharides from Angelica sinensis (Oliv.) Diels. Int J Biol Macromol 2010;46:115-22. [PMID: 19941888 DOI: 10.1016/j.ijbiomac.2009.11.005]
- Saw CL, Wu Q, Su ZY, Wang H, Yang Y, et al. Effects of natural phytochemicals in Angelica sinensis (Danggui) on Nrf2-mediated gene expression of phase II drug metabolizing enzymes and anti-inflammation. Biopharm Drug Dispos 2013;34:303-11. [PMID: 23640758 DOI: 10.1002/bdd.1846]
- Su ZY, Khor TO, Shu L, Lee JH, Saw CL, et al. Epigenetic reactivation of Nrf2 in murine prostate cancer TRAMP C1 cells by natural phytochemicals Z-ligustilide and Radix angelica sinensis via promoter CpG demethylation. Chem Res Toxicol 2013;26:477-85. [PMID: 23441843 DOI: 10.1021/tx300524p]
- Deng W, Fu M, Cao Y, Cao X, Wang M, et al. Angelica sinensis polysaccharide nanoparticles as novel non-viral carriers for gene delivery to mesenchymal stem cells. Nanomedicine 2013;9:1181-91. [PMID: 23727125 DOI: 10.1016/j.nano.2013.05.008]
- Gu P, Xu S, Zhou S, Liu Z, Sun Y, et al. Optimization of Angelica sinensis polysaccharide-loaded Poly (lactic-co-glycolicacid) nanoparticles by RSM and its immunological activity in vitro. Int J Biol Macromol 2018;107(Pt A):222-9. [PMID: 28867235 DOI: 10.1016/j.ijbiomac.2017.08.176]
- Hsu CM, Tsai FJ, Tsai Y. Inhibitory effect of Angelica sinensis extract in the presence of 2-hydroxypropyl-beta-cyclodextrin. Carbohydr Polym 2014;114:115-22. [PMID: 25263871 DOI: 10.1016/j.carbpol.2014.07.042]
- Zhong CY, Zhang L, Yu L, Huang JD, Huang SY, et al. A review for antimicrobial peptides with anticancer properties: re-purposing of potential anticancer agents. BIO Integration 2020;1:151-67. [DOI: 10.15212/bioi-2020-0013]